Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alexandru Enesca and Version 2 by Catherine Yang.
The photoreactors design and concepts vary based on the working regime (static or dynamic), photocatalyst morphology (powders or bulk) and volume.
- reactors
- flow rate
- photocatalysts
1. Introduction
Water represents a vital element for all living organisms and preserving it in a free pollutant state is essential [1]. Due to the high increase of the population, most of them living in the urban area have accelerated the pressure on the treatment water plant to provide fresh and safe water. In the same time the wastewater plants encounter significant difficulties to address the increase of contaminants concentration and structure [2][3][4][2,3,4]. Among these contaminants, the organic pollutants such as phenols [5], pesticides [6], pharmaceutical [7] and dyes [8] raise special issues due to their impact on human health and aquatic life. The organic pollutants removal by conventional methods (adsorption, coagulation, filtration, microorganism and enzymes) showed important limitation due to the reluctance of these molecules [9][10][9,10]. One disadvantage of the traditional wastewater treatments is given by the incomplete mineralization of the organic pollutant, which may result in the formation of other organic molecules with high toxicity potential [11][12][11,12]. Additionally, the conventional methods have failed in removing highly toxic pollutants found most often in low or even trace concentrations. These pollutants originating from medical care or industrial activities may persist for long periods of time into the contaminated environment [13]. Addressing these issues in a sustainable manner require an integrated procedure that includes economic and political factors. An alternative to this issue is represented by the photocatalytic technology considered as an advanced oxidation process (AOP) [14].
The driving force of the photocatalytic process is the light irradiation, able to provide enough energy necessary to produce oxidative species involved in organic pollutant mineralization. Sun is a continuous source of energy sending around 5 × 1022 J each year on the Earth surface [15]. There are several approaches (photobiological, photothermal, photovoltaic and photochemical) aiming to convert the photon energy into an available energy form [16][17][18][16,17,18].
Besides the irradiation source, photocatalyst material and the photoreactors structure play an important role on the photocatalytic performance evaluated based on the pollutant removal efficiency. The additional components such as pumps, valves, pipes and the working regime (static or dynamic) contribute to the overall characteristics of the photocatalytic process [19][20][19,20]. Keeping high standards in wastewater treatment procedures require finding a suitable balance between the technological parameters, chemical substances consumption and economical costs [21][22][21,22]. The photocatalytic processes can be used for the indoor air decontamination as well. Obviously, the photoreactors must be adapted to include the air-proof concept and gas flow dynamic, which are mandatory during the design step. Adapting photocatalytic technologies to the conventional wastewater plant is a key factor to be considered for large scale applications. Both material and process optimizations are required in order to improve the pollutant addressability and the overall photocatalytic efficiency. For example, doping photocatalytic materials can significantly increase the charge carrier’s concentration due to the use of larger light absorption spectra. Coupling semiconductors with other materials (metals, wood, fly ash, etc.) can tune properties such as: conductivity, surface energy, porosity or crystallinity. Finding new pathways that combine the advantages of simple technology, energy sustainability and environmentally friendly materials is a prerequisite for future applications.
2. Photoreactors Design and Concepts
The photoreactors can be characterized based on several parameters: geometrical shape, photocatalyst type or morphology, fluid dynamics or applications. The present paper focus on two most common photoreactor geometrical shapes: cylindrical and rectangular. The analysis includes the influence of flow rate, reactor volume, light properties, photocatalyst and pollutant characteristics (dosage, concentration, etc.) on the overall photocatalytic efficiency of the process. Due to the length limitation, the mini-review cannot include all the representative papers published until now. The papers containing insufficient relevant data or missing experiments were excluded.
2.1. Cylindrical Photoreactors
The cylindrical reactors are usually irradiated by a central lamp or lamps arranged in a circle (Figure 13). The photocatalyst can be dispersed into the liquid volume or immobilized on various substrates (including the lamp cover). Additionally, the set-up often contains a storage tank (with aeration and mixer), pumps, valves, flowmeter and a control system able to manage the entire system. The storage tank aeration is required to ensure oxygen saturation conditions during the oxidative radical’s formation. The cylindrical reactors have the advantage of radial flow distribution, which increases the diffusion homogeneity of the mobile photocatalysts.
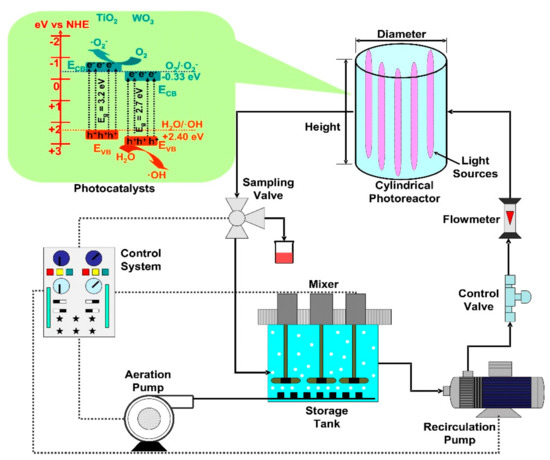

Figure 13.
Cylindrical photoreactors components (TiO
2
/WO
3
heterostructure photocatalyst).
The phenol removal was evaluated using 4.9 L [23][69] and 3.3 L [24][70] reactors and TiO2 as the photocatalyst. The 4.9 L reactor use a flow rate of 19 L/min and the irradiation was done by a UV-A light source able to provide 60 or 90 W intensity. The highest efficiency (55.7%) was recorded when the TiO2 dosage was 3000 mg and the light irradiation was 60W. Using the same photocatalyst dosage, phenol concentration (20 mg/L) and irradiation period (240 min) but higher light intensity (90 W) the photocatalytic efficiency exhibit 49% showing that there is no linear dependence between the photocatalytic pollutant removal and the UV-A light intensity. Basically, at low values of light intensities, the rate of photogenerated electron-hole formation increases and the rate-limiting step is represented by the holes formation. Higher light intensities may induce loss of photonic efficiency due to excessive heating of the reaction solution. However, higher concentration of phenol (30 mg/L) was completely removed by using a 3.3 L reactor with a flow rate of 2 L/min. The results confirm that lower UV light intensity (40 W) and TiO2 dosage can improve the photocatalytic efficiency due to better light penetration into the aqueous environment. The photocatalyst immobilized on rotating substrate will increase the mass transfer favoring the phenol degradation. Similar experiments were done on 4-nitrophenol but in very different experimental conditions. Low UV-C light intensity (11 W) was used to irradiate a 1 L reactor [25][71] during 300 min. At a flow rate of 6 L/min the photocatalytic efficiency toward 4-nitrophenol (15 mg/L) removal in the presence of TiO2 was 95.7%. When the flow rate increases, the liquid phase turbulence increases too, which provide a considerable reduction of the mass transfer resistance in the liquid, simultaneously with the appetency of renewing the photocatalyst surface due to the contaminants diffusion. Using a 0.15 L small reactor size [26][72], lower 4-nitrophenol concentration (10 mg/L) and higher light intensity (90 W) the photocatalytic efficiency after 15 min of irradiation was 80%. These results can be used for large applications considering the energy sustainability as a prerequisite on developing new cost-effective technologies.
A comparative study between the phenol and pharmaceutical active compounds photocatalytic removal was done using a 0.34 L reactor, 0.05 L/min flow rate and g-C3N4/chitosan mediators [27][73]. After 300 min of irradiation with 1000 W UV–Vis light source, the photocatalytic phenol removal was 20%, while for carbamazepine was 10% and for sulfamethoxazole was 30%. However, it is worth mentioning that the pharmaceutics compounds concentration was double compared with phenol concentration. The influence of a slow mass transfer rate on carbamazepine and sulfamethoxazole photodegradation was more predominant, while the g-C3N4 coverage by chitosan has a detrimental impact on phenol degradation. The influence of direct photolysis of oxytetracycline [28][74] and sulfamethazine [29][75] pharmaceutics compounds was evaluated under UV irradiation. The microreactor with a volume of 1.6 × 10−2 L exhibit 97% oxytetracycline photocatalytic removal after 120 min of irradiation with 11 W UV-C light. The photocatalytic system uses 20 mg/L oxytetracycline concentration and 100 mM of H2O2. The increase in flow rate from 50 to 100 Lh−1 will reduce the antibiotic residence time inside the photoreactor. To overcome this aspect the recirculation’s number can be increased considering the overall energetic balance of the photocatalytic system. Using a larger reactor of 3 L, the 10 mg/L sulfamethazine solution was completely removed after 15 min of irradiation with 16 W UV light source. The study indicate that the photocatalytic efficiency improves when the H2O2 concentration increased from 1 to 10 mM due to more ·OH radicals available to participate in the sulfamethazine mineralization. If the H2O2 concentration increases up to 20 mM then the photocatalytic efficiency decreases as H2O2 excess acts as a scavenger for ·OH, generating ·OOH groups.
A microreactor with 1 × 10−4 L volume was tested for the direct photolysis of benzoylecgonine [30][76], a cocaine metabolic product formed by the liver and excreted in the urine. The experimental tests were done at 0.01 L/min flow rates using a low intensity (8 W) UV light source. After 2 min of irradiation the 9.1 mg/L benzoylecgonine concentration was completely removed from the aqueous solution. These results indicate that cylindrical microreactor can be efficiently used for the removal of the metabolic product without the addition of oxidative species and having low energy consumption. The key parameter is the optimized flow rate in accordance with the pollutant concentration, reactor size and light intensity. Two pharmaceutical compounds, acetaminophen [31][77] and metformin [32][78], with the same concentration (50 mg/L) were submitted to photodegradation during 60 min of irradiation. The acetaminophen solution was tested in a 0.12 L reactor under continuous stirring and using a 9 W UV-C radiation source. The metformin degradation was evaluated into a 0.44 L reactor using 0.07 L/min flow rate and 5.7 W UV radiation source. The direct photolysis of acetaminophen aid by S2O82− (0.36 mg/L) reached 84.3% removal efficiency due to the SO4−· production through a proton-catalyzing process. In the case of metformin photodegradation the photocatalytic process was mediated by Fe(II) (0.05 mg/L) and peroxymonosulfate (20 mg/L). The flow rate was adapted according with the rector volume (0.44 L) at 0.07 L/min. Around 99% of the metformin was removed after 60 min of irradiation with a 5.7 W UV light source confirming the enhancement of the degradation performance due to the increased concentration of oxidative radicals. The addition of Fe(II) into the reaction medium will induce parallel Fenton reaction, producing additional HO· and HO2· radicals with positive impact on metformin degradation. Figure 24 indicate the contribution of all participants into pollutant removal during the photocatalytic activity. The photoreactor set-up was optimized to provide certain quantity of mediator and to allow even distribution and perpendicular incidence of UV light.
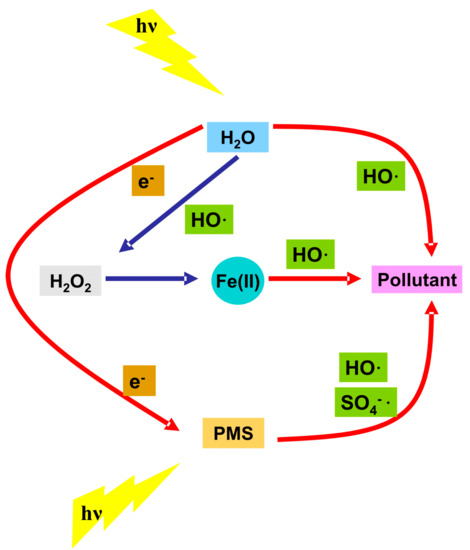

Figure 24.
Generation of oxidative species during the pollutant removal.
Zinc oxide (ZnO) was employed as a photocatalyst for paracetamol and caffeine removal using a 0.2 L reactor [33][79]. Both reference pollutant solutions were kept at the same concentration (12.5 mg/L) and photocatalyst dosage. The samples were irradiated for 240 min with a 14 W UV light source and at a constant flow rate of 144 Ncc/min. The results indicate the complete removal of caffeine, while the paracetamol concentration was reduced with 77%. The cause of the photocatalytic activity differences toward caffeine and paracetamol were not completely elucidated. The influence of surface chemistry and pollutant molecule stability play an important role on the mineralization process. The investigation done on floating and fixed-bed indicate the advantages of flotation, able to ensure uniform ZnO irradiation, which produce more oxidative radicals required for pollutant degradation. A high reactor volume (14.4 L) was tested for a comparative investigation regarding 4-chlorophenol and methylene blue (MB) removal [34][80]. The pollutant concentration was similar (10 mg/L) but the TiO2 photocatalyst dosage varied from 0.25 for MB to 0.5 mg/L for 4-chlorophenol. The same working conditions were applied: 24 L/min flow rate and 70 min of sunlight irradiation. The reactor set-up includes the storage tank, a centrifugal pump and two control valves required to set the recirculation flow rate. The higher MB photodegradation efficiency (99%) comparing with 4-chlorophenol (55%), confirms the previous investigations [23][27][69,73] showing that the phenol compounds are more reluctant to the oxidative radicals’ activity during the photocatalyst light irradiation. Additionally, the results indicate that the degradation increases at higher initial contaminants concentration since the reaction order is above zero. Considering the use of direct solar radiation, the set-up can be easily scaling up for large applications.
A flow reactor and stirring dynamic reactors were used to remove methyl red [35][81] and direct red [36][82] dyes in the presence of TiO2 photocatalyst. The flow dynamic reactor has 7.7 L volume and uses TiO2 catalyst to remove 10 mg/L methyl red pollutant. After 120 min of irradiation and using 12 L/min flow rate the photoreactor was able to degrade 99.5% of methyl red. Optimizing the catalyst dosage based on the photoreactor technical parameters can significantly decrease the chemicals consumption during the photocatalytic activity. The second reactor working in continuous stirring process has a lower volume (0.5 L) and use a high TiO2 dosage. The experiments involve two direct red dye concentrations: 30 mg/L and 40 mg/L. The photocatalytic evaluation indicates an interesting dependence between the dye concentration and irradiation period required to completely eliminate the pollutant. The 30 mg/L pollutant concentration was removed in 140 min, while for the mineralization of 40 mg/L direct red the exposure time increases at 240 min. Consequently, the energy consumption must be correlated with the pollutant concentration in order to ensure the implementation of a cost-effective technology. At high dyes concentrations, the solution become more colored acting as light screening for the irradiation sources. A 3.0 L reactor was also used to evaluate the reactive red dye degradation under continuous stirring [37][83]. The degradation of 100 mg/L reactive red solution was mediated by Na2S2O8 under 16 W UV radiation source. After 60 min of irradiation the reactive red was completely eliminated. This type of photoreactor has the advantage of working at high pollutant concentration due to the oxidative activity of SO4−· radicals formed during UV irradiation with 254 nm wavelength. These results indicate the importance of adapting the light source characteristic at a specific pollutant and mediator’s compounds and a particular dynamic regime. The stirring reactor has the advantage of keeping the solution and light radiation in permanent contact, without requiring the solution recirculation through pipes and storage tank.
The methyl orange (MO) photocatalytic removal was performed under irradiation with 150 W Vis light [38][84] and 10 W UV light [39][85] sources. In the Vis irradiation scenario, the MO concentration was 5 mg/L and the TiO2 photocatalyst was doped with N to extend the light absorbance spectra. After 60 min of irradiation and using a 2.7 L/min flow rate, the photocatalytic efficiency of MO removal reaches 59%. By switching to UV irradiation scenario and undoped TiO2, the photocatalytic efficiency at a lower flow rate (0.05 L/min) and longer exposure time (720 min) increases at 69%, even if the MO concentration was double (10 mg/L). The results were verified on Rhodamine B and the photocatalytic activity increased at 91% in the same experimental conditions. The comparative evaluation indicates that the energy consumption in the UV scenario (120 Wh) was lower than that of Vis light scenario (150 Wh), which use a smaller MO concentration. The energy consumption for the MO degradation using a slurry flow photoreactor is drastically reduced by optimizing the catalyst dose in correspondence with the light scenario and the provided turbulence.
Cylindrical reactors were employed for the removal of oxalic acid [40][86], paraffin [41][87] and poly(vinyl alcohol) [42][88] under UV irradiation. The oxalic acid solution with 0.9 mg/L concentration was inserted into a 1.4 L reactor together with 400 mg/L TiO2 dosage. Keeping a constant flow rate of 16 L/min during 60 min of irradiation with 100 W UV source it was possible to remove 80% from the initial oxalic acid concentration. While lamp orientation showed minimal photocatalytic impact, the reactor volume and flow rate may induce significant changes on the overall pollutant removal efficiency. Larger reactor and high flow rates seem to boost the photocatalytic activity due to a lower density of catalyst particles and higher irradiated surface. TiO2/SiO2 heterostructure was used as photocatalysts for the removal of high concentrated paraffin (500 mg/L) solution. The irradiation was done with a 16 W UV-C light source and the flow rate was lower (2.5 L/min) compared with oxalic acid experiments. After 180 min of irradiation, the photocatalytic efficiency was 86%, which shows that changing one of the key parameters (photocatalyst composition, pollutant type or concentration and irradiation source) it is possible to modify the degradation reaction kinetics based on the system capability to produce oxidizing radicals. The poly(vinyl alcohol) solution with 20 mg/L concentration was placed into a 6 L (0.5 L/min flow rate) reactor and submitted to direct photolysis aided by 0.9 mg/L H2O2. The photocatalytic efficiency reaches 63% after 150 min of irradiation with 13 W UV light source. Direct photolysis can represent a good alternative to photocatalysis, considering the catalysts limitations in terms of active surface and interface chemistry. However, the excessive use of photolysis promoters (i.e., H2O2 and S2O82−) raises serious issues in terms of a green approach and environmental impact.
The cylindrical photoreactors can be fully integrated into large scale wastewater treatment technologies by optimizing the geometrical configuration with the irradiation scenario, pollutant characteristics and technical parameters. Higher reactor volumes and flow rates must consider the energy consumption as a key parameter for proposing a cost-effective technology. A significant limitation is represented by the inability to predict the variation of photocatalytic activity based on the pollutant type and interface chemistry with the catalysts.
2.2. Rectangular Photoreactors
The rectangular reactors allow a higher versatility of the irradiation sources orientation, which can be placed on the internal lateral sides (Figure 35), central position, horizontal or vertical in the corners. The immobilized photocatalysts can be placed on the reactor walls, on the lamps cover or even on individual substrates (e.g., glass, textiles, and composites). If mobile photocatalysts are employed, the flow rate must be adapted to this particular geometry in order to provide a homogenous diffusion through the reactor volume. Consequently, the photoreactor set-up components are chosen to ensure a maximum production of oxidative species required to remove the organic pollutants. Table 2 includes representative studies on the photocatalytic applications of rectangular reactors for the removal of various organic pollutants.
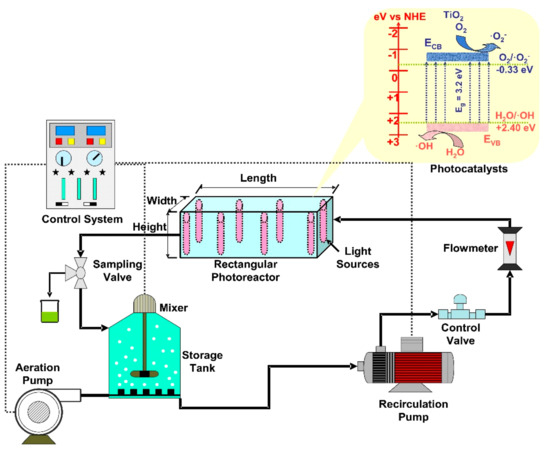

Figure 35.
Rectangular photoreactors components (TiO
2
photocatalyst).
N-doped TiO2 photocatalyst immobilized on glass spheres was involved in the photocatalytic MB removal using two rectangular reactors with different volumes. The 0.375 L reactor [43][89] with 0.04 L/min flow rate was irradiated for 264 min with an 8 W UV light source. The photocatalytic set-up was able to remove 75% from the 32 mg/L MB initial concentration. Due to the flat profile, a plug flow behavior providing a perfect homogenization of the inside fluid was obtained. The presence of structured catalyst will increase the removal rate due to the higher surface exposure to light irradiation. The second reactor has a volume of 0.3 L [44][90] and work in a higher flow rate (0.15 L/min). The photocatalytic efficiency after 180 min of irradiation with a 36 W Vis light source was 70%. However, in this case the MB, the concentration was 4.5 × lower, and the energy consumption was 3× higher. These values indicate the necessity of adopting the most suitable irradiation scenario and flow rate, able to favor the oxygen readsorption on the photocatalyst surface, which could subsequently react with the excess of photogenerated electrons, hence reducing recombination with holes.
Direct exposure to sunlight can be an energy efficient method for MB removal using TiO2 as a photocatalyst. A 3.4 L reactor [45][91] was used to evaluate the photocatalytic efficiency toward 25 mg/L MB solution and the flow rate was established at 7.25 L/min. Around 98% of the MB was removed after 48 h of sunlight exposure, considering that the photoreactor efficiency depends on the ratio between the reactor volume to the total solution volume. A larger ratio of volume will be beneficial, allowing the increase of the catalyst surface (when is immobilized) and leading to a higher photodegradation ability of the photoreactor. These results were confirmed when the same MB concentration was tested in 3.25 and 1.25 L reactors [46][92] using a flow rate of 3.44 L/min. The photocatalytic efficiency after 48 h of sunlight exposure shows a small decrease at 97% for 3.25 L reactor and 96% for 1.25 L reactor. These photocatalytic efficiency differences become quantitatively significant when the technology is scaled-up for a large application. Comparing the rate constants of both photoreactors shows that the 3.25 L reactor (0.117 h−1) was twice faster in removing the MB pollutant, than the 1.25 L reactor (0.05 h−1). The use of sunlight represents an alternative for implementing sustainable technologies but is dependent on the geographical position and climatic changes.
An oscillatory [47][93] and a dynamic [48][94] reactor was employed for MB removal using ZnO photocatalyst. The oscillatory reactor has a volume of 0.1 L and works using a low intensity (4 W) UV light source. The 10 mg/L MB solution was completely removed in the presence ZnO after 110 min of irradiation. The oscillatory motion will minimize the catalyst particles deposition and increase the suspension leading to an improvement of the photocatalytic activity. An additional argument is given by the lower hydraulic resistance of the oscillatory motion, combined with a longer resilience period of the liquid in reactor room. The dynamic microreactor with 3 × 10−3 L volume use a higher ZnO dosage, and the photocatalytic activity is aid by H2O2. Low MB concentration (0.013 mg/L) was completely removed after 30 min of irradiation with a 5 W UV source. The same result was obtained for salicylic acid (0.013 mg/L) removal but after 500 min of irradiation. The results shows that the removal reaction rate for MB is 17 × higher compared with salicylic acid, confirming the photocatalytic efficiency depends on the pollutant molecule characteristics. Using a microreactor bring the advantage of: (1) low quantities of reactants, solvents and catalyst; (2) safe conditions for UV radiation utilization; (3) energy saving and (4) facile operation of the set-up. The photocatalytic removal of high salicylic acid concentration (27.6 mg/L) was tested in a 6 × 10−2 L dynamic reactor using different flow rates and irradiation sources [49][95]. The highest photocatalytic activity (100%) in the presence of 1 mg/L Ag modified TiO2 was obtained after 240 min of irradiation with the 8 W Vis source and using a flow rate of 0.067 L/min. The photocatalytic efficiency decreased at 92% by reducing the flow rate up to 0.033 L/min. By keeping the same flow rate (0.033 L/min) but changing the radiation sources from Vis to UV (same intensity) the photocatalytic efficiency decreased by 90%. Besides doping, silver can be used to form Schottky junctions (Figure 46) able to generate high concentration of oxidative species, enhancing the photocatalytic reactions. In a reaction system mediated by mobile photocatalytic particles, the volumetric photon absorption is a key parameter to increase the local reaction rates. Assuming a uniform distribution of TiO2 particles in the liquid environment, the matches between catalyst particles local velocity and the fluid local velocity in laminar flow will ensure a longitudinal direction of the photocatalyst particles. Hence, to avoid poor illumination regions it is important to properly design the width of rectangular reactors.
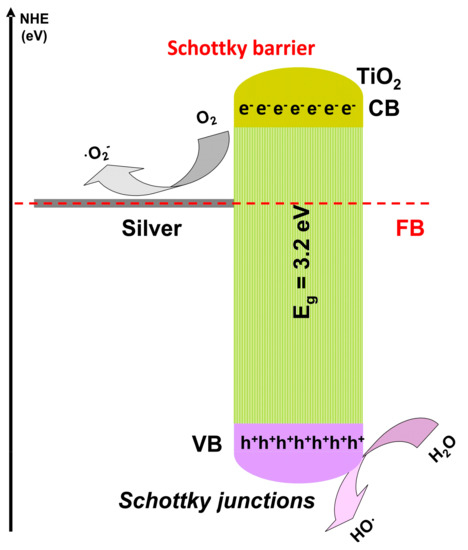

Figure 46.
Oxidative species generation using Schottky junction.
The photodegradation of two antibiotics (penicillin G and flumequine) were tested in the presence of the TiO2 photocatalyst. Penicillin G solution with 5 mg/L concentration was inserted into a 0.5 L reactor, working under continuous stirring [50][96]. The TiO2 photocatalyst was simultaneously doped with C, N and S in order to increase the absorption range in Vis spectra. After 240 min of irradiation with 15 W Vis light spectra, 95% of penicillin G was removed. The penicillin G degradation efficiency increased based on the permeate water volumetric percent recycled by the photoreactor, due to the prolonged hydraulic residence time. The flumequine solution was tested at a 4× higher concentration (20 mg/L) in a 0.6 L reactor using a double flow rate (0.09 L/min) and stirring regime [51][97]. The TiO2 photocatalyst was immobilized on 30 × 10 cm2 textile substrates, with 0.36 g of TiO2 per textile face. Around 93% of flumequine was removed using a 30 W UV radiation source. These results indicate that low transmittance substrates can be successfully involved in the photoreactor set-up development, with the condition of ensuring a homogeneous irradiation on the entire substrate surface. Using luminous textile has the advantage of removing the catalyst separation process from the treated solution, and allows the photoreactor size reduction by light source integration in the photocatalytic support.
High concentrated reactive red solution (118 mg/L) was submitted to photodegradation in a 0.5 L reactor under sunlight irradiation [52][98]. The ZnO photocatalyst was used in form of a thin film coated on a glass substrate. The experiments made 0.03 L/min flow rate indicate that the time required to remove half of the reactive red concentration was 15.8 min and the complete removal was achieved in 100 min. Tartrazine dye solution with different concentrations (10, 20 and 30 mg/L) was tested in a 1 L reactor volume using the TiO2 catalyst and UV radiation [53][99]. After 300 min of irradiation the experiments indicate the dependence between pollutant concentration and photocatalytic efficiency: the reaction rate was double at 10 mg/L concentration than that of 30 mg/L, and 1.66× higher than that of 20 mg/L. Consequently, 77.7% of reactive red was removed at the lowest concentration and only 46.5% at the highest concentration. The degradation rate was correlated with the catalyst active surface, responsible for electron-hole pair’s photogeneration. In this particular case, the amount of catalyst was constant, and the hydroxyl radical’s concentration remains unchanged, while tatrazine dye concentration increases. Therefore, the available hydroxyl radical for each tartrazine molecules decreased with increasing the dye concentrations, leading to lower photodegradation efficiencies. Higher tartrazine concentrations will increase the liquid UV light screening, which is an additional contributor on the decreasing of the hydroxyl radical’s amount. When the flow rate increase from 9.78 to 28 mL/s, the degradation efficiency increases, due to a higher turbulence in the solution, promoting the external mass transfer from the dye solution to photocatalyst surface. A complex study was made to verify the influence of dye molecule, photocatalyst and irradiation exposure period on the photocatalytic activity of a combined flow (0.032 L/min) and stirring reactor with 0.15 L volume [54][100]. In the first step, basic yellow, red and blue dyes with 25 mg/L concentration were irradiated for 480 min with Vis light in the presence of N and S-doped TiO2. In the second step the same dyes concentrations were irradiated for 240 min with Vis light but using Zn, N and S tri-doped TiO2 catalyst. The results indicate similar photocatalytic efficiency for basic blue dye in both cases. However, for basic red and yellow there was a significant increase of the photocatalytic activity in the second scenario (from 68 to 88% for basic red and from 78 to 94% for basic yellow). These variations of the photocatalytic response can be the result of anions doping, such as nitrogen and sulfur in the TiO2 anatase structure, inducing changes of the electrical conductivity or optical properties due to anions p orbitals combination with oxygen 2p orbitals, hence lowering the bandgap energy. Adding Zn as codoping will play the role of electron scavenger, with beneficial consequences on preventing the electron-hole recombinations. The insertion of Zn2+ ions in TiO2 anatase lattice enhance the HO· and O2−· production, playing a significant contribution in the dye degradation.
The photocatalytic removal of 106 colony-forming units/mL was evaluated in a rectangular 0.075 L reactor using a flow rate of 0.04 L/min [55][101]. The sample was irradiated with Vis light (60 W) for 200 min in the presence of N-doped TiO2 photocatalyst. The 50% photocatalytic efficiency was obtained after the optimization of E. coli concentration and flow rate. The study shows that by reducing the E. coli concentration at half of the above value, the photocatalytic activity decreased due to the minimum close proximity between the bacteria and immobilized N-doped TiO2 nanoparticles surface. The flow rate influence was statistically significant, indicating that by increasing the flow rate up to 0.06 L/min the photocatalytic efficiency decreased with more than 20%. This variation is related with the decrease in residence time of the E. coli solution due to the increase in flow rate, inducing an insufficient contact between the bacteria colony and immobilized photocatalyst. Landfill leachate (550 mg/L) considered as recalcitrant wastewater was photocatalytically treated in a 4.5 L reactor using W-C-codoped TiO2 layers as a catalyst [56][102]. The W-C-codoping allow the TiO2 catalyst to be active in the Vis range, which is considered as an advantage when passing to sunlight is envisaged. After 60 h of irradiation with 40 W Vis light source and using a flow rate of 6 L/min the photocatalytic efficiency reached 84%. The photocatalyst morphology in the reactor plays an important role on the overall photocatalytic activity. In the presence of catalyst nanoparticles, two concurrencies’ processes occur: adsorption and photodegradation. If the photocatalyst layer thickness increase, then it will reduce the adsorption mechanism for the nanoparticles located in the bottom layers. However, when the catalyst layer thickness is significantly higher than the optimum value, the porosity bottom layers will decrease, the nanoparticles become more compacted and the internal mass transfer is reduced.
UV-A radiation was used to remove hexacyanocobaltate [57][103] and potassium hexacyanoferrate [58][104] pollutants. A stirring reactor with 1.5 L volume was employed for the removal of 32 mg/L hexacyanocobaltate in the presence of 0.2 g of TiO2. The reactor was irradiated with a high intensity (120 W) UV-A light source during 350 min in order to decompose 40% of the initial hexacyanocobaltate concentration. Results show that the higher photocatalytic activity was obtained when extra oxygen was added into the reactor, since it generated higher free cyanide concentration and induced the oxidation chain up to NO3−, considering that reactive oxygen species are produced in the O2 atmosphere. The potassium hexacyanoferrate removal (100 mg/L) was evaluated into a microreactor (0.04 L) using a low UV-A radiation intensity source (15 W) for a total period of 90 min. Due to the spatial limitation the TiO2 dosage was reduced at 0.1 g and the photocatalytic efficiency was 70%. Using a higher potassium hexacyanoferrate concentration will induce a decrease of photocatalytic activity due to Fe precipitation on the TiO2. These results are in accordance with other papers [48][49][94,95] employing microreactors, which exhibit high photocatalytic activity and possess better process control. However, these technologies raise issues when upscaling for large applications, due to unpredictable changes in term of parameters evolution (flow regime, uniformity, photo distribution, etc.).
Ammonia removal was tested in a large 5 L reactor volume, using UiO-66(Ti)-Fe3O4-WO3 as a photocatalyst [59][105]. The flow rate was optimized at 0.55 L/min and after 60 min of irradiation with 14.4 W UV light source, 91.8% of the ammonia initial concentration (30 mg/L) was removed. At lower flow rates values, the catalyst diffusion in the reactor volume was insufficient while the catalyst residence time in the reactor room would increase, consequently more catalyst light exposure occurred. By increasing the flow rates above the optimized value, the convective mass transfer coefficient between the ammonia solution and photocatalyst surface was substantially enhanced, while the radiation contact time with the photocatalyst was reduced, which decreased the ammonia degradation efficiency. These experiments also highlighted that lower ammonia removal efficiency was obtained at higher pollutant concentration, due to the reduction of available catalyst active sites corresponding to each ammonia molecule.
Finally, rectangular photoreactors were tested for the removal of phenol compounds such as p-nitrophenol [60][106] and bisphenol A [61][107] in the presence of TiO2 photocatalyst. The 50 mg/L p-nitrophenol solution was placed into a 6 L reactor, operating with a flow rate of 7.8 L/min. The sample was irradiated for 360 min with 5 W UV source light and the photocatalytic efficiency was 72%. Comparing with the experiments made in cylindrical reactors [25][26][71,72] the photocatalytic activity was significantly lower. However, it must be considered that in this case the light source intensity was lower and the pollutant concentration was higher. By increasing the TiO2 catalyst amount, the available active sites will increase and the p-nitrophenol degradation is enhanced. On the other hand, high TiO2 quantity will increase the solution opacity and light scattering effect reduces the formation of oxidative species. Additionally, TiO2 particles tend to form an aggregate, which causes a reduction in the interfacial area between the catalyst and reaction solution. All these factors have a direct impact on the photocatalytic efficiency of the reactor set-up. The bisphenol A removal was evaluated in a smaller reactor (0.3 L) under direct exposure to sunlight for 300 min. The photocatalytic efficiency was 78.7% using low bisphenol A concentration. The experiments were repeated with 17 β–estradiol and 17 α-ethynyl estradiol, and the results were in the same range (83.7% for 17 β–estradiol and 79.7% for 17 α-ethynyl estradiol). These results indicate that using direct exposure to sunlight radiation can be a feasible way to remove organic pollutants. The main issues to this type of set-up are represented by the necessity to have a cooling system (to avoid overheating) and the unpredictability in term of light intensity.
The rectangular reactors can be easily upscaled based on a modular design, which allows the increase of solution volume treated each cycle. Due to the shape simplicity and geometrical versatility, the rectangular reactors can be implemented for small indoor or large outdoor applications. The main issues are related with optimizing the flow rate and irradiation parameters to avoid excessive velocities and to allow a uniform photocatalyst irradiation.
