Biosurfactants (BSs) are emerging surface-active molecules with high potential for a wide range of applications in the biomedical and pharmaceutical fields. BSs are extremely attractive due to their significant antimicrobial (against bacteria, fungi and viruses), antiadhesive and biofilm disruptive properties. Their use, either on their own or in combination with other antimicrobial or chemotherapeutic drugs, might pave the way for a future strategy of prevention and counteraction of microbial infections, biofilm formation and proliferation. In addition, BSs have recently attracted the attention of the scientific community as a new potential generation of pharmaceutics to be included in anticancer, immunomodulatory, wound healing, cosmetic and drug delivery agents.
- biosurfactants
- antimicrobials
- biofilm inhibition
- Lactic acid bacteria
- rhamnolipids
1. Introduction
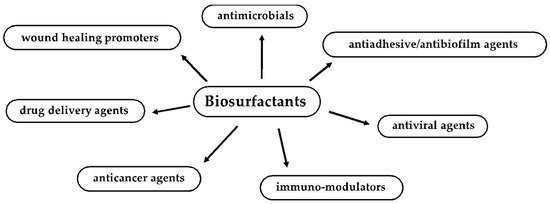
2. Biosurfactant Properties and Biological Activities Useful for Biomedical and Pharmaceutical Applications
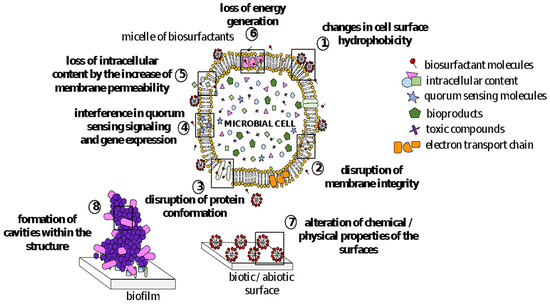
3. Antimicrobial Activity of BSs
3.1. Lipopeptides and Glycolipids as Antimicrobial Agents
3.2. Biosurfactants from Lactic Acid Bacteria with Antimicrobial Activities
References
- Morita, T.; Ishibashi, Y.; Hirose, N.; Wada, K.; Takahashi, M.; Fukuoka, T.; Imura, T.; Sakai, H.; Abe, M.; Kitamoto, D. Production and characterization of a glycolipid biosurfactant, mannosylerythritol lipid B, from sugarcane juice by Ustilago scitaminea NBRC 32730. Biosci. Biotechnol. Biochem. 2011, 75, 1371–1376.
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554.
- Chen, M.L.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; Tucker, I.; et al. Mixing behavior of the biosurfactant, rhamnolipid, with a conventional anionic surfactant, sodium dodecyl benzene sulfonate. Langmuir 2010, 26, 17958–17968.
- Chen, M.L.; Penfold, J.; Thomas, R.K.; Smyth, T.J.P.; Perfumo, A.; Marchant, R.; Banat, I.M.; Stevenson, P.; Parry, A.; Tucker, I.; et al. Solution self-assembly and adsorption at the air-water interface of the monorhamnose and dirhamnose rhamnolipids and their mixtures. Langmuir 2010, 26, 18281–18292.
- Martinotti, M.G.; Allegrone, G.; Cavallo, M.; Fracchia, L. Biosurfactants. In Innovative Technologies for Sustainable Development; Piemonte, V., De Falco, M., Basile, A., Eds.; Wiley: Hoboken, NJ, USA, 2013.
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444.
- Mandal, S.M.; Barbosa, A.E.A.D.; Franco, O.L. Lipopeptides in microbial infection control: Scope and reality for industry. Biotechnol. Adv. 2013, 31, 338–345.
- Singh, A.; Van Hamme, J.D.; Ward, O.P. Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol. Adv. 2007, 25, 99–121.
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.M.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144.
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in utilization of renewable substrates for biosurfactant production. AMB Express 2011, 1, 5.
- Fracchia, L.; Ceresa, C.; Franzetti, A.; Cavallo, M.; Gandolfi, I.; Van Hamme, J.; Gkorezis, P.; Marchant, R.; Banat, I.M. Industrial Applications of Biosurfactants. In Biosurfactants: Production and Utilization-Processes, Technologies and Economics; Kosaric, N., Sukan, F.V., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 245–267.
- Swarnalatha, M.S.; Rani, J.C. Biosurfactants: Unique properties and their versatile applications. Pharma Innovat. J. 2019, 8, 684–687.
- Kłosowska-Chomiczewska, I.E.; Mędrzycka, K.; Karpenko, E. Biosurfactants–biodegradability, toxicity, efficiency in comparison with synthetic surfactants. Adv. Chem. Mech. Eng. 2011, 2, 1–9.
- Lima, T.M.S.; Procópio, L.C.; Brandão, F.D.; Carvalho, A.M.X.; Tótola, M.R.; Borges, A.C. Biodegradability of bacterial surfactants. Biodegradation 2011, 22, 585–592.
- Tripathy, D.B.; Mishra, A. Sustainable Biosurfactants. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 1–11.
- Banat, I.M.; Carboué, Q.; Saucedo-Castañeda, G.; Cázares-Marinero, J.d.J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. Part A 2021, 320, 124222.
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697.
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019, 127, 12–28.
- Patel, S.; Homaei, A.; Patil, S.; Daverey, A. Microbial biosurfactants for oil spill remediation: Pitfalls and potentials. Appl. Microbiol. Biotechnol. 2019, 103, 27–37.
- Decesaro, A.; Machado, T.S.; Cappellaro, Â.C.; Reinehr, C.O.; Thomé, A.; Colla, L.M. Biosurfactants during in situ bioremediation: Factors that influence the production and challenges in evalution. Environ. Sci. Pollut. Res. 2017, 24, 20831–20843.
- Millioli, V.S.; Servulo, E.-L.C.; Sobral, L.G.S.; De Carvalho, D.D. Bioremediation of crude oil-bearing soil: Evaluating the effect of rhamnolipids addition to soil toxicity and to crude oil biodegradation efficiency. Glob. NEST J. 2009, 11, 181–188.
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Biological surfactants vs. polysorbates: Comparison of their emulsifier and surfactant properties. Tenside Surfactants Deterg. 2018, 55, 273–280.
- Fracchia, L.; Banat, J.J.; Cavallo, M.; Ceresa, C.; Banat, I.M. Potential therapeutic applications of microbial surface-active compounds. AIMS Bioeng. 2015, 2, 144.
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401.
- Fracchia, L.; Ceresa, C.; Banat, I.M. Biosurfactants in Cosmetic, Biomedical and Pharmaceutical Industry. In Microbial Biosurfactants and Their Environmental and Industrial Applications; Banat, I.M., Thavasi, R., Eds.; CRS Press: Boca Raton, FL, USA, 2019; pp. 258–288.
- Fracchia, L.; Cavallo, M.; Martinotti, M.G.; Banat, I.M. Biosurfactants and bioemulsifiers: Biomedical and related applications-present status and future potentials. In Biomedical Science, Engineering and Technology; Ghista, D.N., Ed.; InTech: Rijeka, Croatia, 2012; pp. 325–370.
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactants. Environ. Microbiol. 2001, 3, 229–236.
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Physiological aspects Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006, 24, 604–620.
- Chrzanowski, L.; Ławniczak, L.; Czaczyk, K. Why do microorganisms produce rhamnolipids? World J. Microbiol. Biotechnol. 2012, 28, 401–419.
- Pamp, S.J.; Tolker-Nielsen, T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 2531–2539.
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062.
- Satpute, S.K.; Banpurkar, A.G.; Banat, I.M.; Sangshetti, J.N.; Patil, R.H.; Gade, W.N. Multiple Roles of Biosurfactants in Biofilms. Curr. Pharm. Des. 2016, 22, 1429–1448.
- Horn, J.N.; Sengillo, J.D.; Lin, D.; Romo, T.D.; Grossfield, A. Characterization of a potent antimicrobial lipopeptide via coarse-grained molecular dynamics. Biochim. Biophys. Acta 2012, 1818, 212–218.
- de Cortés-Sánchez, A.J.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168, 22–32.
- Walencka, E.; Rozalska, S.; Sadowska, B.; Rozalska, B. The Influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008, 53, 61–66.
- La Fauci, V.; Alessi, V. Antibiotic resistance: Where are we going? Ann. Ig. 2018, 30, 52–57.
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32.
- Robbel, L.; Marahiel, M.A. Daptomycin, a bacterial lipopeptide synthesized by a nonribosomal machinery. J. Biol. Chem. 2010, 285, 27501–27508.
- Ngai, A.L.; Bourque, M.R.; Lupinacci, R.J.; Strohmaier, K.M.; Kartsonis, N.A. Overview of safety experience with caspofungin in clinical trials conducted over the first 15 years: A brief report. Int. J. Antimicrob. Agents 2011, 38, 540–544.
- Emiroglu, M. Micafungin use in children. Expert Rev. Anti Infect. Ther. 2011, 9, 821–834.
- George, J.; Reboli, A.C. Anidulafungin: When and how? The clinician’s view. Mycoses 2012, 55, 36–44.
- Cochrane, S.A.; Vederas, J.C. Lipopeptides from Bacillus and Paenibacillus spp.: A Gold Mine of Antibiotic Candidates. Med. Res. Rev. 2016, 36, 4–31.
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins revisited. Clin. Microbiol. Rev. 2008, 21, 449–465.
- Vater, J.; Kablitz, B.; Wilde, C.; Franke, P.; Mehta, N.; Cameotra, S.S. Matrix-assisted laser desorption ionization--time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl. Environ. Microbiol. 2002, 68, 6210–6219.
- Naruse, N.; Tenmyo, O.; Kobaru, S.; Kamei, H.; Miyaki, T.; Konishi, M.; Oki, T. Pumilacidin, a complex of new antiviral antibiotics. Production, isolation, chemical properties, structure and biological activity. J. Antibiot. 1990, 43, 267–280.
- Grangemard, I.; Wallach, J.; Maget-Dana, R.; Peypoux, F. Lichenysin: A more efficient cation chelator than surfactin. Appl. Biochem. Biotechnol. 2001, 90, 199–210.
- Saini, H.S.; Barragán-Huerta, B.E.; Lebrón-Paler, A.; Pemberton, J.E.; Vázquez, R.R.; Burns, A.M.; Marron, M.T.; Seliga, C.J.; Gunatilaka, A.A.L.; Maier, R.M. Efficient purification of the biosurfactant viscosin from Pseudomonas libanensis strain M9-3 and its physicochemical and biological properties. J. Nat. Prod. 2008, 71, 1011–1015.
- Benincasa, M.; Abalos, A.; Oliveira, I.; Manresa, A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Van Leeuwenhoek 2004, 85, 1–8.
- Díaz De Rienzo, M.A.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015, 32, 720–726.
- Kitamoto, D.; Yanagishita, H.; Shinbo, T.; Nakane, T.; Kamisawa, C.; Nakahara, T. Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J. Biotechnol. 1993, 29, 91–96.
- Yang, X.; Huang, E.; Yuan, C.; Zhang, L.; Yousef, A.E. Isolation and Structural Elucidation of Brevibacillin, an Antimicrobial Lipopeptide from Brevibacillus laterosporus That Combats Drug-Resistant Gram-Positive Bacteria. Appl. Environ. Microbiol. 2016, 82, 2763–2772.
- Ghribi, D.; Abdelkefi-Mesrati, L.; Mnif, I.; Kammoun, R.; Ayadi, I.; Saadaoui, I.; Maktouf, S.; Chaabouni-Ellouze, S. Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J. Biomed. Biotechnol. 2012, 2012, 373682.
- Mnif, I.; Grau-Campistany, A.; Coronel-León, J.; Hammami, I.; Triki, M.A.; Manresa, A.; Ghribi, D. Purification and identification of Bacillus subtilis SPB1 lipopeptide biosurfactant exhibiting antifungal activity against Rhizoctonia bataticola and Rhizoctonia solani. Environ. Sci. Pollut. Res. Int. 2016, 23, 6690–6699.
- Bouassida, M.; Fourati, N.; Krichen, F.; Zouari, R.; Ellouz-Chaabouni, S.; Ghribi, D. Potential application of Bacillus subtilis SPB1 lipopeptides in toothpaste formulation. J. Adv. Res. 2017, 8, 425–433.
- Cordeiro, R.d.A.; Weslley Caracas Cedro, E.; Raquel Colares Andrade, A.; Serpa, R.; José de Jesus Evangelista, A.; Sales de Oliveira, J.; Santos Pereira, V.; Pereira Alencar, L.; Bruna Leite Mendes, P.; Cibelle Soares Farias, B.; et al. Inhibitory effect of a lipopeptide biosurfactant produced by Bacillus subtilis on planktonic and sessile cells of Trichosporon spp. Biofouling 2018, 34, 309–319.
- Basit, M.; Rasool, M.H.; Naqvi, S.A.R.; Waseem, M.; Aslam, B. Biosurfactants production potential of native strains of Bacillus cereus and their antimicrobial, cytotoxic and antioxidant activities. Pak. J. Pharm. Sci. 2018, 31, 251–256.
- Medeot, D.B.; Fernandez, M.; Morales, G.M.; Jofré, E. Fengycins From Bacillus amyloliquefaciens MEP218 Exhibit Antibacterial Activity by Producing Alterations on the Cell Surface of the Pathogens Xanthomonas axonopodis Pv. vesicatoria and Pseudomonas aeruginosa PA01. Front. Microbiol. 2020, 10, 3107.
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 2017, 7, 108.
- Mani, P.; Dineshkumar, G.; Jayaseelan, T.; Deepalakshmi, K.; Ganesh Kumar, C.; Senthil Balan, S. Antimicrobial activities of a promising glycolipid biosurfactant from a novel marine Staphylococcus saprophyticus SBPS 15. 3 Biotech 2016, 6, 163.
- Valotteau, C.; Banat, I.M.; Mitchell, C.A.; Lydon, H.; Marchant, R.; Babonneau, F.; Pradier, C.-M.; Baccile, N.; Humblot, V. Antibacterial properties of sophorolipid-modified gold surfaces against Gram positive and Gram negative pathogens. Colloids Surf. B Biointerfaces 2017, 157, 325–334.
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: Physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnol. 2017, 36, 26–36.
- Elshikh, M.; Moya-Ramírez, I.; Moens, H.; Roelants, S.; Soetaert, W.; Marchant, R.; Banat, I.M. Rhamnolipids and lactonic sophorolipids: Natural antimicrobial surfactants for oral hygiene. J. Appl. Microbiol. 2017, 123, 1111–1123.
- Sen, S.; Borah, S.N.; Kandimalla, R.; Bora, A.; Deka, S. Efficacy of a rhamnolipid biosurfactant to inhibit Trichophyton rubrum in vitro and in a mice model of dermatophytosis. Exp. Dermatol. 2019, 28, 601–608.
- Khan, F.; Oloketuyi, S.F.; Kim, Y.-M. Diversity of bacteria and bacterial products as antibiofilm and antiquorum sensing drugs against pathogenic bacteria. J. Hazard. Mater. 2019, 364, 441–448.
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, Anti-Adhesive and Anti-Biofilm potential of Biosurfactants Isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb. Pathogen. 2019, 127, 12–20.
- Woźniak-Karczewska, M.; Myszka, K.; Sznajdrowska, A.; Szulc, A.; Zgoła-Grześkowiak, A.; Ławniczak, Ł.; Corvini, P.F.-X.; Chrzanowski, Ł. Isolation of rhamnolipids-producing cultures from faeces: Influence of interspecies communication on the yield of rhamnolipid congeners. New Biotechnol. 2017, 36, 17–25.
- Gudiña, E.J.; Fernandes, E.C.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and anti-adhesive activities of cell-bound biosurfactant from Lactobacillus agilis CCUG31450. RSC Adv. 2015, 5, 90960–90968.
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158.
- Fariq, A.; Saeed, A. Production and Biomedical Applications of Probiotic Biosurfactants. Curr. Microbiol. 2016, 72, 489–495.
- Morais, I.M.C.; Cordeiro, A.L.; Teixeira, G.S.; Domingues, V.S.; Nardi, R.M.D.; Monteiro, A.S.; Alves, R.J.; Siqueira, E.P.; Santos, V.L. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P6A and Lactobacillus gasseri P65. Microb. Cell Fact. 2017, 16, 155.
- Vecino, X.; Rodríguez-López, L.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Bioactivity of glycolipopeptide cell-bound biosurfactants against skin pathogens. Int. J. Biol. Macromol. 2018, 109, 971–979.
- Ghasemi, A.; Moosavi-Nasab, M.; Setoodeh, P.; Mesbahi, G.; Yousefi, G. Biosurfactant Production by Lactic Acid Bacterium Pediococcus dextrinicus SHU1593 Grown on different carbon sources: Strain screening followed by product characterization. Sci. Rep. 2019, 9, 5287.
