Terpenes—a diverse group of secondary metabolites—constitute the largest class of natural products abundant in almost every plant species.
- carvone
- carvacrol
- perillyl alcohol
- perillaldehyde
- menthol
- limonene
- deep eutectic solvents
1. Introduction
Natural products derived from terrestrial plants, microorganisms, fungi and also marine organisms have long been sources of medicinal products that have since been exploited in medicine, pharmacology and biology [1]. Terpenes, also referred to as terpenoids or isoprenoids, represent the largest class of natural products with more than 88,000 structurally diverse compounds [2]. These substances are part of the secondary metabolism of vegetal and animal species. Structurally, terpenes are derived from isoprene units (C5H8). Depending on the number of linked isoprene units these chemical compounds are classified as hemi-, mono-, sesqui, -di-, sester-, tri-, sesquar-, tetra- (C5, C10, C15, C20, C25, C30, C35 and C40) and also polyterpenes. Many of these compounds have been extensively characterized and applied in the pharmaceutical, food, perfumery and cosmetic industries [2][3][2,3]. The broad range of the biological properties of terpenoids and their derivatives—including cancer chemopreventive, anti-inflammatory, antihyperglycemic, antibacterial, antifungal, antiviral, analgesic, antioxidant and anti-parasitic activities—have led to their widespread clinical application [4].
The bioactivities of monoterpenes are extensively reviewed in the literature. Many of these describe biomedical applications of essential oils and unmodified terpenes [5][6][7][8][9][10][11][12][5,6,7,8,9,10,11,12]; in contrast, articles dedicated to the biological activity of monoterpene derivatives are particularly rare [3][13][3,13]. Modifications of these natural compounds with proven biological properties are highly desirable. Terpenes can be functionalized by adding an element with confirmed bioactivity or, more simply, introducing a simple group (e.g., ether, ester and epoxide) that, by creating one or more heteroatoms, diametrically changes the activity of said molecule. Given the increasing amount of monoterpene derivatives described in the literature, a review of recent developments on monoterpene derivatives and their specific biological applications is warranted. As such, this work focuses on the clinical potential of promising monocyclic monoterpenes and their derivatives as antitumor agents (Figure 1). Specifically, we explore the following monoterpenes: carvone, carvacrol, perillyl alcohol, perillaldehyde, limonene and menthol.
Figure 1. Division of various monoterpenes and their antitumor activity.
Cancer is a complex, multifactorial disease involving uncontrolled cell growth that can affect nearly every tissue type in the body. Given that its prevalence continues to grow at an unmatched rate, cancer now represents one of the most pressing public health concerns worldwide. Though synthetic anticancer compounds still occupy the largest sector of the current cancer treatment market, more than two-thirds of the drugs currently used clinically are either derived directly from natural products or developed based on their bioactivity [10][14][10,14].
2. Carvone and Its Derivatives
Carvone (2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one; 5-isopropyl-2-methyl-2-cyclohexen-1-one; CVN) is an important cyclic unsaturated monoterpenoid ketone which exhibits promising activities. This monoterpene is presents in large amounts in caraway, dill and spearmint essential oils [6][7][6,7]. This compound exists as two optical isomers that can perform different biological activities. (S)-(+)-Carvone is the main component of caraway (Carum carvi L.) and dill (Anethum graveolens L.) seed oils, with a scent similar to these herbs. In contrast, (R)-(−)-carvone occurs in spearmint oil (Mentha spicata L.), which is steam-distilled from the leaves of the plant and has a minty, sweet scent (Figure 2). Carvone has several established applications: in the food industry, for fragrance and flavor; in agriculture as a sprouting inhibitor; and in medicine, due to its anticonvulsant and antioxidant properties [15][18]. Moreover, it has antimicrobial effects against various microorganisms [7][16][7,19]. Current research supports carvone’s therapeutic application to brain, colon, skin, myeloma and prostate cancer [14].
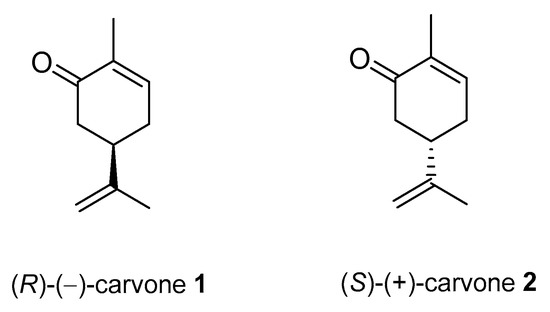
Figure 2. Stereoisomers of carvone.
Mesa-Arango et al. examined the cytotoxicity of CVN in vitro, using human cervix epithelioid carcinoma cells (HeLa cell line ATCC, CCL-2) and Cercopithecus aethiops African green monkey kidney cells (Vero cell line, ATCC CCL-81). CVN was cytotoxic to the HeLa tumor cell line (CC50 = 74.5 μg/mL) and had little effect on the Vero non-tumor cell line (CC50 > 200 μg/mL) (Table 1). Furthermore, the cytotoxic effects of CVN on HeLa cells were dose-dependent [17][20].
Zyad and co-workers evaluated the antitumor activity of carvone in vitro, using five tumor cell lines: P-815, K-562, CEM, MCF-7 and MCF-7 gem. They found that this monoterpene has an important effect, especially against the P-815, K-562 and CEM tumor cell lines with IC50 values ranging from 0.11 to 0.17 μM (Table 1) [18][21].
The anticancer potential of carvone was tested by Aydin et al. in cultured primary rat neurons and N2a neuroblastoma (NB) cells. The study revealed that high doses of CVN (≤ 100 mg/L) conferred a strong cytotoxic effect on healthy rat neurons and NB cells. The examinations also demonstrated weak antioxidant functions and little anticancer potentials in vitro. Studies suggest that CVN can be useful as a brain cancer chemopreventive and chemotherapeutic agent [19][22].
The biological effect of (S)-(+)- and (R)-(−)-carvone enantiomers on human colon tumor cells (HT29) and healthy colonic epithelial cells (CCD 841 CoTr) was evaluated by Paduch et al. [20][23]. The cytotoxicity and cell sensitivity of these stereoisomers were determined by using NR and MTT assays. The finding suggest that the divergent activity of enantiomers depends on the assay method used. (R)-(−)-Carvone demonstrated less toxic activity in tumor and healthy cells compared with its (S)-(+) enantiomer in the NR assay. (+)-Carvone was 59.4% and 27.1% more cytotoxic to tumor and healthy cells, respectively, than its (−)-stereoisomer. Tumor cells were much more sensitive to (R)-(−)-carvone whereas healthy cells were a little more susceptible to (S)-(+)-enantiomer in the MTT method. Both isomers were less cytotoxic to healthy cells (IC50 = 475 and 310 μg/mL, respectively). Similar results were obtained for this pair of enantiomers in all of the cancer lines (OVCAR-8, HCT-116 and SF-295) tested (Table 1) [21][24].
Another group of scientists, Nalini and co-workers [22][25] examined the chemopreventive potential of (S)-(+)-carvone against colon carcinogenesis. The considerable inhibitory effects of (+)-CVN on 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis was observed. The authors suggest that this effect could be related to the pro-oxidant–antioxidant balance, carcinogen detoxification and also its anti-proliferative effects. A dose of 10 mg/kg body weight prevented chemically induced colon carcinogenesis probably by reducing the neoplastic and inflammatory responses induced by DMH.
(S)-(+)-Carvone also efficaciously protected against DMBA induced skin carcinogenesis by modulating the activities of phase I and phase II detoxification enzymes and inducing cancer cell apoptosis. Oral administration of (+)-CVN restored the molecules involved in the apoptosis process [23][26].
Ding and Chen [24][27] demonstrated a significant anticancer effect on the myeloma cancer cells of CVN in a dose-dependent manner (IC50 = 20 μM against KMS-5 myeloma cells) (Table 1). The antiproliferative effect was related to induction of apoptosis and G2/M cell cycle arrest. CVN could inhibit cell invasion and the expression of the p-P38 protein at this IC50.
Studies conducted by Wang et al. [25][28] showed that (S)-(+)-CVN significantly inhibited cell proliferation and induced apoptosis in a DMBA-induced tumor hamster model of oral mucosal carcinogenesis.
A series of carvone derivatives with lipophilic benzoates and hydrophilic amines linked to the terpenoid moiety were synthesized by Dong and co-workers (Figure 3) [26][29].
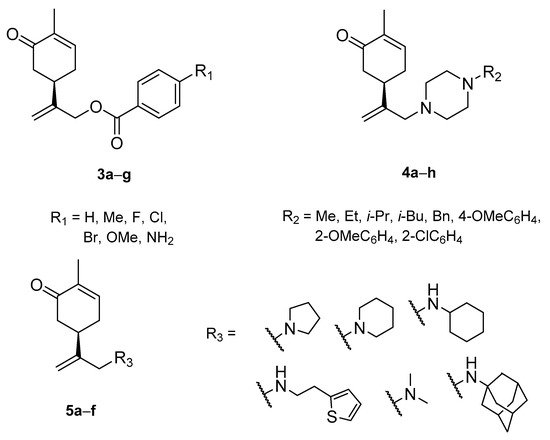
Figure 3. Carvone derivatives with anticancer activities.
All of the prepared compounds including (R)-(−)-carvone (L-carvone) were screened in vitro for their antiproliferative activities against human prostate (LNCaP) cancer cells, using MTT assay (Table 1). It was found that the presence of 4-methylbenzoyl 3b, 4-methoxybenzoyl (3f) and 4-aminobenzoyl groups (3g) in CVN notably increased the antiproliferative activity compared with non-substituted (−)-carvone. The incorporation of N-alkylpiperazine or N-benzylpiperazine into the

carvone moiety (4a–e) did not increase the antiproliferative effect of (−)-CVN noticeably. However, the presence of N-arylpiperazine scaffold in the structure of (R)-(−)-carvone (4f–h) significantly increased the antiproliferative activity of (−)-CVN. Amine substituted compounds 5a–f did not improve the antiproliferative properties except for compound 5d with a 2-thiopheneethylamine group (2-methyl-5-{1-[(2-thiophen-2-ylethylamino)methyl]vinyl}cyclohex-2-enone). The cell growth inhibitory effect of these compounds correlates with ERK activation and p21waf1 induction.
Structurally related sets of (S)-(+)-carvone-based hydroisobenzofuran analogues of sclerophytin A 6 were synthesized by Chambers and co-workers via an aldol-cycloaldol sequence (Figure 4) [27][30].
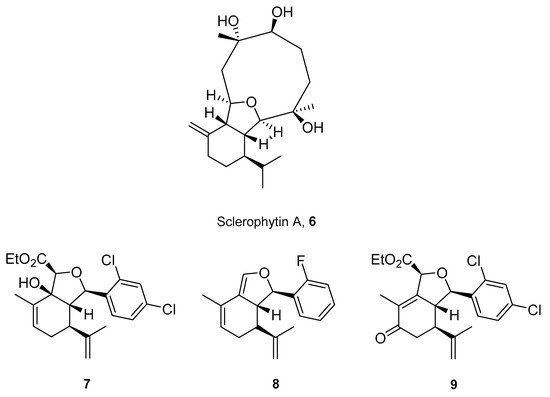
Figure 4.
The most active analogues of sclerophytin A
6
exhibited anticancer activity.
These compounds were screened for their antitumor activity against the human epithelial carcinoma cell line (KB-3), using the MTT assay. Among the tested classes of the compound, promising results were obtained for alcohol 7, diene 8 and enone 9 (Figure 4). Carvone derivative 9 showed significant differential activity against RPMI-8226 leukemia and the prostate cancer cell lines(PC-3). The results indicated that the most active was diene 8, having considerably varied activity against the entire leukemia panel and the non-small lung cancer line. A subsequent five-dose testing of 8 revealed GI50 = 0.148 μM and LC50 = 9.36 μM for RPMI-8226 leukemia cell line and GI50 = 0.552 μM and LC50 = 26.8 μM for the HOP-92 non-small-cell lung cancer line (Table 1).
De Sousa and co-workers selected eighteen monoterpenes including carvone derivatives 10–13 and evaluated their cytotoxic activity against tumor cell lines: HCT-116 (colon), OVCAR-8 (ovarian) and SF-295 (brain), using the MTT assay to establish the corresponding structure–activity relationships (SARs) (Figure 5) [21][24].
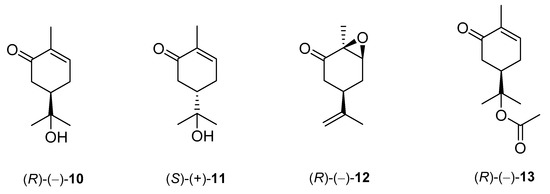
Figure 5. Carvone derivatives examined against tumor cell lines: HCT-116 (colon), OVCAR-8 (ovarian) and SF-295 (brain).
The authors examined the influence of hydroxyl and epoxide groups and ester function in the structure of (R)-(−)- and (S)-(+)-carvone enantiomers in terms of cytotoxic activity. Only the hydroxylation of a double bond in (−)-carvone significantly enhanced its anti-proliferative effect. (−)-8-Hydroxycarvotanacetone 10 was the most cytotoxic compound (GI = 61.59–94.01% at the concentration of 25 μg/mL) among the carvone derivatives tested (Table 1).
Many studies have demonstrated that metal complexes with thiosemicarbazones exhibit also anticancer activity [28][31]. Recently synthesized copper(II) complexes with various thiosemicarbazones are highly potent against HepG-2 (human liver hepatocellular carcinoma), NCI-H460 (human large-cell lung carcinoma) and HeLa (human cervical carcinoma) cells with significantly lower IC50 values than cis-platin [29][32]. Kokina et al. prepared Cu(I) and Pd(II) complexes with chiral thiosemicarbazones containing fragments of (−)-carvone and determined the cytotoxicity of the ligand L1 (14) and the complex PdL1Cl2 (15) for the adenocarcinoma Hep2 cell line (Figure 6) [30][33]. The results showed that complex 15 was more cytotoxic than thiosemicarbazone 14 (Table 1).
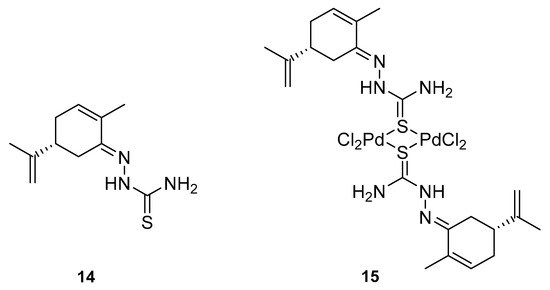
Figure 6.
(
R
)-(−)-Carvone thiosemicarbazone
14
(L
1
) and Pd(II) complex
15
(PdL
1
Cl
2
).
In summary, carvone and two carvone enantiomers decreased the viability of cancer cells. The (+)-carvone and the opposite (−)-isomer expressed diverse activity against healthy and tumor cells, depending on the cytotoxicity measurement method used (MTT or NR assay). Both isomers induced less cytotoxicity against healthy cells. Furthermore, this study demonstrated that carvone derivatives, in many cases, exhibit more potent cytotoxic effects on certain cancer cells compared with the starting terpene. The insertion of, for example, a hydroxyl or 4-substituted benzoyl groups as well N-arylpiperazine scaffold in the structure of (R)-(−)-carvone significantly increases the antiproliferative capacity of (−)-CVN.
