Photocatalysis is a classical solution to energy conversion and environmental pollution control problems. In photocatalysis, the development and exploration of new visible light catalysts and their synthesis and modification strategies are crucial. It is also essential to understand the mechanism of these reactions in the various reaction media. Recently, bismuth and graphene’s unique geometrical and electronic properties have attracted considerable attention in photocatalysis.
- bismuth/graphene
- nanohybrids
- photocatalysis
- reaction mechanisms
- energy
- pollution
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
The increase in pollution due to urbanization and industrialization has become a significant challenge for the sustainability of human society. The waste generated in different industries during crude oil storage, transportation, and refinery has become a global problem [1,2][1][2]. The water and soil pollution caused by several pollutants’ discharge is a critical public health concern due to their toxicity. These pollutants can cause many health effects such as neurological toxicity, lung cancer, lethargy, fatigue, depression, headaches, nausea, dizziness, throat and eye irritation, and acute and chronic respiratory effects [3]. Toluene, benzene, xylene, ethyl benzene, and phenolic compounds some of the main compounds categorized as pollutants posing severe threats to our environment [4,5,6][4][5][6]. In the present situation, environmental pollution has increased several-fold due to the mismanagement of industrial waste. This can negatively affect the ecosystem and make lands unusable for agriculture and many other purposes [7]. Therefore, it is essential to remediate these toxic pollutants in our environment [8,9,10][8][9][10].
To eliminate organic pollutants from the environment, numerous technologies have recently been established for their degradation. Organic pollutants can be degraded by different methods, such as physical, chemical, biological treatments and advanced oxidation techniques [9,11,12,13,14,15][9][11][12][13][14][15]. Organic pollutant photodegradation is an attractive “green” chemical technology to control pollution, where photocatalysis is the most widely and potentially applied method used for demineralization and degradation of such pollutants [16,17][16][17].
Various light sources have been applied for the excitation of heterogeneous catalysts [18], but the photodegradation approach is more economical if sunlight can be used compared to ultraviolet light [16,19,20][16][19][20]. The evolution of the term “photocatalysis” shows the development of certain fundamental concepts of photochemistry. The point where photochemistry became a discipline was when it became differentiated from thermal chemistry. Indeed, several researchers saw irradiation as one of the many methods available to catalyze a response that makes it quicker by, for example, heating or processing it with certain chemicals until the beginning of the 20th century [21]. Ciamician, the first scientist to systematically understand the chemical effect of light, took great pains in finding out if he had “initiated heat” alone rather than “light” [22]. This was appropriately allotted the term “photochemical,” whilst the word “photocatalytic” applied to reactions caused by light, but with the same result as thermal reactions. Another step further was the identification of electronically excited states, which became a general idea in 1914 and were part of Bodenstein’s photochemical reactions along with reactivity and thermodynamics. In an early stage, more distinction was made in the thermochemistry of the process itself. This allowed for photosynthesis to occur when part of photon energy in the products rose [22,23][22][23].
Around 43% of visible-light energy is solar, so visible-light catalysts are chosen in photoelectrocatalysis and photocatalysis processes. Until now, several semiconductive products have been utilized, including metal oxides (Ag2O, TiO2, Cu2O, ZnO, Fe2O3, Ta2O5), metal selenides (CdSe and MOSe2), metal phosphides (Ni2P), metal sulfides (Bi2S3, ZnS, MoS2, and CdS), multi-structure oxides (Sr TiO3WO), metal halides and oxyhalides (AgBr, BiOBr) and metal-free materials (SiC, Si and g-C3N4), [24,25,26,27,28][24][25][26][27][28]. Those with a bandgap (Eg) greater than 3 eV, e.g., SrTiO3, TiO2, ZnO, KTaO3, ZnS, and SrTiO3, are called wide-bandgap photocatalysts, whereas catalysts with an Eg of less than 4 eV, e.g., Si, SiC, Ag2O, Bi2WO6, CdSe, InTaO4, Ag3VO4, CoO, Fe2O3, Cu2O, TaON, Ta3N5, CdS, Bi2S3, g-C3N4, and BiVO4, are photocatalysts that react to visible light [25,29][25][29].
Heterogeneous catalysts play a vital role in environmental pollution control [30,31,32][30][31][32]. Powdered semiconductor photocatalysts are commonly used in various areas, such as carbon reduction [33], selective organic transformations, environmental remediation [34], and water splitting [35]. There has been, in numerous applications, a growing interest in the use of semiconductors as photocatalysts. In 2015, around 5500 documents about photocatalytic applications were published, indicating that interest in heterogeneous photocatalysis was enormous and highly important in diverse research fields. This number has recently grown to over 13,000. A country-specific view of the increase in the number of publications on “photocatalytic degradation” is listed in Table 1. No commercially accessible material can currently meet all application requirements, such as cost-effectiveness, stability, high visible-light quantum efficiency, and security [36]. For such tasks to be completed, a highly effective architecture and system for environmental remediation and energy supply are needed to examine new visible-light semiconductor materials.
Table 1. Country-wise publications growth on the photocatalytic degradation of organic pollutants. (Data acquired from SciFinder).
| S. No. | Country | No. of Publications |
|---|---|---|
| 1 | China | 8838 |
| 2 | India | 1090 |
| 3 | Iran | 676 |
| 4 | South Korea | 384 |
| 5 | United States of America | 178 |
| 6 | Japan | 175 |
| 7 | Malaysia | 158 |
| 8 | Saudi Arabia | 103 |
| 9 | Pakistan | 84 |
| 10 | Italy | 77 |
| 11 | Australia | 73 |
| 12 | Spain | 72 |
| 13 | Brazil | 57 |
| 14 | United Kingdom | 48 |
The development of nanomaterials has progressed from the synthesis of single-particles to multicomponent assemblies or hierarchical structures, where two or more pre-synthesized nanomaterials are coupled to obtain multifunctionality. Such multicomponent assemblies are termed nanohybrids. The development and use of these nanohybrids requires interdisciplinary knowledge from the energy and environmental sectors, including the applications reported in references [37,38,39,40,41,42,43][37][38][39][40][41][42][43]. There are previously published review articles on some types and uses of nanohybrids, including gold-graphene oxide nanohybrids [39], organic/inorganic nanohybrids [44], polymer nanohybrids for oil recovery [45], nanohybrids of epoxy/polyamide with carbon nanotubes [46], protein-inorganic nanohybrids [47], gold-based inorganic nanohybrids [48] and polymer-inorganic supramolecular nanohybrids [49].
Graphene is the basic structure of all other carbon allotropes. It is well noted that the potential applications of graphene its derivatives are mainly driven by progressive production of different graphene materials such as graphene oxide (GO), reduced graphene oxide (rGO), functionalized graphene oxide (fGO), and functionalized reduced graphene oxide (frGO) with specific attention to precise applications and this is expected to continue for at least a couple of decades as promising applications and requirements are disclosed [50,51][50][51]. Various literature reports on the synthesis, modification and application of photocatalysts based on graphene for energy and environment solutions have already been published [52]. Graphene, graphene and its derivatives [53[53][54],54], graphene in photocatalysis [55], graphene doping [56], graphene and graphene oxide sponge [57], nitrogen-doped graphene [58], structure of graphene and its disorders [59], strain engineering of graphene [60], mechanics of graphene nanocomposites [61], chemical vapor deposition of graphene [62], functional modification of graphene/graphene oxide [63], graphene-based fibers [64], and graphene-based electrochemical micro-supercapacitors [65] are some of the subjects that have been reviewed.
Considering the stability, reactivity, reusability, and light-responsive effect of bismuth (Bi) it has been widely used as a photocatalyst. Several state-of-the-art review articles on topics including barium potassium bismuth oxide [66], bismuth-based composite oxides [67], bismuth ferrite nanoparticles [68], bismuth vanadate-based materials [69], bismuth tungstate photocatalysts [70], and bismuth oxyhalides [71] have been published. Annual numbers of publications on graphene photocatalysts in the last ten years are shown in Figure 1a. Similarly, bismuth-containing compounds are significant photocatalysts that react to visible light and fascinating research has been published in the field of bismuth photocatalysis over the last ten years (Figure 1b).
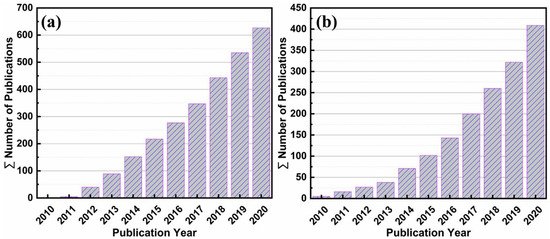
Figure 1. Annual numbers of published items in the last 10 years identified in SciFinder using the keywords: “Graphene-based photocatalysts” (a) and “Bismuth-based photocatalysts” (b).
This review, therefore, summarizes and discusses recent Bi-graphene photocatalysts and their energy and environmental sector applications. The choice of bismuth with graphene is due to the vast available literature, as shown in Figure 1. Furthermore, most bismuth-based photocatalysts are stable, reusable, photoactive, cheaper, and more environmentally friendly that other alternatives. Besides, due to some shortcomings of the pristine photocatalysts, such as charge carrier recombination, slow migration of charge carriers, and low visible light absorption [72,73][72][73].
2. Bismuth-Graphene Based Photocatalytic Materials
2.1. Bi2O3 and Bi2S3/Graphene Composites
A significant and the simplest bismuth compound is bismuth trioxide (Bi
O
). It can be used in various ceramics, fuel cells, and gas sensors [74][75]. It has also been used as a photocatalyst in organic pollutant decomposition and water splitting [76]. Bi
O
is a visible-light-responding photocatalyst when acting as a semiconductor, and its bandgap ranges between 2.1 eV and 2.8 eV. Doping with noble materials and combination with other components have been used to increase graphene’s activity in photocatalytic (PC) form [77][78].
In recent times, the PC activity of some Bi-based semiconductors, e.g., BiVO
[79], Bi
MoO
[80][81], BiOX (X = Cl, Br, I), Bi
Sn
O
[82], Bi
O
[83], and BiSbO
[84] in the degradation of pollutants has been described. Bismuth oxide was shown to be a strong candidate among the various Bi-based semiconductors because of its good PC and appropriate bandgap properties. Bi
O
’s PC activity is however restricted by quick recombination of the photogenerated carriers and by its susceptibility to photocorrosion. Because of the short distance between the conduction band (CB) of Bi
O
and the valence band (VB), graphene can be designed for the sharing of Bi
O
and graphene [85]. Under such conditions, electrons generated in the CB of Bi
O
would quickly be coupled with graphene VB holes [86]. Therefore, the photogenerated electrons accumulated on the CB of graphene display strong reduction ability, and the photogenerated holes on the VB of Bi
O
exhibit excellent oxidation ability [87][88]. The Z-Scheme PC activities are more effective than one component in terms of reduction and oxidation and advanced photocatalytic performance in the traditional photocatalysts [89][90]. Cui has reported a novel Z-scheme Bi
O
/graphene photocatalyst. Bi
S
has a 1.7 eV bandgap and is a perfect photocatalytic material for light-harvesting due to its near-IR and visible light activation [91]. A number of Bi
S
nanocrystal forms ranging from 1D nanorods and 2D nanosheets have been created with hot injection and standard non-oxidation techniques [92][93], while a solvothermal method produces 3D sea-urchin-like spheres [94].
Bismuth sulfide (Bi
S
) is a priviledged nontoxic inorganic semiconductor with excellent photocatalytic activity and chemical stability because of its good visible light response. It has been exploited and investigated mostly for optoelectronic applications. The photogenerated holes and hydroxyl radicals (-OH) in the VB of Bi
S
(1.62 eV) are mostly utilized in dye pollutant decomposition [92]. In combination with many other photocatalysts such as CdS [95], TiO
WO
[97], the recombination rate of electron-hole pairs could be lowered. An increase in visible light absorption enhances the photocatalytic activity.
A graphene/Bi
S
nanocomposite with narrow bandwidth was recently synthesized. Compared with the individual components, the PC of this nanocomposite was much higher. Zhou et al. stated that the well-matched bandgap of graphene/Bi
S
heterojunction could be tailored to increase the transfer and separation efficiency of photoinduced carriers and the visible light response. These graphene/Bi
S
composites are effective photocatalysts for the photocatalytic degradation of environmental pollutants [74].
2.2. Bi2MO6 (M = Cr, Mo, W)/Graphene Composites
Bi
MO
(M = Mo, Cr, W) is considered the most common member of the Aurivillius family, Bi
A
B
O
(A = Sr, Ca, Ba, Bi, Pb, K, Na; B = Nb, Ti, Ta, Fe, W, Mo) is the general formula for Bi
MO
. The Bi
MO
electronic structure is theoretically based on density functional theory (DFT) [98], while the Bi
MO
crystal structure falls under orthorhombic space group Pca2(1). It was seen that both VB and CB of Bi
MO
are composed of hybridized orbitals Bi
p, O
p, and M
d (n = 3, 4, and 5) for Bi
CrO
, Bi
MoO
and Bi
MO
, respectively [99]. Bi
MO
compounds are suitable as visible-light-activated photocatalysts. Among all Bi
MO
species Bi
CrO
has a narrower bandgap, thus, it easily undergoes recombination of photogenerated holes and electrons and is thus not considered suitable as a photocatalyst and consequently few Bi
CrO
studies are available in the field of photocatalysis. For the preparation of Bi
MoO
samples with a wider special surface area, smaller particles, and higher photocatalytic function, the solvothermal and hydrothermal methods are effective. Several Bi
MoO
morphologies have been described, including floral hollow spheres (solvothermal process) and nanoplates (hydrothermal method). Moreover, microwave heating was applied to synthesize Bi
