Electrochemical enzyme-based biosensors are analytical devices composed of a bio-recognition material and a transducer.
- enzyme immobilization
- covalent bonding
- adsorption
- cross-linking
- entrapment
- self-assembled monolayers
- gold nanoparticles
- biosensor
1. Introduction
The working electrode can be modified with, for example, Au nanoparticles (NPs) before enzyme immobilization, or AuNPs can be deposited together with molecules. Enzymes working in the frame of the key and lock analogy can selectively recognize analyte and induce a redox reaction that is finally responsible for the generation of an electric signal. The electric signal correlates to the substrate concentration, but this relationship is strongly affected by the sensor construction and the environment of the measurement. The electrochemical biosensor can be evaluated based on its linear range, the limit of detection (LOD) and sensitivity, recognized as three major parameters describing sensing device characteristics [1]. Electrochemical techniques used for glucose detection can usually be divided into three categories: potential, current and impedance-based methods.
At the same time, the enzymatic reaction which is monitored during measurement can cause charge accumulation or a potential drop/increase (potentiometric), charge generation (amperometric) or changes in resistance (impedimetric) [1]. In addition, the electrochemical biosensors can be divided into first-, second-, and third-generation sensors depending on the type of mechanism occurring during the period when the glucose molecule interacts with the sensing electrode [2]. In order to prepare the perfect biosensor, characterized by a low limit of detection, wide linear range, high sensitivity, good selectivity and the absence of non-specific bindings, effective electron transfer between the enzyme and transducer, as well as high stability and an excellent understanding of the interactions between the enzyme modification components are required. The immobilization of an enzyme can have various effects on enzyme activity [3]. It is valuable because this makes it possible to reuse an enzyme multiple times, thereby extending its life span as well as reducing its degradation [4]. In addition, this immobilization influences the improvement of its pH and temperature stability [5]. The selectivity, specificity and activity can be improved after immobilization by changing the conformation of the enzyme [6]. Another benefit of enzyme immobilization is the ability to catalyze reactions in non-aqueous environments. The homogenous product instead of a mixture of enantiomers and isomers can also be obtained [7]. This immobilization increases operational stability [8]. Suitable separation of the enzyme from the platform, medium and product allows the reduction of costs [9]. It should also be emphasized that biocatalytic processes involving enzymes are environmentally friendly [10]. However, enzyme distortion due to the multi-interactions between the support and molecules can possibly occur. Consequently, the enzyme can change properties or even lose its activity. Other examples are the blockage of active centers coming as a result of the unsuitable enzyme orientation and the diffusion limitations [11]. The selection of an appropriate immobilization method for the specific substrate can help prevent these problems.
Gold nanoparticles provide an excellent platform for solving health problems in cancer therapy as well as for chemical and biological sensing [12]. The properties of AuNPs can be tuned by changing their shape, size, and aggregation. The modification of the nanoparticle surface with various types of molecules provides unique properties for further applications, among others, in drugs, nucleic acids, proteins, bacteria and virus detection. Currently, many nanoparticles include the noble metals Au, Ag, Pt, Pd [13][14][15], and oxides (CuO, Cu2O, NiO, Fe2O3 [16][17][18]), as well as bimetallic systems (Au-Pt, Au-Pd, and Cu-Ag [19][20][21]), have been used for glucose detection. Nevertheless, the major benefit of using gold is a higher glucose oxidation current than for other noble metals [22]. Transition metals and their oxides, such as Cu and Ni, have been extensively examined because of their good catalytic performance. However, in the case of Cu and its oxides, high background current and the competitive oxygen evolution reaction can disturb glucose oxidation [22].
2. Types of Immobilization Methods
[23]
[24]. There are five most commonly applied methods: covalent bonding, adsorption, cross-linking, entrapment and self-assembled monolayers. The schematic representation of each method is shown in Figure 1.
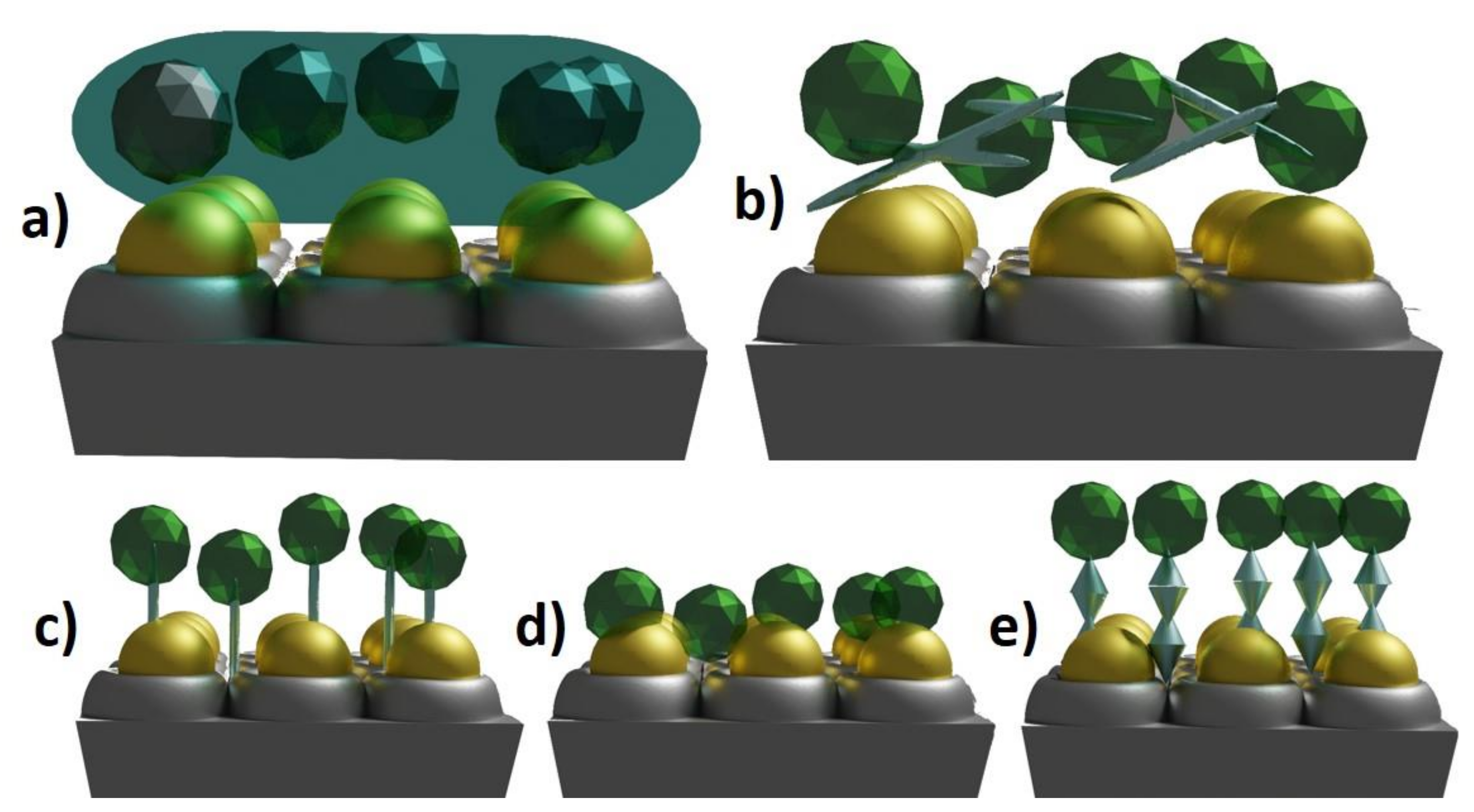
Figure 12.
a
b
c
d
e
Covalent bonding, adsorption, cross-linking, entrapment and self-assembled monolayers are recognized as the most frequently applied methods for enzyme immobilization. Electrodes modified using covalent linking and self-assembled monolayer techniques achieved the lowest limit of detection among all types of immobilization. This could be the result of ordered molecular arrangements and fast electron transfer along the chains. At the same time, the highest sensitivity and the widest range of linear response were obtained when cross-linking and entrapment techniques were used. In this case, the number of enzymes immobilized on the electrode surface and the thickness of the functionalization layer can be greater than for adsorption, covalent bonding and SAMs modifications. As a result of the utilization of highly advanced methods of nanomaterials fabrication and novel characterization techniques [25] as well as progress in theoretical and computational methods [26], the inevitable and fast development of enzyme immobilization and sensing systems is expected. The multianalyte detection of glucose, lactose, fructose, cortisol, dopamine, vitamin C or paracetamol, and more, will be expanded [27].
We believe that the development of protective biofouling materials [28], as well as the minimization of sensors and wearable technology [29], will receive great attention. The huge number of articles as well as projects concerning sensors, among others glucose biosensors, lead us to the conclusion that they will become an integral part of human life. We also strongly believe that sensors will contribute particularly to the development of rapid medical diagnostics. Of course, one cannot forget that the way to transfer the proof-of-concept devices that are described in the literature to real-life applications should occur first, as it still remains the most critical issue to be solved. In such a case scenario, electrochemical sensors will be used to their full potential. We are confident that in the near future it will be possible for each individual to be able to examine the analytes in his/her body at the most convenient time. Moreover, the obtained results will be simultaneously sent to the doctor, enabling the fast implementation of treatment. Collecting health data, fast analysis and medical advice will become a daily reality.
References
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763.
- Hatada, M.; Loew, N.; Inose-Takahashi, Y.; Okuda-Shimazaki, J.; Tsugawa, W.; Mulchandani, A.K. Sode, Development of a glucose sensor employing quick and easy modification method with mediator for altering electron acceptor preference. Bioelectrochemistry 2018, 121, 185.
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904.
- Spahn, C.; Minteer, S. Enzyme Immobilization in Biotechnology. Recent Pat. Eng. 2008, 2, 195–200.
- Mazlan, S.Z.; Hanifah, S.A. Effects of Temperature and pH on Immobilized Laccase Activity in Conjugated Methacrylate-Acrylate Microspheres. Int. J. Polym. Sci. 2017, 2017, 1–8.
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; de Oliveira, D. Nanomaterials for biocatalyst immobilization–state of the art and future trends. RSC Adv. 2016, 6, 104675–104692.
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821.
- Nisha, S.; Arun Karthick, S.; Gobi, N. A Review on Methods, Application and Properties of Immobilized Enzyme. Chem. Sci. Rev. Lett. 2012, 1, 148–155.
- Liang, S.; Wu, X.-L.; Xiong, J.; Zong, M.H.; Lou, W.Y. Metal-organic frameworks as novel matrices for efficient enzyme immobilization: An update review. Coord. Chem. Rev. 2020, 406, 213149–213173.
- Engelmann, C.; Ekambaram, N.; Johannsen, J.; Fellechner, O.; Waluga, T.; Fieg, G.; Liese, A.; Bubenheim, P. Enzyme Immobilization on synthesized Nanoporous Silica Particles and their Application in a Bi-enzymatic Reaction. ChemCatChem 2020, 12, 2245–2252.
- Geormalar, C.; Seenuvasan, M.; Kumar, K.S.; Kumar, A.; Parthiban, R. Review on surface modification of nanocarriers to overcome diffusion limitations: An enzyme immobilization aspect. Biochem. Eng. J. 2020, 158, 107574.
- Zhang, J.; Mou, L.; Jiang, X. Surface chemistry of gold nanoparticles for health-related applications. Chem. Sci. 2020, 11, 923–936.
- Baghayeri, M.; Nodehi, M.; Amiri, A.; Amirzadeh, N.; Behazin, R.; Iqbal, M.Z. Electrode designed with a nanocomposite film of CuO Honeycombs/Ag nanoparticles electrogenerated on a magnetic platform as an amperometric glucose sensor. Anal. Chim. Acta 2020, 1111, 49–59.
- Chen, H.; Yuan, C.; Yang, X.; Cheng, X.; Elzatahry, A.; Alghamdi, A.; Deng, Y. Hollow Mesoporous Carbon Nanospheres Loaded with Pt Nanoparticles for Colorimetric Detection of Ascorbic Acid and Glucose. ACS Appl. Nano Mater. 2020, 3, 4586–4598.
- Chen, Z.; Zhao, B.; Fu, X.Z.; Sun, R.; Wong, C.P. CuO nanorods supported Pd nanoparticles as high performance electrocatalysts for glucose detection. J. Electroanal. Chem. 2017, 807, 220–227.
- Yang, P.; Wang, X.; Ge, C.; Fu, X.; Liu, X.Y.; Chai, H.; Chen, K. Fabrication of CuO nanosheets-built microtubes via Kirkendall effect for non-enzymatic glucose sensor. Appl. Surf. Sci. 2019, 494, 484–491.
- Lin, L.Y.; Karakocak, B.B.; Kavadiya, S.; Soundappan, T.; Biswas, P. A highly sensitive non-enzymatic glucose sensor based on Cu/Cu2O/CuO ternary composite hollow spheres prepared in a furnace aerosol reactor. Sens. Actuators B Chem. 2018, 259, 745–752.
- Kailasa, S.; Rani, B.G.; Bhargava Reddy, M.S.; Jayrambabu, N.; Munindra, P.; Sharma, S.; Venkateswara Rao, K. NiO nanoparticles-decorated conductive polyaniline nanosheets for amperometric glucose biosensor. Mater. Chem. Phys. 2019, 181, 1581–1589.
- Shim, K.; Lee, W.C.; Park, M.S.; Shahabuddin, M.; Yamauchi, Y.; Hossain, M.S.A.; Kim, J.H. Au decorated core-shell structured for the glucose oxidation reaction. Sens. Actuators B Chem. 2018, 278, 88–96.
- Li, X.; Du, X. Molybdenum disulfide nanosheets supported Au-Pd bimetallic nanoparticles for non-enzymatic electrochemical sensing of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 239, 536–543.
- Chawla, M.; Pramanick, B.; Kaur Randhawa, J.; Prem Felix, S. Effect of composition and calcination on the enzymeless glucose detection of Cu-Ag bimetallic nanocomposites. Mater. Today 2021, 26, 101815–101828.
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34.
- Nguyena, H.H.; Kim, M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163.
- Bashir, N.; Sood, M.; Bandral, J.D. Enzyme immobilization and its applications in food processing: A review. Int. J. Chem. Stud. 2020, 8, 254–261.
- Lipińska, W.; Ryl, J.; Ślepski, P.; Siuzdak, K.; Grochowska, K. Exploring multi-step glucose oxidation kinetics at GOx-functionalized nanotextured gold surfaces with differential impedimetric technique. Measurement 2021, 174, 109015.
- Xie, Y.; Li, Z.; Zhou, J. Hamiltonian replica exchange simulations of glucose oxidase adsorption on charged surfaces. Phys. Chem. Chem. Phys. 2018, 20, 14587–14596.
- Aksorn, J.; Teepoo, S. Development of the simultaneous colorimetric enzymatic detection of sucrose, fructose and glucose using a microfluidic paper-based analytical device. Talanta 2020, 207, 120302.
- Song, Z.; Ma, Y.; Chen, M.; Ambrosi, A.; Ding, C.; Luo, X. Electrochemical Biosensor with Enhanced Antifouling Capability for COVID-19 Nucleic Acid Detection in Complex. Anal. Chem. 2021, 93, 5963–5971.
- Min, J.; Sempionatto, J.R.; Teymourian, H.; Wang, J.; Gao, W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. 2020, 172, 112750–112800.
