Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Rosa Maria Vitale and Version 2 by Catherine Yang.
Cannabidiol (CBD), the major nonpsychoactive Cannabis constituent, has been proposed for the treatment of a wide panel of neurological and neuropsychiatric disorders, including anxiety, schizophrenia, epilepsy and drug addiction due to the ability of its versatile scaffold to interact with diverse molecular targets that are not restricted to the endocannabinoid system.
- cannabidiol
- pharmacology
- neuropsychiatric disorders
- neurological disorders
- Molecular targets
1. Cannabidiol
Cannabidiol (CBD, Figure 1), along with Δ9-tetrahydrocannabinol (Δ9-THC), is the most abundant bioactive compound of Cannabis sativa. Differently from Δ9-THC, it is devoid of any psychotropic effects [1]. Interestingly, Δ9-THC and CBD are often considered the yin and the yang of cannabis extract for their antithetic effects: Δ9-THC binds with high affinity and activates cannabinoids receptors, responsible for the rewarding effects of cannabis, while CBD has a low affinity for the orthosteric sites of those receptors and acts as negative allosteric modulator (NAM) at Cannabinoid receptor 1 (CB1R) in the nanomolar range [2]. The NAM effect of CBD at CB1R was confirmed in a recent study by Tham et al. [3] while at CB2R it behaves as partial agonist. Moreover, CBD counteracts the anxiogenic effects of Δ9-THC and, due to its effects in inhibiting drug relapse, is currently under investigation for the treatment of addiction disorders [4]. CBD, whose structure was elucidated by Mechoulam and Shvo [5] in 1963, has been recently approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as an antiepileptic drug (Epidiolex) for the treatment of patients affected by refractory epilepsy such as Dravet [6] and Lennox–Gastaut syndromes [7]. Here, we discuss the therapeutic potential of CBD in neurological and neuropsychiatric disorders with particular emphasis on the involved molecular targets and mechanisms. The review is organized in two main sections: the first one reports an overview of the pharmacological effects of CBD in neurological and neuropsychiatric disorders, the second one describes the molecular targets and the molecular mechanisms involved in these effects. Structural details from experimental structures of the ligand-binding sites are also discussed, along with mutagenesis data.
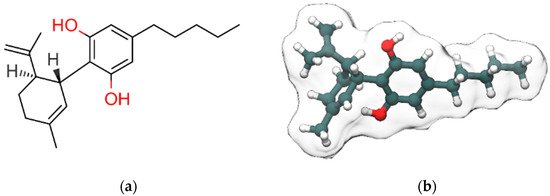
Figure 1. 2D (a) and 3D (b) representations of cannabidiol (CBD). Hydroxyl group (2D view)/oxygen atoms, carbon atoms and hydrogen atoms (3D view) are colored red, dark slate gray and white, respectively. Only polar and stereo hydrogen atoms (the latter colored in gray) are shown in (a).
2. CBD Molecular Targets and Mechanisms Involved in the Neurological and Neuropsychiatric Disorders
2.1. Cys-Loop Superfamily of Ligand-Gated Ion Channels
2.1.1. GABAARs
GABA is the major inhibitory neurotransmitter in the central nervous system (CNS), acting through its cognate receptors GABAARs and GABABRs. While GABABRs are metabotropic G protein-coupled receptors (GPCR), GABAARs are chloride-selective ion channels, belonging to the Cys-loop superfamily of ligand-gated ion channels, which include nicotinic acetylcholine receptors, 5-HT3Rs and GlyRs (Figure 2).
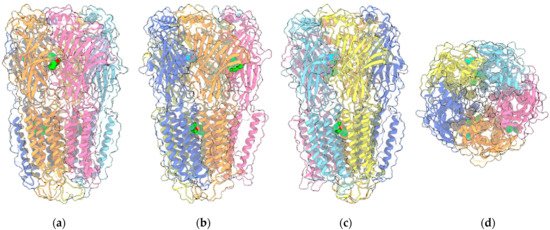
Figure 2. Four views of the 6HUP PDB entry corresponding to the complex of an α2β2γ GABAAR (ribbon plus transparent surface) with three molecules of diazepam (“sphere” representation with green C atoms) and two molecules of GABA (“sphere” representation with cyan C atoms). The two α1, two β3 and the single γ2L monomers are painted light/medium blue, yellow/orange and pink, respectively. Oxygen and nitrogen ligand atoms are painted red and blue, respectively. (a–c) are perpendicular to the channel axis and aligned either with the α1-γ2L interface (a), or the two β3-α1 interfaces (b,c). (d) shows the view along the channel axis. Other ligands included in the entry are omitted.
2.1.2. GlyRs
GlyRs, as other members of Cys-loop superfamily, are pentameric ligand-gated ion channels, which enable the influx of chloride ions. Functional GlyRs arise from different combinations of their four α subunit isoforms α1–4 and the single subunit isoform β. GlyRs mainly occur as heteromers formed by two α and three β subunits [8][65], anchored to the post-synaptic membranes through the protein gephyrin [9][66]. The α subunits are characterized by a different temporal distribution: while α2 and α4 subunits are involved in neuronal development, being mainly expressed in embryonic CNS, α1 and α3 mediate the majority of glycinergic inhibitory neurotransmission in the adult spinal cord and brain stem [10][11][67,68]. Additionally, α3 is expressed in the hippocampus where it is implicated in temporal lobe epilepsy [12][69]. Alterations of GlyRs functionality have been associated with autism spectrum disorder (ASD) [11][68]. CBD was shown to potentiate glycine currents in HEK293 cells expressing α1 and α3 subunits [13][14][70,71]. NMR studies carried out on purified α3 GlyRs allowed the identification of the binding site of CBD, unveiling a direct interaction between CBD and S296 residue, located on the third transmembrane domain [14][71]. The crucial role of S267 in the transmembrane region of α1 subunit in mediating the glycine-enhancing effect of CBD was shown by Foadi and co-workers [15][72] since the modulatory effect of CBD is lost in HEK293 cells expressing the homomeric mutated form of the receptor α1(S267I).
2.1.3. 5-HT3Rs
5-HT3Rs are the only serotonin (5-hydroxytryptamine, 5-HT) receptors belonging to the Cys-loop family of ligand-gated ion channels, since the other 5-HTRs are coupled to G proteins [16][73]. They mediate the fast excitatory neurotransmission of serotonin. Besides the well-known effect of 5-HT3R antagonists in the prevention of nausea and vomiting in chemotherapy, many studies have suggested a potential role in neurodegenerative and neuropsychiatric disorders including seizure, memory disorders, eating disorders schizophrenia, depression, anxiety, and drug addiction, as recently reviewed by Fakhfouri and coll. [16][73]. 5-HT3Rs, as other members of the Cys-loop family, are composed of five subunits arranged around a central membrane-spanning pore permeable to sodium, potassium and calcium ions. So far, five 5-HT3R subunits (A-E) have been identified, even though those most extensively characterized are 5-HT3AR and 5-HT3BR. 5-HT3Rs are expressed both in the central and in the peripheral nervous system. Activation of presynaptic 5-HT3Rs induces a rapid influx of calcium, causing a release of neurotransmitters and neuropeptides, while postsynaptic 5-HT3Rs are associated with fast excitatory sodium and potassium-dependent depolarization. Due to the involvement of 5-HT3Rs in processes related to cognition and emotion, the use of 5-HT3R antagonists has been proven to be beneficial in the treatment of various psychiatric disorders [17][74], as recently reviewed by Juza et al. [18][75]. CBD was found to act as an allosteric inhibitor at 5-HT3AR expressed in Xenopus laevis oocytes, inhibiting the currents evoked by 1μM 5-HT in a concentration-dependent manner with an IC50 = 0.6 μM [19][76].
2.2. TRP Channels
2.2.1. TRPV1
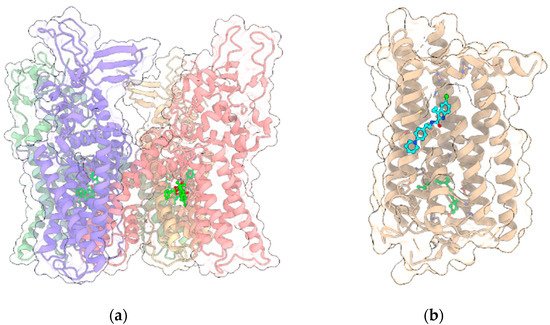
TRPV1 was the first member of the large family of TRP channels (Figure 3a) to be discovered and cloned [20][77]. All TRPV members (TRPV1-TRPV6) are homotetramers sharing a similar architecture, with the transmembrane region of each monomer arranged in a voltage-sensor-like domain (VSLD) and forming the pore channel, while the cytosolic N-terminus contains ankyrin repeat domains (ARDs). The three-dimensional structures of TRPV1 in both apo-form and complexed to agonist/antagonists have been recently elucidated by cryo-electron microscopy [21][22][23][78,79,80]. As with other members of the family, TRPV1 is expressed in various types of both excitable and non-excitable cells and, upon activation, behaves as a non-selective cation channel, conducting divalent as well as monovalent cations, with a preference for the formers and, in particular, Ca2+, showing the overall permeability order: Ca2+ > Mg2+ > Na+ ≈ K+ [20][77]. The channel is activated by various stimuli including exogenous vanilloids such as capsaicin, endogenous lipid molecules such as endocannabinoids, eicosanoids, temperature and/or low pH [24][81]. The binding site of capsaicin and other TRPV1 modulators is located in the so-called vanilloid pocket, formed by the transmembrane helices S3, S4, the S4–S5 linker of one monomer and S6 helix of the adjacent monomer. The importance of TRPV1 channels in the nervous system has been well documented. In particular, TRPV1 expression is restricted to specific brain regions including the hippocampus and cortex with additional expression in the hypothalamus, olfactory nuclei, dentate gyrus, locus coeruleus, superior colliculus and spinal cord [25][26][43,44], where it plays a critical role in regulating the cell excitability, long-term depression (LTD), synaptic plasticity and reactive oxygen species production triggering cell death [27][28][29][30][82,83,84,85].

Figure 3. (a) Crystal structure (PDB entry: 5IRX) of the 4:4 complex between rat TRPV1 (light green, red, yellow and violet ribbon and transparent surface for the four protein monomers) and the agonist resiniferatoxin (ball and stick with bright green C atoms); (b) Crystal structure (PDB entry: 6KQI) of the complex of CB1R (tan ribbon and transparent surface) with the negative allosteric modulator ORG27569 (ball and stick with cyan C atoms) and the agonist CP55940 (ball and stick with bright green C atoms). In all panels, other ligands and non-receptor protein sequences are omitted; protein sidechains contacting ligands within 5 Å are shown as sticks; oxygen, nitrogen, sulfur and chlorine atoms in ligands and visible protein sidechains are colored red, blue, yellow and green, respectively.
2.2.2. Other TRP Channels
Iannotti et al. [31][92] demonstrated that, besides TRPV1, CBD and CBDV activate and rapidly desensitize other TRP channels including TRPV2 and TRPA1. While this study represents, to the best of our knowledge, the first report where TRPV2 has been directly associated with models of epileptiform activity and acute seizure, in a recent study Günaydın et al. [32][93] showed that the long term activation of TRPA1 channels by its agonist trans-cinnamaldehyde (TCA) causes an exacerbated PTZ-induced seizure activity in rats. Based on these pieces of evidence, although drugs interacting with multiple targets have long been flagged as undesirable, the story of CBD as an antiepileptic drug has so far demonstrated that molecules hitting more than one target may show not only a higher efficacy compared to the canonical single-target AED drugs, but also a better safety profile [33][34][94,95].
2.3. GPCRs
2.3.1. CB1R
As stated before, the psychotropic effects of Δ9-THC and the synthetic cannabinoids are due to the activation of the cannabinoid receptor CB1R, one of the most highly expressed GPCRs (Figure 3b) in the CNS, where it mediates the signaling of the endocannabinoids N-arachidonoylethanolamine (AEA) and 2-AG. CB1R is also widely expressed in the peripheral nervous system and it primarily couples to Gi/o protein, inhibiting the production of cAMP and adenylyl cyclase. However, in certain conditions, CB1R can also couple to Gs and Gq proteins [35][96]. CB1R regulates the activity of various ion channels and modulates the release of neurotransmitters by inhibiting the synaptic release of glutamate and GABA, the latter through inhibition of N-type voltage-gated calcium channels [36][37][38][97,98,99]. The reduction of glutamate release due to CB1R activation in presynapses contributes to NMDAR hypofunction. Moreover, NMDA receptors are also negatively regulated by CB1R in postsynapses [39][100]. Indeed, the C-terminus of CB1R associates with the C1 segment of NMDAR NR1 subunit and the protein HINT1 stabilizes this complex. The activation of CB1R results in a reduction of the NMDAR activity. Due to its wide distribution in the nervous system, CB1R is involved in various physio-pathological processes and is considered a relevant molecular target for the pharmacological treatment of pain, inflammation, multiple sclerosis, nausea, obesity and substance abuse disorders. For example, the Δ9-THC/CBD association is the active principle of sativex, approved for the treatment of spasticity, nausea and pain [40][101]. Recently, the crystallographic structures of CB1R in complex with either agonists or antagonists and negative allosteric modulators have been solved [41][42][43][102,103,104], shedding light on the molecular mechanisms of activation/regulation. While the orthosteric binding site is located in a pocket formed by the transmembrane helices near the N-terminus, the allosteric binding site for the negative allosteric modulator (NAM) ORG27569 is extrahelical, toward the C-terminus, and overlaps a conserved site for cholesterol. The studies of Laprairie et al. [2] showed that CBD behaves as NAM at CB1R and such activity, as for ORG27569, is influenced by the occurrence of polar residues at positions 98 and 107, corresponding to two cysteine residues.
2.3.2. 5-HT1AR
Among the large number of serotonin receptors, 5-HT1A subtype has gained considerable attention for its role in the etiology and treatment of anxiety disorders. 5-HT1AR is coupled to various inhibitory Gi/o proteins, widely expressed in the nervous system and classified in two populations, based on their localization: presynaptic autoreceptors and postsynaptic heteroreceptors. The autoreceptors are distributed on 5-HT neurons in the raphe nuclei where they act as inhibitory feedback, negatively regulating 5-HT release, whereas heteroreceptors mediate 5-HT effects on mood, emotion and stress on target neurons expressed in the hippocampus, septum, amygdala and prefrontal cortex [44][105]. Alterations of 5-HT1AR expression or its pharmacological or genetic blockade lead to anxiety- and depression-like behaviors in animal models, while its overexpression reduces anxiety in mice [45][106]. In humans, mood disorders such as anxiety and depression have been associated with alteration in the expression pattern of 5-HT1AR, with an upregulation of autoreceptors and a downregulation of heteroreceptors [45][46][106,107]. Russo et al. [47][108] reported that CBD is an orthosteric ligand of 5-HT1AR, being able to displace, in a concentration-dependent manner, the classical agonist [3H]8-OH-DPAT from human 5-HT1AR-expressing CHO cells membranes, with a displacement of 73% at 16 μM. CBD acts as an agonist at this receptor, since at the same concentration, it increases [35S]GTPγS binding and reduces forskolin (FSK)-stimulated cAMP, an effect counteracted by the specific 5-HT1AR antagonist NAN-190. Later, Rock et al. [48][109] evaluated in vitro the ability of CBD to activate 5-HT1AR expressed at physiological levels in rat brainstem membranes in a 1 nM-10 μM range. At concentrations up to 10 μM, CBD was not able to displace [3H]8-OH-DPAT from specific binding sites on rat brainstem membranes. Then, they compared the ability of CBD and 8-OH-DPAT to stimulate [35S]GTPγS binding to rat brainstem membranes in a concentration-related manner. While 8-OH-DPAT induced such stimulation, no response was observed for CBD at any used concentration. Then, it was investigated whether CBD could act as positive allosteric modulator of 8-OH-DPAT in this concentration range. Indeed, CBD enhanced the ability of this agonist to stimulate [35S]GTPγS binding to rat brainstem membranes, since 100nM of CBD produced an upward shift in the concentration response curve of 8-OH-DPAT with a significant increase in Emax but not in EC50. The potentiating effect of CBD on 8-OH-DPAT was also confirmed in vivo, since CBD and 8-OH-DPAT synergistically suppress, at subthreshold doses, the LiCl-induced conditioned gaping reactions in rats. The involvement of 5-HT1AR in the anxiolytic and antidepressant-like effects of CBD has been documented in diverse studies [49][50][51][110,111,112]. For example, Zanelati et al. [49][110] showed that CBD induces antidepressant-like effects comparable to imipramine, an effect blocked by the 5-HT1AR antagonist WAY100635. The synergistic effect of CBD at 5-HT1AR was also demonstrated by Sales et al. [52][113], who showed that ineffective doses of CBD and fluoxetine, a serotoninergic anti-depressant, resulted in a significant anti-depressant-like effect. Moreover, the pretreatment with PCPA, an inhibitor of serotonin synthesis, abolishes CBD-induced behavioral effects in the forced swimming test, indicating the involvement of serotonin in CBD action. These results strongly corroborate the positive allosteric activity of CBD at 5-HT1AR. Norris et al. [53][114] reported that using targeted microinfusions of CBD into the shell region of the mesolimbic nucleus accumbens (NASh), CBD blocks the formation of fear-related memory and decreases ventral tegmental area (VTA) dopaminergic neuronal frequency and bursting activity through a mechanism mediated by 5-HT1AR, since both effects are reversed by the selective 5-HT1AR antagonist NAD 299. Moreover, intra-NASh CBD induces significant increases in non-dopaminergic, presumptive VTA GABAergic neurons. It was demonstrated, by a functional contralateral disconnection procedure, that the ability of intra-NASh CBD to block the formation of conditioned freezing behaviors was dependent on intra-VTA GABAergic transmission. These findings disclosed a novel circuit in the mesolimbic system between nucleus accumbens (NAc) and VTA, responsible for the observed effects of CBD on associative fear memory formation.
2.3.3. GPR3 and GPR6
GPR3 and GPR6 are constitutionally active orphan receptors coupled to Gs protein, phylogenetically related to cannabinoid receptors [54][115]. They are widely expressed in the CNS where they are involved in several physio-pathological processes. GPR3 has been reported to play a role in the modulation of behavioral responses to stress in animal models used to evaluate emotional disorders including anxiety, depression-like disorders, and aggressiveness, by altering monoamine levels in various brain regions [55][116]. GPR6 is involved in instrumental learning and has been proposed as a therapeutic target for PD [56][117] and schizophrenia [57][118]. Laun and coll. [54][115] found, by screening a panel of cannabinoids against these receptors by a β-arrestin2 recruitment assay, that CBD acts, in a concentration-dependent manner, as an inverse agonist at both receptors, with higher activity at GPR6 (EC50 0.18 vs. 1.22 μM). Such activity could contribute to rationalize the molecular mechanisms underlying the neuroprotective effect of CBD in animal models of PD [58][23]. Later, the same authors showed that CBD exhibits biased activity for GPR6 toward the β-arrestin2 recruitment pathway, being unable to significantly affect GPR6-mediated cAMP accumulation [59][119].
2.3.4. GPR55
GPR55 is considered a novel cannabinoid-like receptor due to its nanomolar affinity for many endo-, synthetic- and phyto-cannabinoids [60][120]. GPR55 couples with Gα13 protein and its stimulation leads to the downstream signaling pathway activation of rhoA, cdc42 and rac1 [60][120]. GPR55 is widely expressed in brain [61][121] and it has been proposed as a potential target for the treatment of anxiety and depression since its activation alleviates anxiety-like symptoms in mice subjected to acute stress [62][122]. However, it is unlikely that the anxiolytic effects of CBD are GPR55-mediated, due to its antagonist profile at this receptor (IC50 of 445 nM against the GPR55 agonist CP55940) [60][120]. Conversely, Kaplan et al. [63][123] showed that the efficacy of CBD in reducing seizures in a mouse genetic model of DS is mediated, at least in part, by its antagonism at GPR55, since the observed increase of inhibitory interneuron excitability is mimicked by CID16020046, a selective GPR55 antagonist. Moreover, GPR55 blockade by antagonists prevents the effects of CBD, further corroborating the role of GPR55 in the efficacy of CBD in such a DS model. In particular, high doses of CBD protect against seizures, while low doses improve autistic-like social deficit behaviors in DS mice, effects lost at higher doses. Such biphasic effect is similar to that exhibited by clonazepam. This latter compound acts as a positive allosteric modulator of GABAARs specific for α2/α3 subunit-containing receptor at low doses, while at higher doses it binds α1 subunit-containing receptor, inducing opposite effects in social behavior [64][124]. Thus, it is possible to speculate that low-dose effects of CBD reflect its positive allosteric modulation at α2 subunit-containing GABAARs (see Section 3.1.2) while at higher doses it targets GPR55, which is effective in controlling seizures.
2.4. σ1R
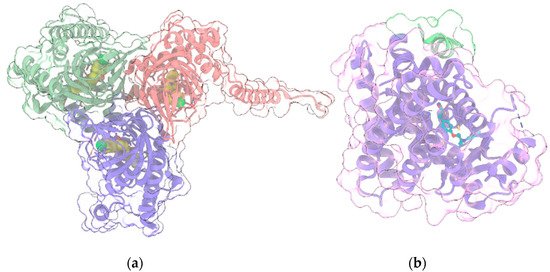
The human σ1R (Figure 4a) is a transmembrane regulatory protein involved in a wide range of physiological processes, including neurotransmission, calcium signaling and regulation of a large array of ion channels, G-protein coupled receptors and transcription factors.

Figure 4. (a) Crystal structure (PDB entry: 6DJZ) of the 3:3 complex between σ1R (light green, red and violet ribbon and transparent surface for the three protein monomers) and the antagonist haloperidol (“sphere” representation with yellow C atoms); (b) Crystal structure (PDB entry: 5YCP) of the complex of PPARγ (light violet ribbon and transparent surface) with the agonist rosiglitazone (ball and stick with cyan C atoms) and the nuclear receptor coactivator 1 peptide (light green ribbon and transparent surface). In all panels, protein sidechains contacting ligands within 5 Å are shown as sticks. Oxygen, nitrogen, sulfur and chlorine atoms in ligands and visible protein sidechains are colored red, blue, yellow and green, respectively.
Its crystallographic structure has been recently solved in complex with two ligands with different pharmacological profiles, i.e., the antagonist PD144418 and the agonist/inverse-agonist 4-IBP [65][125], unveiling a trimeric organization, with a single transmembrane helix for each protomer, located at each corner of the triangular trimer. The cytosolic domain of each protomer has a cupin-like β-barrel fold, which hosts a ligand in the central pocket, flanked by four α-helices. σ1R dysfunction has been implicated in a variety of neurological and neuropsychiatric disorders [66][126]. In particular, it has been shown [67][127] that σ1R, in tandem with the histidine triad nucleotide-binding protein 1 (HINT1), acts as on/off switch to control the association between GPCRs and NMDAR: σ1R agonists promote the interaction of GPCRs and NMDAR, whereas σ1R antagonists disrupt this association and prevent GPCRs from enhancing NMDAR function. Thus, σ1R antagonists represent a promising therapeutic strategy for the treatment of neuropsychiatric disorders where the NMDAR-mediated glutamatergic signaling plays a critical role [68][128]. Since some therapeutic indications for σ1R antagonists are common to those of CBD, Rodríguez-Muñoz and coll. [68][128] evaluated the effects of CBD in vitro and in animal models characterized by elevated activity of NMDAR, such as opioid analgesia attenuation, NMDA-induced convulsive syndrome and ischemic stroke. Indeed, in in vitro assay, CBD was shown to act similarly to a σ1R antagonist, disrupting the association of σ1R with the NR1 subunit of NMDAR, an effect prevented by σ1R agonists. Moreover, the in vivo positive effects of CBD on the aforementioned models are reduced by σ1R agonists and absent in σ1R−/− mice. Collectively, these data suggest that CBD acts as σ1R antagonist by disrupting the association between NMDAR and GPCRs.
2.5. PPARγ
PPARγ (Figure 4b), along with the other two isoforms PPARα and PPARβ/δ, belongs to the group of Peroxisome proliferator-activated receptors (PPARs), a family of nuclear receptors [69][129] widely distributed in various organs and tissues. PPARs are ligand-activated transcription factors, which regulate the expression of their target genes upon heterodimerization with retinoid-X receptors (RXRs). Ligands bind to the ligand-binding pocket (LBP) of PPAR, inducing conformational changes that promote the recruitment of co-activators and the release of corepressors. Canonical agonists form a network of hydrogen bonds which stabilize the conformation of a short helix, the helix12 (H12), whereas partial agonists bind to distinct sub-regions or allosteric sites and activate these receptors through H12-independent mechanisms. Besides their well-consolidated role in glucose and lipid metabolism, PPARs also have anti-inflammatory and neuroprotective effects [70][130]. In particular, PPARγ is highly expressed in brain areas involved in the regulation of motivational and emotional behaviors [71][131]. Starting from the occurrence of PPARγ in neurons of the VTA, de Guglielmo et al. [72][132] demonstrated that PPARγ activation attenuates opioid consumption by reducing both the opioid motivation and its rewarding properties. These effects are associated with both a drastic reduction of heroin-induced elevation of the phosphorylation of DARPP-32 protein - a regulator of the efficacy of dopaminergic (DAergic) neurotransmission - in NAc, and a reduction of the acute heroin-induced NAc DA levels increase. Moreover, PPARγ activation attenuates the opioid-induced DA activity in the VTA. Domi et al. [71][131] investigated the role of PPARγ in anxiety and stress response in mice, showing that its activation prevents the anxiogenic effect of acute stress, while its ablation or the administration of a specific antagonist exacerbates basal anxiety and enhances sensitivity to stress. These results, combined with other studies including preclinical and clinical trials [73][74][75][76][77][133,134,135,136,137], demonstrate the therapeutic potential of PPARγ agonists for various neuropsychiatric disorders and drug dependencies. Interestingly, since CBD is a selective PPARγ agonist [78][79][19,138], many of its therapeutic effects in alleviating neuropsychiatric disorders could be mediated by PPARγ signaling and by its regulation of the mesolimbic DA activity [80][139]. Indeed, an increasing amount of evidence demonstrated that CBD modulates the mesolimbic DA system and that it represents a promising compound for the treatment of schizophrenia, due to its antipsychotic effects [81][140]. However, the modulation of VTA DA activity by CBD could also involve multiple mechanisms, including 5HT1A activation [53][114] (see above).
