In electric and hybrid vehicles Life Cycle Assessments (LCAs), batteries play a central role and are in the spotlight of scientific community and public opinion. Automotive batteries constitute, together with the powertrain, the main differences between electric vehicles and internal combustion engine vehicles. For this reason, many decision makers and researchers wondered whether energy and environmental impacts from batteries production, can exceed the benefits generated during the vehicle’s use phase. In this framework, the purpose of the present literature review is to understand how large and variable the main impacts are due to automotive batteries’ life cycle, with particular attention to climate change impacts, and to support researchers with some methodological suggestions in the field of automotive batteries’ LCA. The results show that there is high variability in environmental impact assessment; CO
2
eq emissions per kWh of battery capacity range from 50 to 313 g CO
2
eq/kWh. Nevertheless, either using the lower or upper bounds of this range, electric vehicles result less carbon-intensive in their life cycle than corresponding diesel or petrol vehicles.
- battery electric vehicles
- environmental impacts
- life cycle assessment
- review
1. Life Cycle Inventory—LCI
Inventory includes data collection and calculation procedures to quantify relevant inputs and outputs in the assessed system. Data collection includes their validation, data and process units’ relationship, the relationship between data and reference flow and functional unit. In our work, we focus our attention on data quality. Data for the analysis can be divided in two categories: primary data, directly collected from producers and users of the systems, and secondary data, derived from the existing literature (including databases). Although technical guidelines and two literature reviews realized by the authors and strongly recommend using primary data, most of the existing studies use secondary information. Only six of the analyzed works use primary data obtained thanks to direct collaboration with batteries manufacturers, which provided information about the amount of material for each component, energy consumption for battery production, waste, percentage of recycled material used and battery maintenance operations. One study, uses primary data only to evaluate battery energy consumption during the use phase, while only secondary data, obtained from the available literature and from the Ecoinvent database. In general, it is possible to observe a lack of primary data that either are absent or cannot be presented in the studies due to industrial confidentiality reasons. Although this critical issue is justified by the high rate of competition and innovation of the sector, the lack of information related to primary data affects the transparency and replicability of many studies. Furthermore, it is difficult to update studies based on outdated databases or to check the results against different energy mixes.
2. Life Cycle Impact Assessment—LCIA
In an LCA, the impacts evaluation phase (Life Cycle Impact Assessment—LCIA) allows the assessment of potential impacts extent using data collected in the LCI. This operation links inventory data with specific impact categories and indicators, in order to better evaluate these impacts. The LCIA phase gives important information for life cycle results interpretation. Since different studies rely on different hypotheses, make use of different databases for background data and, above all, use different Life Cycle Impact Assessment Methods with their own unit, results cannot be compared easily with each other [1]. Nevertheless, some general conclusions may be drawn. First of all, for almost all impact categories, results show that the environmental major impacts of batteries life cycle occur during the production phase [2] and are due to energy consumption during materials and component production [3][4]. In particular, anode production process is responsible for the greatest impacts for impact categories such as eutrophication and acidification, whereas the cathode has major impacts for global warming and abiotic depletion [5]. Coming to the amount of the environmental impacts, results show great variability. Variability is due to, as mentioned, the use of different hypotheses and databases, but it is also linked to the different batteries’ chemistry. As discussed, global warming is the most investigated impact category, since EV market penetration is mainly driven by transport sector decarbonization. Figure 1 summarizes results variability linked to greenhouse gas emissions per kWh of batteries capacity, relating to batteries production phase. These values are extracted or inferred by the assessed studies in this literature review. Depending on the different technologies and on the age of the studies, greenhouse gas emissions per kWh batteries capacity can range from 53 kg CO
2
2eq/kWh.
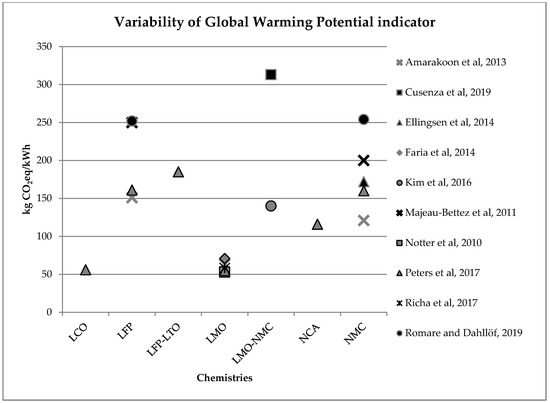
Figure 12.
2eq/kWh) for batteries production phase (LCO: Lithium Cobalt Oxide; LFP: Lithium iron phosphate; LFP-LTO: Lithium iron phosphate-Lithium Titanate; LMO: Lithium Manganese Oxide; LMO-NCM: Lithium Manganese Oxide-Lithium Nickel Cobalt Manganese; NCA: Lithium Nickel Cobalt Aluminum Oxide; NCM: Lithium Nickel Cobalt Manganese Oxide).
Considering a modern EV equipped with a 40 kWh battery lasting for 210,000 km [6], the lower and the upper values in Figure 1 correspond to an emission per km ranging from less than 10 g CO
2
2
2eq/km reported in reference [6], the total CO
2
2eq emission variability (see Table 1).
Table 13.
2
2eq emission per km derives from Figure 1
,
2eq emission per km of remaining life cycle phases are taken from reference [7].
| Vehicle Production | Battery Production | Maintenance | Road | Fuel/Electricity Production | Use | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| g CO | 2 | eq /km |
(w/out battery) | Min | Max | IT marg. Mix | Urban Cycle | Min | Max | ||
| Diesel | 38.2 | 0.0 | 0.0 | 7.7 | 0.6 | 41.1 | 198.5 | 286.1 | 286.1 |
| Electric | 37.7 | 9.5 | 59.6 | 6.2 | 0.6 | 92.5 | 0.0 | 146.6 | 196.7 |
| Petrol | 41.5 | 0.0 | 0.0 | 7.4 | 0.5 | 59.1 | 221.6 | 330.0 | 330.0 |
A similar range of variability can be found for other, less investigated, environmental impact categories (see Figure 2, Figure 3, Figure 4 and Figure 5). Again, if we consider a 40 kWh battery lasting for 210,000 km, and we consider the results from reference [7] for the other life stages, we can see that while for some impact categories for which EV perform worst, like eutrophication [7], the variability of the impacts associated with battery production does not affect the environmental ranking among EV and the corresponding ICE Vehicle. For categories like acidification, the use of the lower bound value implies that EV performs better than ICE Vehicle, while the use of the upper bound value implies that the ICE Vehicle is the best performer (see Table 2).
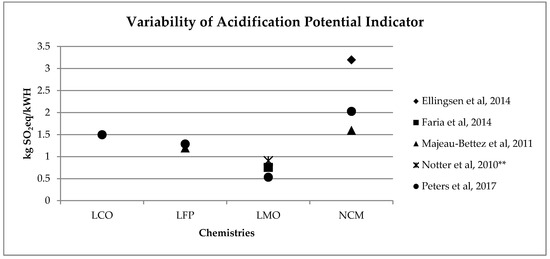
Figure 23. Variability of acidification potential (kg SO2eq/kWh) for batteries production phase (LCO: Lithium Cobalt Oxide; LFP: Lithium iron phosphate; LMO: Lithium Manganese Oxide; NCM: Lithium Nickel Cobalt Manganese Oxide); ** data from reference [3] have been updated using Ecoinvent v 3.5.
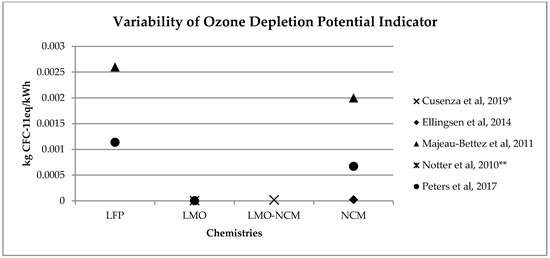
Figure 34. Variability of ozone depletion potential (kg CFC11eq/kWh) for batteries production phase (LFP: Lithium iron phosphate; LMO: Lithium Manganese Oxide; LMO-NCM: Lithium Manganese Oxide-Lithium Nickel Cobalt Manganese; NCM: Lithium Nickel Cobalt Manganese Oxide); * data from reference [2] are calculated on the basis of the total amount and the percentage for battery production, ** data from reference [3] have been updated using Ecoinvent v. 3.5.
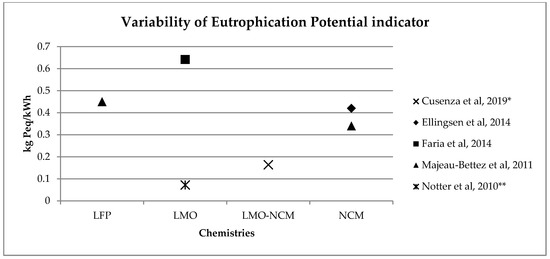
Figure 45. Variability of eutrophication potential (kg Peq/kWh) for batteries production phase (LFP: Lithium iron phosphate; LMO: Lithium Manganese Oxide; LMO-NCM: Lithium Manganese Oxide-Lithium Nickel Cobalt Manganese; NCM: Lithium Nickel Cobalt Manganese Oxide); * data from reference [2] are calculated on the basis of the total amount and the percentage for battery production, ** data from reference [3] have been updated using Ecoinvent v 3.5.
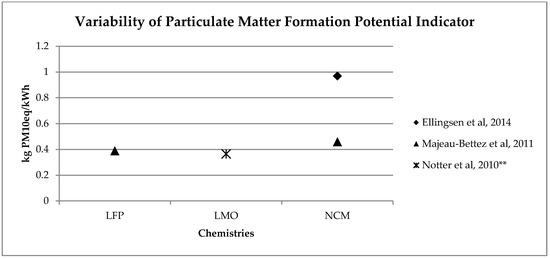
Figure 56. Variability of particulate matter formation potential (kg PM10eq/kWh) for batteries production phase (LFP: Lithium iron phosphate; LMO: Lithium Manganese Oxide; NCM: Lithium Nickel Cobalt Manganese Oxide); ** data from reference [3] have been updated using Ecoinvent v 3.5.
Table 24.
2
4eq) per kWh of battery on the life cycle comparison among a middle size electric, diesel and petrol car. Battery Emissions per km derives from Figure 2 and Figure 4, considering 40 kWh of capacity and 210,000 km of life. Emissions per km of remaining life cycle phases are taken from reference [7].
| Vehicle | Vehicle Production & Disposal | Battery Production & Disposal | Fuel/Electricity Production & Supply | Use & Maintenance | Total | |||
|---|---|---|---|---|---|---|---|---|
| Impacts/km | Type | Min | Max | Min | Max | |||
| Acidification Potential | EV | 0.10 | 0.10 | 0.70 | 0.23 | 0.02 | 0.46 | 1.00 | ||
| g SO | 2 | eq | ICE | 0.14 | - | - | 0.55 | 0.11 | 0.79 | 0.79 |
| Eutrophication Potential | EV | 0.06 | 0.01 | 0.12 | 0.06 | 0.01 | 0.27 | 0.25 | ||
| g PO | 4 | eq | ICE | 0.06 | - | - | 0.07 | 0.03 | 0.16 | 0.16 |
Of course, many of the impacts associated with battery production could be lowered by recycling battery components and using recycled materials for battery production. Recycling may reduce material production energy demand up to 50% and can help to decrease environmental impacts for all the impact categories assessed [8]. Although there are a number of technologies and combinations of technologies being developed for batteries recycling (hydrometallurgy is close at hand, and can potentially extract more materials than pyrometallurgy) [9], battery recycling options are not always included in the analysis, due to the lack of relevant and reliable information [1]. Recently, a very careful recycle phase analysis has been realized in reference [2]. This work states that the environmental credits associated with materials recovered through battery recycling processes exceed the environmental impacts associated with recycling processes in all the impact categories examined, with the exception of ozone depletion, ionizing radiation and freshwater ecotoxicity. The environmental credits are particularly relevant for some impact categories such as: marine eutrophication (−27%), human toxicity (about −20% for human toxicity no cancer effect and −40% for human toxicity cancer effect), particulate matter (−17%) and abiotic depletion (−16.4%). In particular, the environmental credits related to cobalt, nickel and manganese sulphates, copper and steel are really significant and rise up to almost 80% for an important category such as abiotic depletion. Moreover, the environmental benefits linked to recycling could be increased if other cell components/materials, such as graphite, electrolyte and aluminum, are recovered, i.e., by designing battery cells to make disassembling and separating the cell components easier and more secure [2]. Additionally, for climate change impact, recycling can gain relevant positive effects, and the saved emission can be in a range of 16–32 kg CO
- Notter, D.A.; Gauch, M.; Widmer, R.; Wager, P.; Stamp, A.; Zah, R.; Althaus, H.J. Contribution of Li-ion batteries to the environmental impact of electric vehicles. Environ. Sci. Technol. 2010, 44, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Romare, M.; Dahllöf, L. The life cycle energy consumption and greenhouse gas emissions from lithium-ion batteries. Stockholm Zugriff Am 2017, 23. Available online: https://www.ivl.se/download/18.5922281715bdaebede9559/1496046218976/C243+The+life+cycle+energy+consumption+and+CO2+emissions+from+lithium+ion+batteries+.pdf (accessed on 30 March 2020).
- Dunn, J.B.; Gaines, L.; Barnes, M.; Sullivan, J.L.; Wang, M. Material and Energy Flows in the Materials Production, Assembly, and End-of-Life Stages of the Automotive Lithium-Ion Battery Life Cycle; Argonne: Lemont, IL, USA, 2014. [Google Scholar]
- Amarakoon, S.; Smith, J.; Segal, B. Application of Life-Cycle Assessment to Nanoscale Technology: Lithium-Ion Batteries for Electric Vehicles; No. EPA 744-R-12-001; Environmental Protection Agency: Washington, DC, USA, 2013. [Google Scholar]
- ReCharge. PEFCR—Product Environmental Footprint Category Rules for High Specific Energy Rechargeable Batteries for Mobile Applications; Recharge: Brussels, Belgium, 2018. [Google Scholar]
- Richa, K.; Babbitt, C.W.; Nenadic, N.G.; Gaustad, G. Environmental trade-offs across cascading lithium-ion battery life cycles. Int. J. Life Cycle Assess. 2017, 22, 66–81. [Google Scholar] [CrossRef]
- Faria, R.; Marques, P.; Garcia, R.; Moura, P.; Freire, F.; Delgado, J.; de Almeida, A.T. Primary and secondary use of electric mobility batteries from a life cycle perspective. J. Power Sources 2014, 262, 169–177. [Google Scholar] [CrossRef]
- Oliveira, L.; Messagie, M.; Rangaraju, S.; Sanfelix, J.; Rivas, M.H.; Van Mierlo, J. Key issues of lithium-ion batteries–from resource depletion to environmental performance indicators. J. Clean. Prod. 2015, 108, 354–362. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Peters, J.F.; Baumann, M.; Zimmermann, B.; Braun, J.; Weil, M. The environmental impact of Li-Ion batteries and the role of key parameters–A review. Renew. Sustain. Energy Rev. 2017, 67, 491–506. [Google Scholar] [CrossRef]
- Majeau-Bettez, G.; Hawkins, T.R.; Strømman, A.H. Life cycle environmental assessment of lithium-ion and nickel metal hydride batteries for plug-in hybrid and battery electric vehicles. Environ. Sci. Technol. 2011, 45, 4548–4554. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Kelly, J.C.; Gaines, L.; Wang, M. Life cycle analysis of lithium-ion batteries for automotive applications. Batteries 2019, 5, 48. [Google Scholar] [CrossRef]
- ISO—The International Organization for Standardization. ISO 14040:2006: Environmental Management—Life Cycle Assessment—Principles and Framework; ISO—The International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- ISO—The International Organization for Standardization. ISO 14044:2006: Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO—The International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- European Commission—Joint Research Centre—Institute for Environment and Sustainability. International Reference Life Cycle Data System (ILCD) Handbook—Recommendations for Life Cycle Impact Assessment in the European Context, 1st ed.; EUR 24571 EN; Publications Office of the European Union: Luxembourg, 2011. [Google Scholar]
- Ellingsen, L.; Hung, C.; Strømman, A. Identifying key assumptions and differences in life cycle assessment studies of lithium-ion traction batteries with focus on greenhouse gas emissions. Transp. Res. Part D Transp. Environ. 2017, 55, 82–90. [Google Scholar] [CrossRef]
