You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Chou Min Chong and Version 2 by Conner Chen.
Marine sponges are sessile invertebrates that can be found in temperate, polar and tropical regions. They are known to be major contributors of bioactive compounds, which are discovered in and extracted from the marine environment. The compounds extracted from these sponges are known to exhibit various bioactivities, such as antimicrobial, antitumor and general cytotoxicity.
- marine sponge
- bioactive compounds
- microbial symbionts
1. Background
The open oceans and deep seas cover about 70% of the earth’s surface and are a natural habitat to approximately 80% of the world’s plant and animal species [1][2][1,2]. The ocean is known to accommodate various living organisms, ranging from prokaryotic bacteria and marine invertebrates to multicellular complex organisms such as sharks and whales [3]. Despite the extreme conditions of the marine environment, the accessibility of new compounds from marine organisms from the various depths of the sea is now possible due to the advancement in technologies such as scuba diving equipment, remotely operated vehicles, closed-circuit computerized mixed gas rebreathers and manned submersibles [4][5][4,5]. More than 5000 novel natural products have been extracted from marine organisms living in these extreme environments, having a wide thermal range from freezing temperatures to as high as 350 °C in deep hydrothermal vents, pressures ranging from 1 to 1000 atm, varying nutrient ranges and photic and non-photic zones [6][7][6,7].
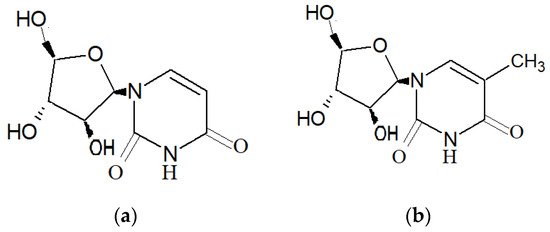
Research involving natural compounds from marine organisms began in the 1950s with Bergmann and coworkers’ discovery of the compounds spongothymidine and spongouridine (Figure 1), extracted from the Caribbean sponge Tectitethya crypta (de Laubenfels, 1949) [4]. In the past few years, several marine taxa, such as sea slugs, sponges and soft corals, have been a major source of novel natural bioactive compounds [8][9][10][8,9,10]. From the initial stage of natural product marine research to the current trend of drug discovery, most of the natural compounds that have been discovered are from marine invertebrates, especially marine sponges, with hundreds of compounds discovered yearly from this source [3][11][3,11]. Figure 2 below showcases the data that were retrieved from https://www.lens.org/ using keywords “marine sponge” and “bioactive compounds”, illustrating the publication of scholarly works regarding the discovery of marine sponge–derived compounds and their application in the form of books, book chapters, journal articles, conference proceedings, dissertations and patents. A sponge is an invertebrate, sessile organism that can be widely found in temperate, tropical and polar habitats [4][12][4,12]. Marine sponges belong to the phylum Porifera, and there are more than 8000 species of sponges that have been discovered so far [11].
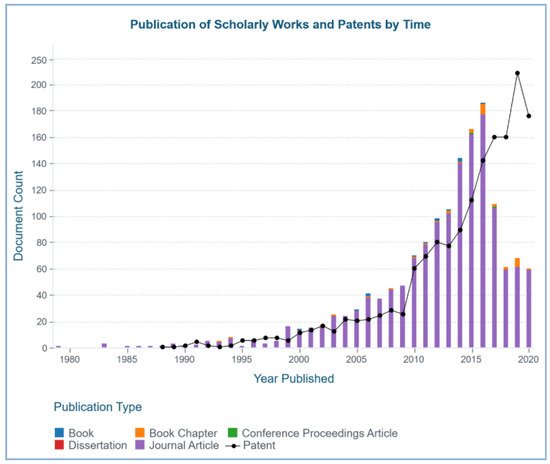
Figure 2. Publication of scholarly works (books, book chapters, journal articles, conference proceedings, dissertations) and patents that reported the discovery and/or applications of marine sponge–derived bioactive compounds retrieved from https://www.lens.org/ using keywords “marine sponge” and “bioactive compounds”.
Marine sponges are made up of a jelly-like layer between two thin layers of cells and might contain the skeleton of spicules made of silica, calcium carbonate, and a protein known as spongin [14]. The identification of sponge species is based on the size and shape of the spicules [14]. Marine sponges are filter feeders; they cope with potentially hazardous particles by producing neutralizing bioactive compounds. Around 11 genera of sponges have been discovered to contribute to the discovery of bioactive compounds, including the three genera Haliclona, Petrosia and Discodemia, which are known to produce compounds with powerful anti-inflammatory and anti-cancer activities [7][15][7,15]. Bioactive compounds synthesized by sponges are chemically diverse and can be grouped as nucleosides, terpenes, sterols, cyclic peptides and alkaloids [11].This diverse range of bioactive compounds are not completely synthesized by sponges alone but are also synthesized by their microbial symbionts [3][14][3,14]. Table 1 below lists a few marine sponge species and their bioactivities, along with the chemical structure of the compounds.
Table 1.
Compounds isolated from marine sponges and their bioactivity.
| Marine Sponge | Compound | Bioactivity | References |
|---|
| Agelas oroides (Schmidt, 1864) associated with Streptomyces sp. SBT345 |  Compound quinolone ageloline A |
|
[16][17][16,17] |
| Dysidea avara (Schmidt, 1862) |  6′-Hydroxy avarol (R1: OH) 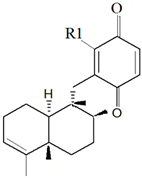 3′-hydroxy avarone (R1: OH) |
|
[18][18[19],19] |
| Hamigera tarangaensis (Bergquist and Fromont, 1988) | 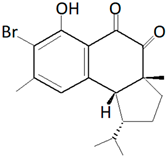 Compound Hamigeran B |
|
[20][21][20,21] |
| Leuconia nivea (Grant, 1826) Synthesized by the symbiont bacteria Microbulbifer sp. |
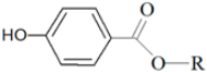 Natural paraben (R:-(CH2)9 –(CH3) |
|
[17][22][17,22] |
2. Ecological and Biological Factors Driving the High Abundance of Bioactive Compounds from Marine Sponges
Marine sponges have two different life cycles. At the initial stage, a larva is released from the female sponge into the water; it will eventually attach to a suitable substrate and will grow into a non-motile (sessile) adult [23]. Marine sponges are known as excellent filter feeders; the surrounding water is drawn into the sponge through small pores known as ostia, and the water is ejected through a larger opening known as osculum [24].
There are specific cells present in the marine sponge that perform particular functions. Flagellated cells (choanocytes) allow water movement in one direction through the coordination of the flagella. Particulate matter present in the water is engulfed by the amoebocyte cells [25]. Figure 3 below depicts the ecological and life-history-related factors that drive the high production of bioactive compounds in marine sponges.
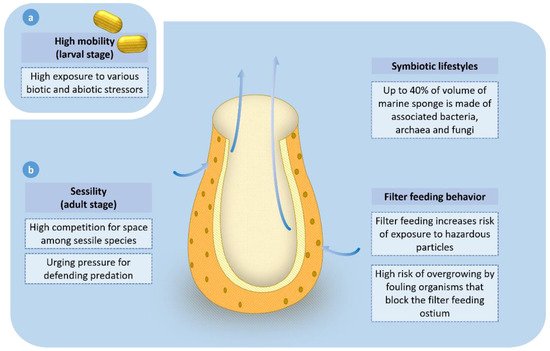
Figure 3. A summary of ecological and life-history-related factors that cause high production of bioactive compounds in marine sponges. (a) The larval stage is highly motile; hence, the larva is exposed to a high variety of pathogenic and abiotic stressors. (b) The non-motile adult experiences high competition for space among sessile organisms and predation by natural predators. Adults have symbionts attached to them. Filter feeding behavior of the adult increases the risk of exposure to hazardous materials. Overgrowing by fouling organisms like barnacles on the marine sponge has a lethal effect by blocking ostia. These factors have driven the high production of bioactive compounds in marine sponges.
Microorganisms passed through the ostia remain in the mesohyl, which is the inner sponge of the tissue, thus forming a symbiotic interaction with the sponge. The symbionts consist mainly of bacteria but also archaea, eukaryotes or larger organisms [26]. The bacterial population found in the extracellular mesohyl matrix of the sponges harbors dense bacterial assemblages that could make up to 38% of the biomass of the sponge [23][27][28][23,27,28]. In some sponges, the bacterial density may even range up to 108 to 1010 or 105 to 106 bacteria/g of the sponge’s wet weight [29]. Due to the difference in range between microbial population densities, marine sponges can be classified as “bacteriosponges” or “highly-microbial-abundance (HMA) and low-microbial-abundance (LMA)” sponges [23][29][23,29].
The ecological and evolutionary importance of the associations between sponge and microorganism can be determined by their ability to produce various bioactive compounds, which can be utilized in the biotechnology industry [30]. The advantage of secondary metabolites in the sponges can be identified based on the presence of antifouling products. For example, sponges can protect the water-pumping ability by preventing biofilm formation and the settlement of barnacles or bryozoans on their surface [31][32][31,32]. Sponges produce high-level cytotoxic chemicals by producing mucus-containing toxins, which create a clear zone around the sponge as a defense against other marine species. This ensures that sponges are able to conquer densely populated rocks or corals, enabling them to compete with organisms that grow rapidly. However, they are also able to selectively use these toxins without destroying themselves [30].
Secondary bioactive metabolites found in marine sponges are generally of microbial origin [33][34][35][33,34,35]. This can be proven through the structural similarity of some of the natural products that have been discovered from the sponges, such as complex polyketides and non-ribosomal peptides, which are exclusively known to microorganisms. However, some metabolites are produced by the sponges. For example, avarol is a compound found in the sponge Dysidea avara, stevensine is from Axinella corrugate (George and Wilson, 1919) and an array of cytotoxic brominated isoxazoline alkaloids are the natural products of the sponges themselves [36][37][38][36,37,38]. In addition, some enzymes with potential therapeutic applications have been isolated from sponges. For instance, ATP N-glycosidase is an enzyme discovered in Axinella polypoides (Schmidt, 1862) that converts adenosine-5′-tri-phosphate (ATP) into adenine and ribose-5-triphosphate [39]. The hydrolysis of ATP takes place by hydrolyzing the N-glycosidic bond that exists in the ATP molecule, thus leaving the energy-rich triphosphate moiety. The cleavage results in ribose-5-phosphate, which is utilized in the synthesis of nucleic acids deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) [40].
3. Chemistry of Compounds from Marine Sponges
The extracts obtained from marine sponges consist of a collection of secondary metabolites that are synthesized by the sponges and also by the symbionts attached to the sponges [17][41][17,41]. These secondary metabolites synthesized by the sponges and the symbionts function as a defense mechanism; the synthesis of these metabolites depends on various stress factors, such as predators, overgrowth of fouling organisms and competition for space with other organisms [3][42][3,42]. These secondary metabolites are chemically diverse, and there are various factors that influence the compounds that can be extracted from the marine sponges [3][43][3,43]. For example, sponges from the genus Haliclona synthesize a wide range of metabolites ranging from class terpenes, such as sterols, sesquiterpenoid quinols, glycosphingolipids, and alkaloid bioactive secondary metabolites [44]. Table 2 below shows examples of compounds from marine sponges and the chemical structure of such compounds. The extraction process applied to obtain the compounds will be discussed further, including the parameters that affect the extraction and the types of extraction that can be applied.
Table 2.
Compounds extracted from marine sponges and the chemical structure of the compounds.
| Marine Sponge | Classification | Compound | Chemical Structure | References |
|---|
| Agelas oroides (Schmidt, 1864) | Bromopyrrole alkaloid | Oroidin | 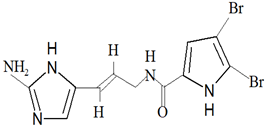 |
[3][45][3,45] |
| Discodermia kiiensis (Hoshino, 1977) | Peptide | Discodermin A | 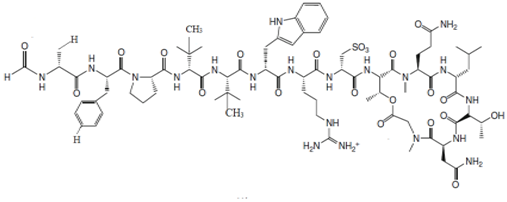 |
[46] |
| Jaspis stellifera (Carter, 1879) Stelleta tenuis (Lindgren, 1897) |
Triterpene | Stelletin A | 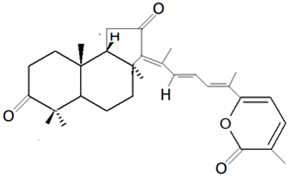 |
[47] |
| Haliclona sp. | Polycyclic, β-carboline-derived alkaloid | Manzamine A | 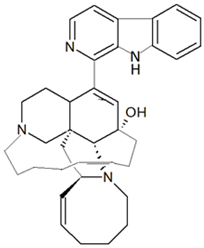 |
[48][49][48,49] |
| Luffariella variabilis (Polejaeff, 1884) | Sesterterpene | Manoalide | 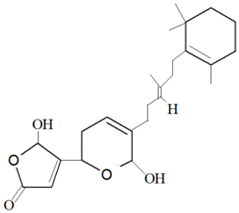 |
[50] |
3.1. Extraction Process
The extraction process is a step that involves the separation of the desired natural products from the raw materials, i.e., the marine sponges [51]. Various methods can be applied to obtain the crude extract [52]. The crude extract is known to be composed of a complex mixture of various metabolites, including alkaloids, terpenoids, peptides and quinones [53][54][53,54]. The extraction process can be divided into two categories, which are the traditional extraction method and newly emerging technologies based on the energy or mechanism [52].
3.1.1. Traditional Extraction Method
The traditional extraction method consists primarily of extraction via solvent or solid-liquid extraction [52]. The Soxhlet method involves boiling the sample along with the solvent, with or without stirring, for a certain duration, whereas maceration involves the sample being soaked in the solvent and stirring from time to time [55]. These techniques are examples of solid-liquid extraction methods that can be practiced. These methods have been practiced widely for a long period. However, there are few disadvantages to applying these techniques, which include that they are time-consuming, they require a large volume of solvents, there can be a loss of compounds during the concentration step due to volatilization, there can be hydrolysis of the compounds due to harsh conditions such as high temperature, and solvent wastes can cause environmental pollution [56].
The solvents used in these techniques consist of either a single solvent or a mixture of solvents with a wide range of polarity [57][58][57,58]. For example, methanol, ethanol, trichloromethane, acetone and water can be utilized individually as a solvent or they can be utilized with a mixture of organic solvents such as ethanol and acetone [59][60][59,60].
3.1.2. Emerging Technology
As there are various limitations to the traditional extraction method, new methods are being practiced that are less time-consuming and that have been found to be more efficient in the extraction process. Microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE) and pressurized solvent extraction (PSE) are few new techniques that are being widely used as alternative extraction procedures [52][56][52,56].
MAE is a technique that utilizes microwave irradiation to speed up the removal of various compounds from the natural matrices [52]. In this method, both heat and mass gradients work in the same direction; that is, friction between the polar molecules results in heat production within the matrix [61][62][61,62]. The friction between the molecules is caused by the electric field, resulting in the vibration and or oscillation of the molecules and causing inter- and intra-molecular friction [56]. This results in rapid heating (in seconds) of the matrix, leading to the pressurized effect and causing rupture of the cell wall and membrane. As a consequence, the transfer rate of the compounds from the cells to the solvent occurs at a faster rate [52].
UAE is another technique that utilizes ultrasound waves to alter the physical and chemical properties after interacting with the material [63][64][63,64]. The advantages of the technique are that it reduces the extraction duration significantly while simultaneously increasing the extraction yield [65]. This can be achieved due to the formation of cavitation bubbles in the solvent [65]. The cavitation bubble is known to be formed in the liquid during the expansion phase. The ability to form a cavitation bubble depends on the characteristics of the ultrasound wave, the property of the solvents, and the surrounding conditions [66][67][66,67]. After a cavitation bubble is formed, it collapses during the compression cycle, resulting in the liquid molecules being pushed together and creating a high-speed micro-jet that moves towards the matrix particle and results in the mixture of the solvent with the matrix [61][66][61,66]. In this procedure, the pressure and temperature can reach up to 1000 bar and 4726.85 °C, respectively [66][67][66,67]. This results in the breakage of the cell wall and membranes, thus allowing the solvent to penetrate the solid particle easily, releasing the intracellular compounds.
PSE is one of the emerging techniques that utilizes temperature and pressure ranging from 50–200 °C and 35–200 bars, respectively. The temperature and pressure applied in this procedure are set to be lower than the critical temperature (Tc) and critical pressure (Pc) of the solvents being used; hence, the solvents stay in the liquid state [68][69][68,69]. The high pressure applied in the procedure results in the rise of the solvent above its boiling temperature; this increase in temperature enhances the solubility and mass transfer rate and simultaneously reduces the viscosity and the surface tension of the solvent [69][70][69,70]. The most commonly used solvent in this procedure consists of water, propane and dimethyl ether [60][61][62][60,61,62].
3.1.3. Factors Affecting the Extraction
The extraction process of the bioactive metabolites from marine sponges depends on the type of extraction process that will be applied along parameters such as solvent polarity, temperature and pressure [53][71][53,71]. These factors influence the types of metabolites found predominantly in the crude extract. The type of solvents used for extraction can be categorized based on polarity, ranging from non-polar solvents such as hexane and trichloromethane to the highly polar solvent of water [54][72][54,72]. A mixture of water and an organic solvent such as ethanol will result in an increase in the yield of phenolic compounds compared to the percentage yield when the solvents are used individually [52].
A study was conducted to identify the influence of the solvents on the percentage yield of the crude extracts by testing the sponges Stylotella aurantium (Kelly-Borges and Bergquist, 1988) and Halicona molitba (de Laubenfels, 1949) using methanol and water as solvents [73]. The methanol extract of both the sponges exhibited a better antimicrobial activity against B. cereus compared to the water extract; however, the water solvent extract of both the sponges exhibited a higher percentage yield. The difference in the yield percentage between the two solvents is due to the polarity. As water is higher in polarity compared to methanol, more polar compounds from the sponges can be extracted, thus increasing the yield percentage [73]. The difference in the antimicrobial activity between the crude extracts suggests that the bioactive compounds that are responsible for the inhibition of the bacterial cell growth are mostly semi-polar; thus the extraction of these compounds was possible with methanol, as it is a semi-polar solvent.
A study was conducted on the marine sponge Xestospongia sp. (Laubenfels, 1932) by Bayona et al. (2018) looking at the correlation between the parameter pressure, temperature and solvent polarity and the diversity in the extracted compounds [53]. It was found that the solvent polarity and temperature applied during the extraction process are the most important factors influencing the metabolic diversity in the extract. As mentioned above, an increase in the solvent polarity was found to be positively correlated with the diversity in the compounds extracted. Ethanol was found to be a more suitable solvent, as it resulted in a wide range of compounds compared to the solvent with a mixture of ethanol and dichloromethane. The ethanol solvent was identified to be more proficient in the extraction of polar compounds and also lipophilic fatty acid compounds, thus resulting in a wide range of yield. When the solvent dichloromethane was used, the extract was found to contain compounds belonging to sterols and fatty acids.
However, when the extraction was conducted at a higher temperature (60–80 °C), there was a reduction in the chemical diversity compared to the lower temperature (30–50 °C). The extraction was performed using 100% ethanol to determine the effect of the temperature [53]. The extraction at different temperature ranges resulted in a specific group of compounds being extracted. At the high temperature, specific groups such as aromatic-based compounds were extracted efficiently, whereas at low temperatures, the extracts were identified to contain significant chemical diversity.
Another factor that was reported to significantly influence extraction was the number of cycles used to perform the extraction; this showed an interaction with the temperature at which the extraction process was conducted [53]. The study found that there was no difference in the diversity of compounds obtained when the extraction was performed at low temperature and one or three cycles. However, when the temperature was increased, the diversity in the extract decreased when one cycle was used, though increasing the number of cycles increased the compound diversity.
3.2. Classification of Compounds
While the crude extract of known organisms has been identified to contain a wide range of compounds from various chemistry classes, three major classes have been predominantly identified among the marine sponge extracts. A few examples of compounds extracted from sponges will be discussed in this review.
3.2.1. Terpenes
Secondary metabolites consist of terpenes in a large number, where they are synthesized in the form of polymers of isoprene (C5H8), joined in a repetitive head-to-tail manner [3][74][75][3,74,75]. The modification in the structures of terpene-based metabolites results in a diverse range of derivatives, where there is a wide range of chemical structure and, at the same time, biological properties. Terpenes have an incredible range of structures that can be extracted based on the polarity of the solvent [76]. The terpenes consist of non-polar terpenes, which are linear and cyclized terpenes, and polar terpenoids. The first terpene that was discovered in the marine sponge was steroidal terpenoids. Sesterterpenoid (C25) and triterpenoids (C30) are two major classes of terpenoids that have been commonly isolated from marine sponges and are known to have various bioactivities [42][77][42,77].
For example, manoalide is a parent compound of various sponge metabolites belonging to the sesterterpene class and was isolated from sponge L. variabilis in Palau [50]. The compound exhibited various bioactivities, such as antibacterial activity against Streptomyces pyogenes and S. aureus, and is a potent inhibitor of phospholipase A2 (PLA2) enzyme, which is responsible for providing a substrate of pro-inflammatory mediators [78][79][78,79]. Furthermore, it was found that the compound manoalide and its analogues’ bioactivities are contributed by the presence of various functional groups in the compounds [3].
Isomalabaricanes are triterpenes that were first identified from sponges Jaspis stellifera (Carter, 1879) in Fiji and Stelletta sp. (Schmidt, 1862) in Somalia [80][81][80,81]. The isomalabaricane triterpenes can be classified into three groups based on the presence of polyene conjugated functionality: (i) stelletins, which possess γ-pyrone; (ii) stelliferins, which can be oxygenated at C-22; (iii) globostellatic acids, which have a carboxyl group at C-4 [3]. Stelletins isolated from marine sponges such as J. stellifera and Stelleta tenuis (Lindgren, 1897) have exhibited cytotoxic activity against cancer cells such as murine leukemia P388 cell lines [47].
3.2.2. Alkaloids
Alkaloid-based compounds are another class of metabolites that have been extensively identified from marine sponges. The manzamines are polycyclic, β-carboline-derived alkaloids; manzamine A was the first isolated from marine sponge Haliclona sp. in 1986. The manzamine compounds are characterized by a fused and bridged tetra- or penta-cyclic ring system linked to the β-carboline [48]. The manzamine alkaloids have various bioactivities, such as antibacterial, anti-malarial and cytotoxic [82][83][82,83].
Furthermore, bromopyrrole alkaloids contain compounds that can only be found among marine sponges. Oroidin was the first compound isolated from this group, being isolated from Agelas oroides (Schmidt, 1864) in 1971. For the compounds classified as bromopyrrole alkaloids, oroidin is the precursor, as these compounds contain a pyrrole-imidazole unit that is a derivative of oroidin [3][45][3,45]. Hymenidin, clathrodin and sventrin are examples of bromopyrrole alkaloids that have been isolated from marine sponges, where the bioactivity is attributed to the bromination pattern of the pyrrole moiety [3].
3.2.3. Peptides
Bioactive peptides are a well-established sector of marine natural product-based research, and the peptides from marine sponges have been discovered to contain unique structures compared to the bioactive peptides from different sources. These peptides are cyclic or linear in shape and have amino acids that are either rare or absent in terrestrial-based and microbial peptides [84][85][84,85]. Peptides isolated from marine sponges are known to be safe, inexpensive and having a wide range of antimicrobial properties [86].
Antimicrobial peptides (AMPs) are an important source of new antibiotics, which can be used as an alternative to the available antibiotics or in combination with these antibiotics [87]. An example of AMP would be Discodermin A, a tetra-decapeptide; it is the first bioactive peptide isolated from Discodermia kiiensis (Hoshino, 1977) from Shikine Island, Japan [46]. Further investigation has resulted in the discovery of various analogues, including discodermin B, C and D [88][89][88,89]. Discodermins A–D has exhibited antibacterial activity and is also a potent inhibitor of enzyme PLA2 [3][90][3,90]. Tetra-decapeptide AMPs are characterized based on the presence of anomalous amino acid residues, which include formyl-D alanine, 3-methyl-L-proline, and 3-methyl-D-valine [87]. The genus Theonella (Gray, 1868) is known as a source of unusual peptides, such as Theonellamide F, which is a bicyclic peptide consisting of a histidino-alanine bridge and exhibiting antifungal properties against various pathogenic fungal strains, such as Candida sp., Trichophyton sp. and Aspergillus sp. [91].
Other examples of peptides are the proline-rich cyclopeptides, which exhibit bioactivities such as anti-cancer, immunosuppressor, and as anti-inflammatory [87]. The proline residue found in the peptide molecules is important for structural stability by reducing the conformational flexibility. This is due to the rigidity provided by the proline ring, leading to the maintenance of the rigid structure of the peptide [65]. Examples of proline-rich cyclopeptides are Callyaerins A–F and H, which are extracted from ethyl acetate extract of marine sponge Callyspongia aerizusa (Desqueyroux-Faùndez, 1984) [87]. One of the compounds, Callyaerin A, showed potent antifungal activity against Candida albicans.