Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ridha Djellabi and Version 2 by Catherine Yang.
Photocatalysis since a long time has been recognized as a eco-technology to fight air pollution. It is focused on the generation of reactive oxygen species (ROSs) under light on the surface of a photocatalytic material. It could be applied in different manners such as for the purification of industrial polluted air, photoconversion of toxic gas into valuable products, self-cleaning systems, photocatalytic filters, etc. However, it faces some technological issues limiting its wide application in real-world.
- photocatalysis
- reactors
- Air purification
- Disinfection
- Environmental remediation
1. Introduction
For more than three decades, the scientific community has devoted a great deal of effort to developing photocatalytic processes for the removal of a range of air pollutants [1][2][3][4][5][6][92,93,94,95,96,97]. Photocatalysis was demonstrated to be effective for the removal of pollutants in gas phase at relatively low concentrations [7][98]. Different photocatalytic materials and photoreactors have been proposed [3][7][8][9][94,98,99,100]. However, similar to the case of photocatalytic water treatment [10], the process scale-up for the photocatalytic air purification is still an open challenge [11][101].
When it comes to the application of photocatalysis in a real air system, different approaches can be envisaged:
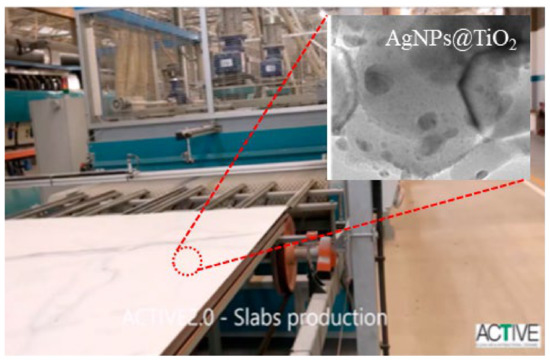
(i) Photocatalytic self-cleaning and antimicrobial materials to be used in indoor/outdoor areas: Under natural solar light (outdoor) or LED irradiation (indoor), the photoactive self-cleaning materials can initiate photocatalytic reactions able to clean continuously their surface and/or treat air pollutants [12][13][14][102,103,104]. This system is very promising and has received a lot attention from the industrial community [15][16][17][105,106,107]. Numerous types of self-cleaning coatings and building materials have been developed and commercialized for both indoor and outdoor applications. For instance, Bianchi et al. [17][18][19][107,108,109] reported a novel digital printing technology for the coating of tiles by visible light-responsive Ag nanoparticle-decorated TiO2 (Figure 14). This scalable technology allows obtaining a homogenous coating without the loss of photoactivity.
(ii) Photocatalytic purification of contaminated air in indoor, small spaces, such as aircraft cabins [20][32] and cold rooms [21][110]: while some air purifier devices based on photocatalytic materials are already on the market, this technology has some drawbacks. Since toxic by-products can be formed during the photocatalytic process if contact times are not properly tuned, the undesired accumulation of toxic by-products in an enclosed environment can occur [22][23][24][111,112,113].
(iii) Photocatalytic reactors for the direct purification of industrial exhausts: photocatalytic reactors for this application have some scale-up issues and the gap between research at a laboratory scale and industrial needs is still substantial.
(iv) Giant solar photoreactors for CO2 reduction have been suggested as a possible strategy against climate change [25][114]: this approach has the additional benefit of producing value-added chemicals or fuels from the reduction process. However, research in this field is still in the early stages.
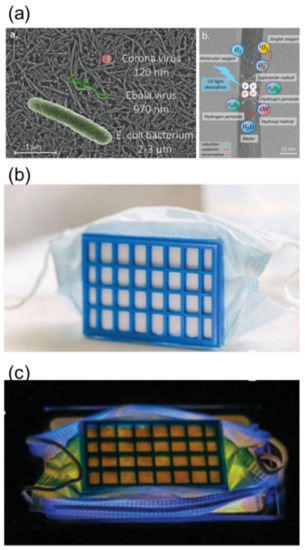
New applications are also emerging. In this respect, Horváth et al. [26][115] recently reported a photocatalytic mask filter based on TiO2 nanowires, which can be used as a protection against airborne viruses, including COVID-19, and can be photocatalytically sanitized under UV irradiation (Figure 25). Li et al. [27][116] fabricated a photocatalytic metal–organic framework (MOF)-based personal mask that can be self-disinfected under visible light.
2. Photocatalytic Reactors for Air Treatment
A successful photoreactor ensures, on one hand, an excellent interaction between the gas pollutants and the immobilized photocatalyst by an enhanced surface-to-volume ratio and, on the other hand, an excellent irradiation of the photocatalyst surface [9][28][29][30][31][100,117,118,119,120].
Mass transfer and contact time are key parameters in photocatalytic air processing, since in air systems only adsorbed pollutants can undergo degradation by direct oxidation by positive holes or surface photoproduced ROS. While this issue is generally less felt in lab scale batch reactors, where contact times range from seconds to minutes [32][121], the design of photocatalytic reactors for treating large air volumes requires a careful optimization of mass transfer issues [33][122] in order to achieve a fast degradation of pollutants without the accumulation of undesired by-products. In real applications, high flow rates are needed to maximize mass transfer, but this comes at the cost of decreasing the contact time between the pollutant and the photocatalyst surface to less than 1 s, which often results in an incomplete degradation of the pollutant. To solve this problem, different strategies have been reported, including modifications in the reactor geometry to decrease face velocity [24][113] or increases in the photocatalytic surface area and irradiance [28][117]. It should be noted that the ideal contact time depends on the adsorption affinity between the reactants and the photocatalyst/support [34][123].
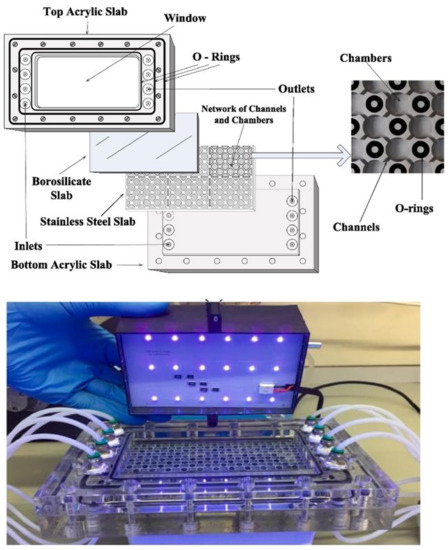
Costa Filho et al. [28][117] proposed a micro-meso-structured photoreactor for the removal of gas phase n-decane by simulated solar light (Figure 36). The adopted geometry allowed a uniform irradiance of the TiO2 photocatalyst, yielding a degradation rate of 6.6 mmol·m−3·s−1 and no loss of activity after 72 h of consecutive use.
Fluidized bed photoreactors have also shown a great potential for the photocatalytic purification of contaminated air. Light irradiation can be external (Figure 47e) [35][36][124,125] or internal (Figure 74f) [37][126], and can also utilize LED lamps [34][123]. However, the leaching of photocatalysts from the bed was observed in this type of reactor [38][127].
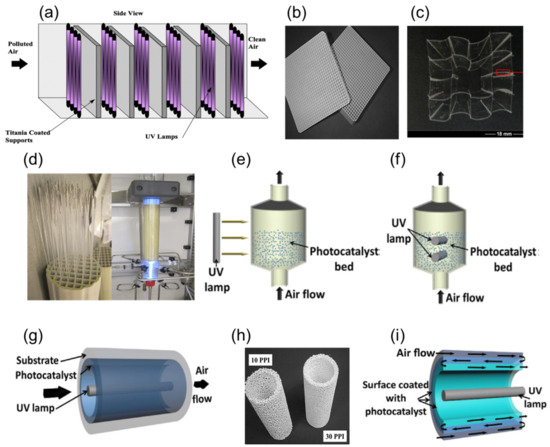
Figure 47. (a) Schematic representation of commercial-scale monolith-based reactor for air purification, reproduced with permission from reference [1][92]. (b) Square-channeled honeycomb monoliths, reproduced with permission from reference [39][130]. (c) TiO2-coated transparent monolith for air purification, reproduced with permission from reference [40][132]. (d) Photographs of monolith reactor equipped with light-emitting fibers inserted into the monoliths, for multiphase photocatalytic treatment, adapted with permission from [41][128]. (e–f) Fluidized bed photoreactor with external (e) and internal (f) irradiation, reproduced with permission from reference [38][127]. (g) Tubular flow photoreactor enclosing lamps with internal-wall-coated photocatalyst, reproduced with permission from reference [38][127]. (h) Alumina reticulated foam monoliths for the use in a photocatalytic tubular flow photoreactor, reproduced with permission from reference [39][130]. (i) Multi-walled tubular reactor flow photoreactor for enhanced mass transfer, reproduced with permission from reference [38][127].
Very recently, Saoud et al. reported a pilot-scale reactor for gas-phase VOC remediation and Escherichia coli inactivation based on a photocatalytic textile, based on optical fiber irradiated by UV LED [34][123]. A 66% degradation of 5 mg·m−3 butane-2,3-dione was measured when the system operated at 2 m3·h−1.
More conventional geometries adopting honeycomb monoliths have already been commercialized for the photocatalytic purification of air (Figure 47a,b). Dijk et al. [41][128] designed an internally illuminated honeycomb-based photoreactor for air treatment (Figure 47d) with high surface-to-volume ratio for air purification. This reactor is made for multiphase use [29][118], wherein the photocatalyst is coated via sol-gel in the inner walls of monolith channels. However, the drawback of this system is the need for separated illumination of each monolith channel. Transport cellulosic monoliths allow the penetration of artificial or natural solar light through all monolith channels (Figure 47c) [42][39][43][40][44][129,130,131,132,133].
Another reactor geometry for air treatment is tubular flow photoreactors enclosing lamps (Figure 47g,h) [45][46][134,135]; for enhanced mass transfer and surface-to-volume ratio, multi-wall-based reactors were constructed as shown in (Figure 47i) [47][48][49][136,137,138].
Hybrid techniques combining photocatalysis and other AOPs hold promise for the treatment of large volumes of air due to their synergistic effects. Filho et al. [50][139] combined O3/UV/TiO2 for the oxidation of VOC in air streams in a NETmix micro-photoreactor system (Figure 58a). While single ozonation exhibited fast oxidation toward n-decane but very slow mineralization, the hybrid system O3/UV/TiO2 showed a synergistic removal and mineralization. Zadi et al. [21][110] combined non-thermal plasma and photocatalysis for the oxidation of propionic acid and benzene in refrigerated food chambers (Figure 58b). By the use of photocatalysis as a single process, the authors reported serious gas toxicity. The combination of photocatalysis with non-thermal plasma overcomes such a toxicity issue, and on top of that, an enhanced photocatalytic efficiency was observed together with an excellent regeneration of the photocatalytic surface.
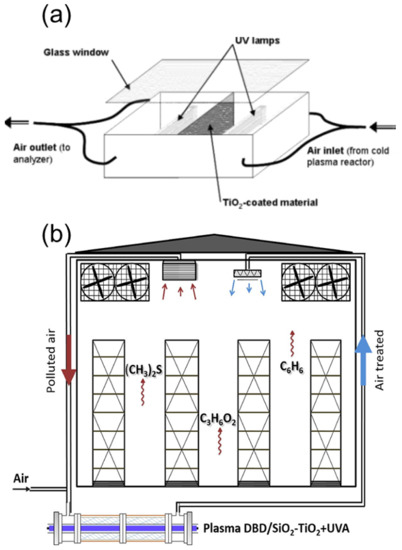
Figure 58. (a) Scheme of cold plasma and TiO2 combined system for the purification of ethanol-contaminated air, reproduced with permission from reference [51][140], Copyright 2007, American Chemical Society. (b) Combination of photocatalysis with non-thermal dielectric barrier discharge (DBD) plasma for the oxidation of propionic acid and benzene in refrigerated food chambers, reproduced with permission from reference [21][110], Copyright 2020, Elsevier.
3. Tests in Realistic Conditions
The efficiency of air purification by photocatalysis depends on a series of operational parameters and environmental conditions. However, comparatively few studies have considered these all-important parameters in photocatalytic tests [24][28][113,117] and even fewer have reported actual field tests of pilot-scale reactors [52][141].
First of all, the role of relative humidity should be considered. Water vapor is a ubiquitous component of air and industrial exhaust. The relative humidity of the treated effluent can vary greatly depending on the environmental conditions and process parameters, but it is generally present in concentrations far higher than those of the pollutants. Water vapor is also known to greatly affect the photocatalytic process [32][121]. However, there is no consensus on the effect of air humidity on the photocatalytic performance [34][123]. Water vapor seems to play different, conflicting roles in gas-phase photocatalysis and the overall beneficial or detrimental effect depends on parameters such as the type of pollutant and its concentration, the photocatalyst adsorption capacity and air relative humidity. It is worth mentioning that water competes for adsorption at the photocatalyst active sites, which can lead to a detrimental effect in terms of pollutant adsorption and, hence, photocatalytic activity [52][141]. Competition for adsorption between the reaction intermediates and water can also result in a detrimental effect in terms of mineralization [52][141]. On the other hand, water can react with photogenerated charges to generate reactive radicals, promoting the photocatalytic degradation. The injection of water vapor can enhance the formation of HO• species on the surface of the photocatalyst [53][142]. Conversely, in a dry air system, the yield of photoproduced ROS can be reduced and it is mostly related to O2−• species.
Air samples also contain a mixture of pollutants, generally with individual concentrations in the order of the (parts-per-billion-volume) ppbv. Most literature studies have involved photocatalytic tests with a single gas pollutant, often with concentrations in the ppm range. Higher pollutant concentrations are generally associated with improved reaction rates (until the rate reaches its plateau), poorer efficiency of removal and lower mineralization [34][123]. In the case of pollutant mixtures, the individual components are generally simultaneously degraded when the individual concentrations are in the ppbv range. However, the co-presence of some species, such as SO2 [52][141] and NOx [52][141], can lead to detrimental effects on the degradation of VOCs.
4. Photocatalyst Deactivation
The deactivation of photocatalyst systems is a serious issue that should be solved for a continuous processing [54][34]. It takes place during the photocatalytic reaction as a result of irreversible adsorption of recalcitrant by-products or site blocking by carbonaceous residues or dust particles on the surface [41][128]. In gas-phase photocatalysis, deactivation is a more pressing concern than it is in liquid phase, as there is no water solvent to help to remove products and intermediates from the surface.
Throughout the literature, the deactivation process has depended mainly on the characteristics of the photocatalysts, the type and concentration of pollutants. Deactivation is most severe in the presence of aromatic compounds [55][56][143,144]; however, it has been reported for a wide range of species [57][145]. For instance, van Dijk et al. reported photocatalyst deactivation within 80 min of photocatalytic operation for the oxidation of cyclohexane [41][128]. Despite the importance of this topic for the commercial application of photocatalytic technologies, comparatively few studies have investigated the mechanisms of deactivation, regeneration and the photocatalyst lifetime.
Several strategies to reactivate the photocatalyst have been proposed, including treatment with water vapor [41][128], high temperature treatment [41][57][128,145], oxidation with H2O2 [57][145] and UV irradiation [58][146]. The use of a more oxidizing atmosphere and better mass transfer have been reported to slow down deactivation [56][144]. Simple washing with water or reagents and organic solvents can be used also to regenerate the deactivated photocatalysts. Djellabi et al. reported the deactivation of TiO2 P25 during the photocatalytic reduction of Cr(VI) under natural solar light [59][147]. It was found that 39% of reduced Cr(VI) was deposited as Cr(III) on the surface of P25, which turned the surface of P25 green. The regeneration of the P25 surface was carried out by the sequential extraction, and the leaching of Cr(III) was found to be 90% and 42% after three washings by citric acid and EDTA, respectively. However, the regeneration of photocatalysts at large scale is not recommended because it is time consuming and not cost effective. The use of vacuum ultraviolet or non-thermal plasma have been suggested to prevent the deactivation of photocatalysts or to enhance their lifetime. In fact, the application of the last two routes is quite hard at large scale for the treatment of large volumes. Engineering of photocatalytic materials may reduce the deactivation process. Chen et al. reported that the deactivation of TiO2 P25 during the decomposition of VOCs in air phase is due to the adsorption of benzaldehyde intermediate on P25 surface, which is characterized by the high reaction energy of the aromatic ring opening [60][148]. The deactivation causes a dramatic decrease in VOC reduction from 72.4% to 12.3% with prolonged time. However, under the same conditions, β-Ga2O3 remains photocatalytically active along with the experiment without a decrease in the efficiency. The authors reported that β-Ga2O3 is more effective for opening the aromatic ring of intermediates, which prevents the deactivation of the photoactive surface.
Combining photocatalysis with another AOP generally promotes the lifetime of the photocatalyst. Zadi et al. [21][110] reported that the introduction of non-thermal plasma to the photocatalytic system can lead to continuous regeneration of the photocatalyst in air system. It was found by Ribeiro et al. [61][149] that addition of ozone allows the use of TiO2 without deactivation up to 77 h.
