Light-initiated polymerization processes are currently an important tool in various industrial fields. The advancement of technology has resulted in the use of photopolymerization in various biomedical applications, such as the production of 3D hydrogel structures, the encapsulation of cells, and in drug delivery systems. The use of photopolymerization processes requires an appropriate initiating system which, in biomedical applications, must meet additional criteria: high water solubility, non-toxicity to cells, and compatibility with visible low-power light sources. This article is a literature review on those compounds that act as photoinitiators of photopolymerization processes in biomedical applications. The division of initiators according to the method of photoinitiation was described and the related mechanisms were discussed. Examples from each group of photoinitiators are presented, and their benefits, limitations and applications are outlined.
- water-soluble photoinitiators
- type I photoinitiators
- type II photoinitiators
- two-photon initiators (2PP), photopolymerization
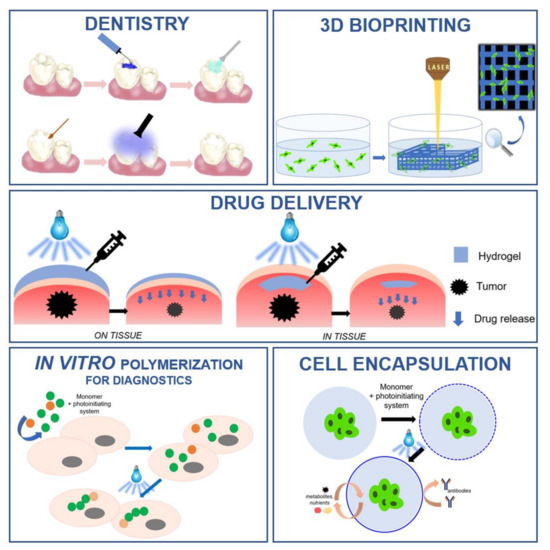
- biomedical applications
- free-radical photopolymerization
- cationic photopolymerization
- compatibility between the absorption characteristics of photoinitiators and the emission characteristics of the light source
- high quantum efficiency
- good solubility in the polymerized composition – for biomedical applications – good water solubility
- non-cytotoxicity
- should not cause yellowing of the cured product
- thermal and temporal stability
- compatibility between the absorption characteristics of photoinitiators and the emission characteristics of the light source
- high quantum efficiency
- good solubility in the polymerized composition – for biomedical applications – good water solubility
- non-cytotoxicity
- should not cause yellowing of the cured product
- thermal and temporal stability
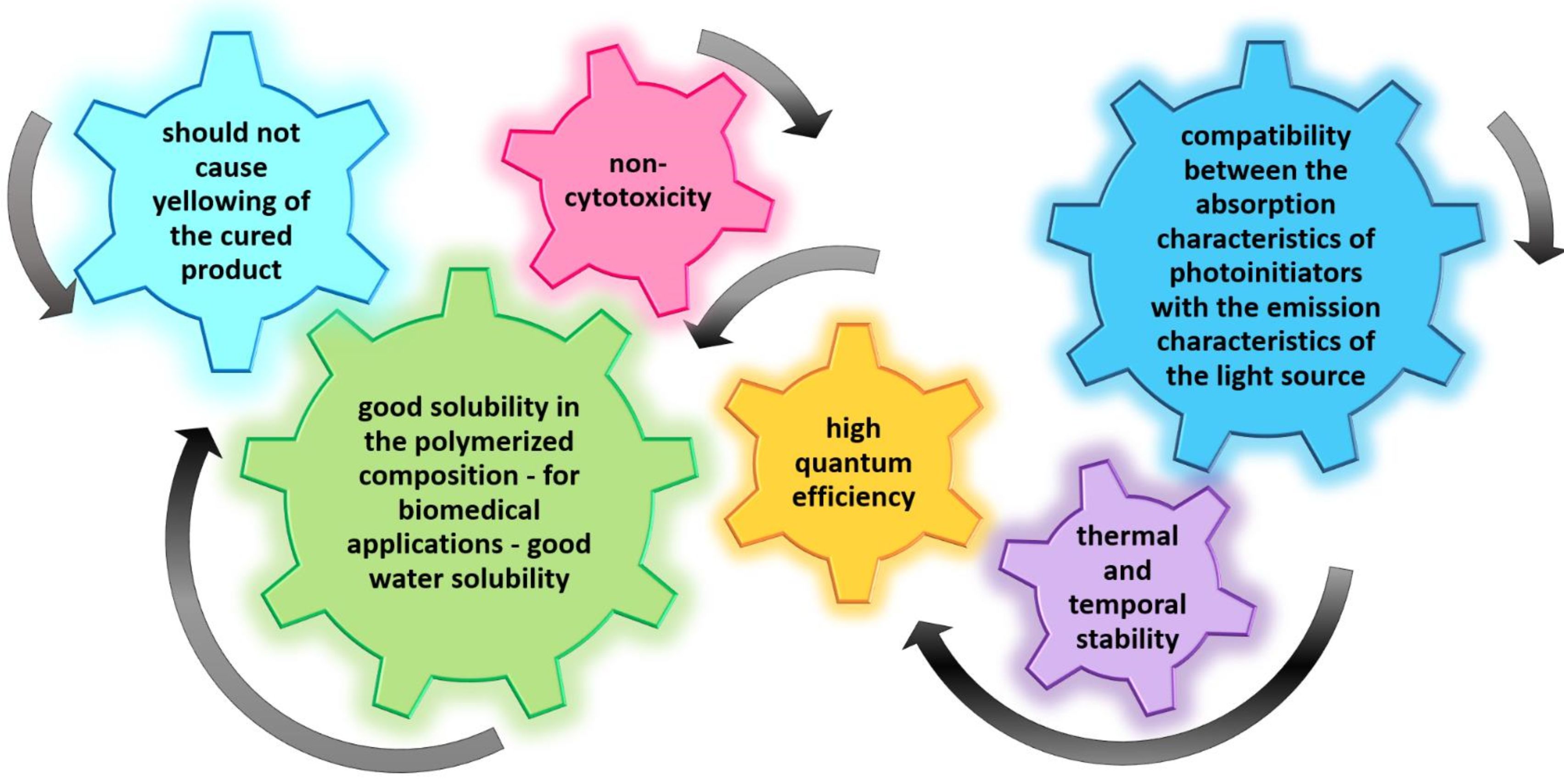
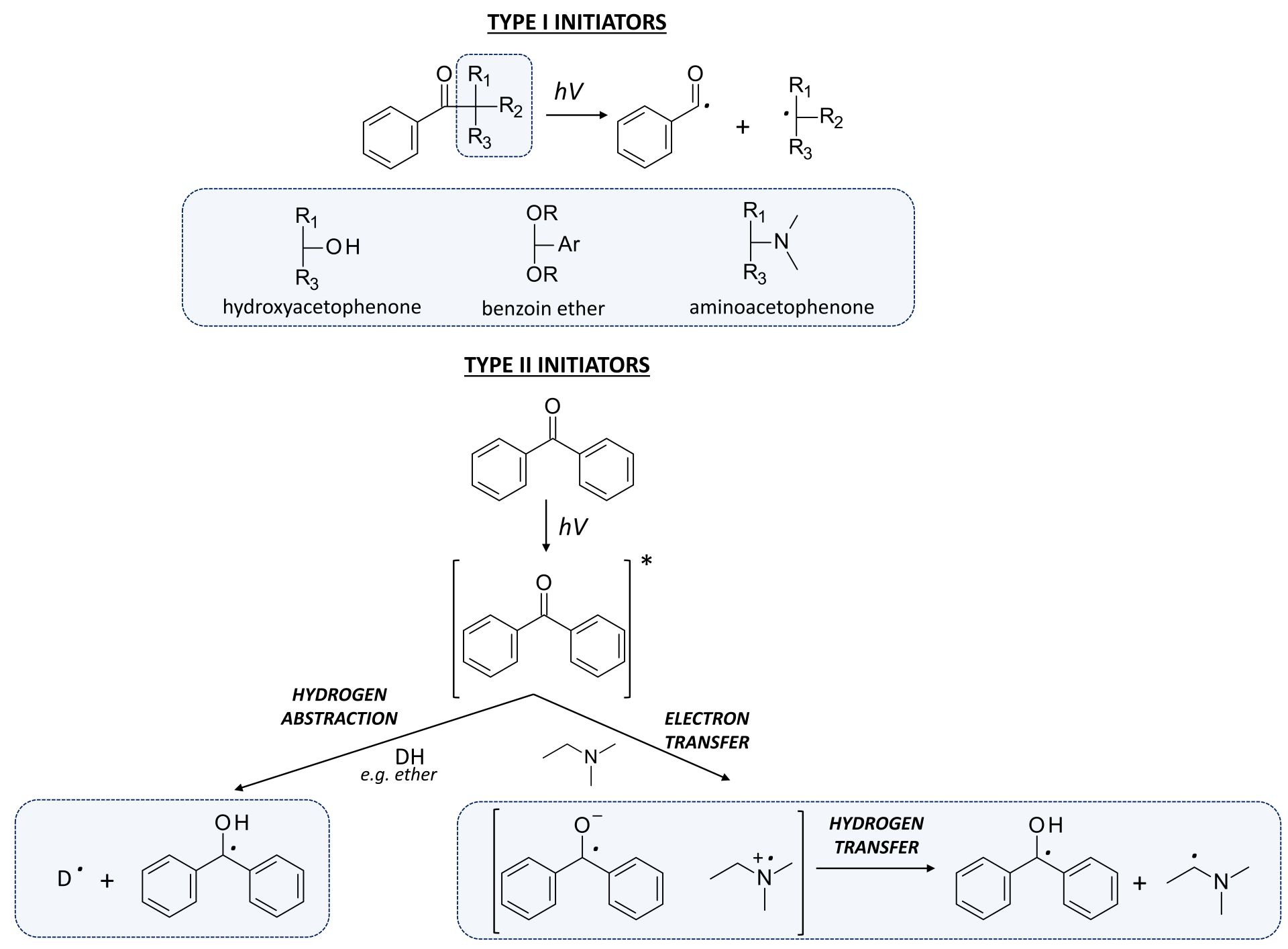
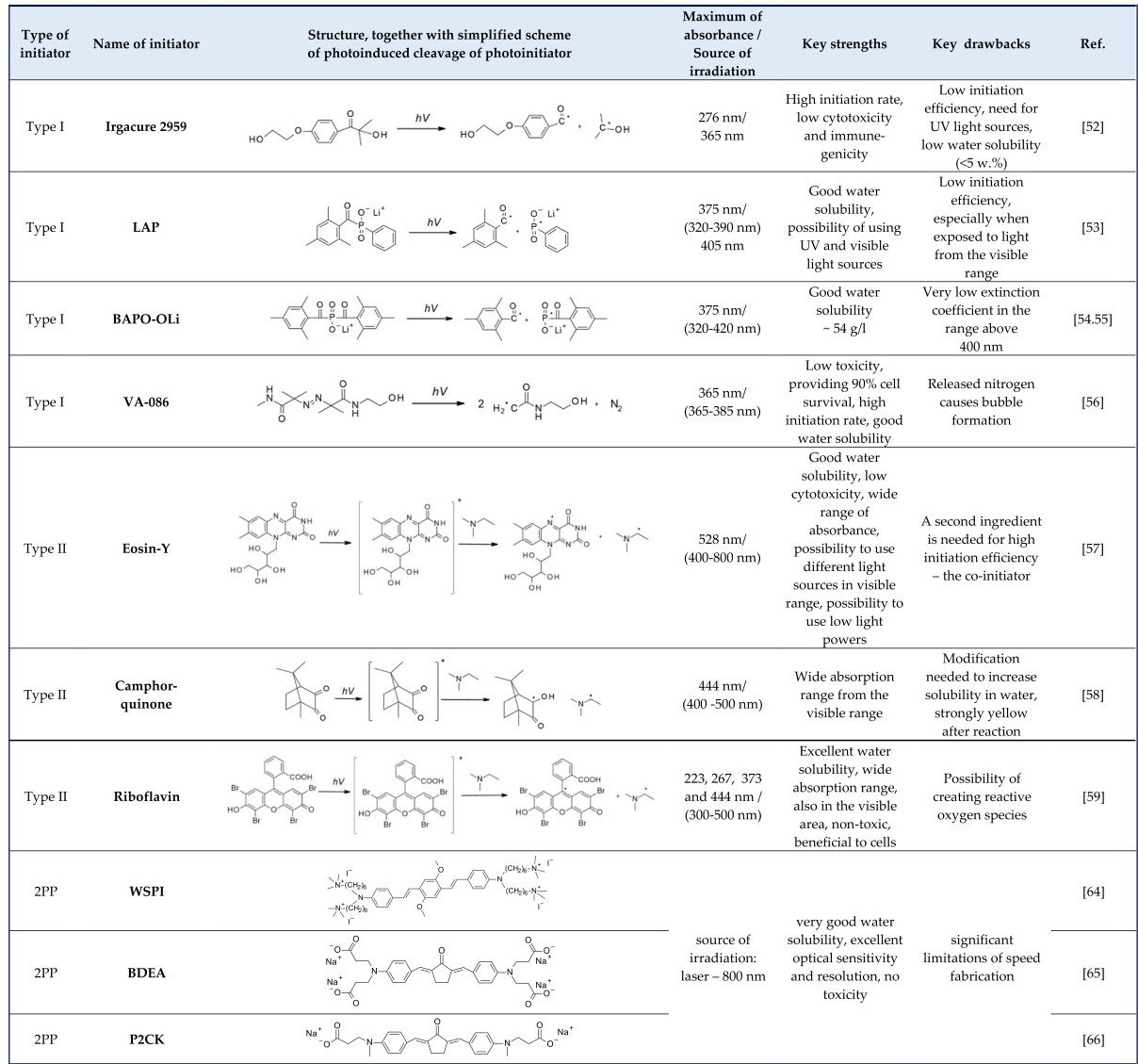
Figure 4. The photoinitiation process using: A. type I initiator; B. type II initiator.
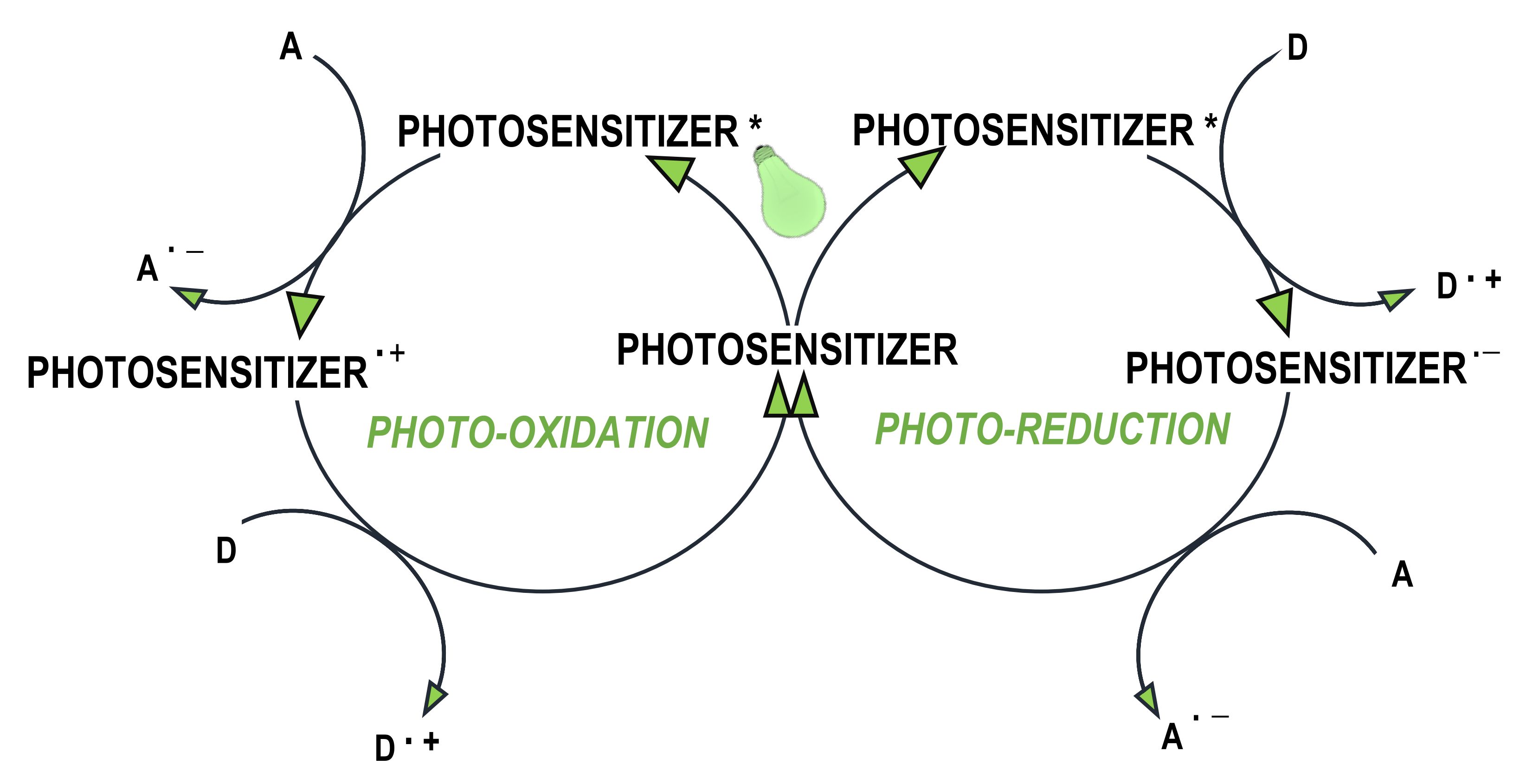
- where the photosensitiser acts as an electron donor, the transfer of the electron to the co-initiator creates a cationic radical of the sensitiser particle and an anionic radical of the co-initiator;
- where the photosensitiser is an electron acceptor, it undergoes photoreduction, and the electron transfer products are the anionic radical formed on the sensitiser molecule and the cationic radical formed on the co-initiator
- where the photosensitiser acts as an electron donor, the transfer of the electron to the co-initiator creates a cationic radical of the sensitiser particle and an anionic radical of the co-initiator;
- where the photosensitiser is an electron acceptor, it undergoes photoreduction, and the electron transfer products are the anionic radical formed on the sensitiser molecule and the cationic radical formed on the co-initiator
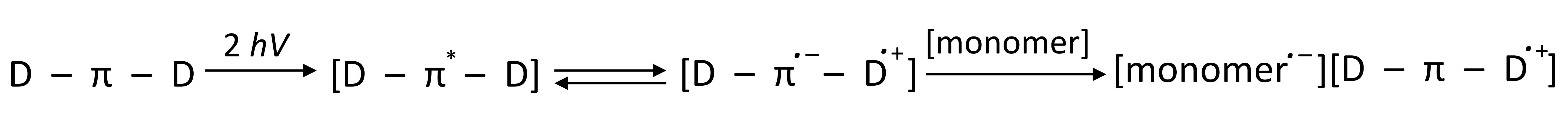
Fields of application for water-soluble photoinitiators
Conclusion and outlook
In conclusion, interest in water-soluble photoinitiators has been ongoing for almost half a century. Significant developments in medicine, including nanomedicine, promote the advancement of photopolymerization processes, as well as the necessary initiating systems in the near future. The currently available modern technologies of nanomedicine, such as targeted drug therapy, modern analysis and diagnostics of diseases, and the production of materials for cell or tissue culture, will require new and increasingly improved initiators that will meet all the criteria for the introduction of materials into the medical market.The development of water-soluble initiating systems is likely to take two directions. First, it will be based on the synthesis of completely new Type I or Type II photoinitiators, with a wide absorption range reaching the visible range and, additionally, fulfilling a number of other requirements, such as lack of cytotoxicity, biocompatibility and high initiation efficiency. Such photoinitiators can be applied, among others, in the processes of in situ polymerization, in targeted drug delivery and in cell encapsulation, which may positively affect the treatment of some diseases, such as type I diabetes by the encapsulation of islets of Langerhans.The second direction of development is the study of two-photon photoinitiators (2PP), which will allow the effective production of hydrogel materials containing living cells with the use of 3D laser printing with extremely high resolution. The constant challenge is to obtain initiators with a simple and inexpensive synthesis path, the scale of which can be easily transferred to the industry.This literature review has presented previous achievements in the field of water-soluble initiators in biomedical applications and has pointed at likely development paths and potential applications of photopolymerization processes.
References
- Frederic Dumur; Recent advances on carbazole-based photoinitiators of polymerization. EuYusuf Yagci; Steffen Jockusch; Nicholas J. Turro; Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macropean Pmolymer Journaecul es 20210, 125, 109503, 10.1016/j.eurpolymj.2020.109503., 43, 6245-6260, 10.1021/ma1007545.
- Junyi Zhou; Xavier Allonas; Ahmad Ibrahim; Xiaoxuan Liu; Progress in the development of polymeric and multifunctional photoinitiators. Shunsuke Chatani; Christopher J. Kloxin; Christopher N. Bowman; The power of light in polymer science: photochemical processes to manipulate polymer formation, structure, and properties. Progress in Polymer Science Chemistry 2019, 99, 101165, 10.1016/j.progpolymsci.2019.101165.4, 5, 2187-2201, 10.1039/c3py01334k.
- D. J. Lougnot; C. Turck; J. P. Fouassier; Water-soluble polymerization initiators based on the thioxanthone structure: a spectroscopic and laser photolysis study. MacrIwona Kamińska; Joanna Ortyl; Roman Popielarz; Mechanism of interaction of coumarin-based fluorescent molecular probes with polymerizing medium during free radical polymerization of a monomer. Polymoleculer Tes ting 201989, 22, 108-116, 10.1021/ma00191a022.6, 55, 310-317, 10.1016/j.polymertesting.2016.09.013.
- Anna Eibel; David E. Fast; Georg Gescheidt; Choosing the ideal photoinitiator for free radical photopolymerizations: predictions based on simulations using established data. Katarzyna Kostrzewska; Joanna Ortyl; Robert Dobosz; Janina Kabatc; Squarylium dye and onium salts as highly sensitive photoradical generators for blue light. Polymer Chemistry 2018, 9, 5107-5115, 10.1039/c8py01195h.7, 8, 3464-3474, 10.1039/C7PY00621G.
- Adina I. Ciuciu; Piotr J. Cywiński; Two-photon polymerization of hydrogels – versatile solutions to fabricate well-defined 3D structures. RSRobert Liska; M. Schuster; R. Inführ; C. Turecek; C. Fritscher; B. Seidl; V. Schmidt; L. Kuna; Anja Haase; F. Varga; et al.Helga LichteneggerJ. Stampfl Photopolymers for rapid prototyping. Journal of Coatings AdvancTechnology and Res earch 20014, 7, 4, 45504-45516, 10.1039/c4ra06892k., 505-510, 10.1007/s11998-007-9059-3.
- Kytai Truong Nguyen; Jennifer L. West; Photopolymerizable hydrogels for tissue engineering applications. BAli Bagheri; Jianyong Jin; Photopolymerization in 3D Printing. ACS Applied Pomlymer Materials 2002, 23, 4307-4314, 10.1016/s0142-9612(02)00175-8.19, 1, 593-611, 10.1021/acsapm.8b00165.
- Andrit Allushi; Ceren Kütahya; Cansu Aydogan; Johannes Kreutzer; Gorkem Yilmaz; Yusuf Yagci; Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. PoWiktoria Tomal; Maciej Pilch; Chachaj- Brekiesz; Joanna Ortyl; Anna Chachaj-Brekiesz; Development of New High-Performance Biphenyl and Terphenyl Derivatives as Versatile Photoredox Photoinitiating Systems and Their Applications in 3D Printing Photopolymerization Processes. Catalymer Chemistry s 2017, 8, 1972-1977, 10.1039/C7PY00114B.9, 9, 827, 10.3390/catal9100827.
- Stephanie J. Bryant; Charles R. Nuttelman; Kristi S. Anseth; Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro.. JournalEmilia Hola; Monika Topa; Anna Chachaj-Brekiesz; Maciej Pilch; Paweł Fiedor; Mariusz Galek; Joanna Ortyl; New, highly versatile bimolecular photoinitiating systems for free-radical, cationic and thiol–ene photopolymerization processes under low light intensity UV and visible LEDs for 3D printing application. RSC of BiomAdvaterials Science, Polymer Edition nces 20200, , 11, 439-457, 10.1163/156856200743805.0, 7509-7522, 10.1039/c9ra10212d.
- Benjamin D. Fairbanks; Michael Schwartz; Christopher N. Bowman; Kristi S. Anseth; Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. BJohn P Fisher; David Dean; Paul S. Engel; Antonios G. Mikos; Photoinitiated Polymerization of Biomaterials. Annual Review omf Materials Research 2009, 1, 30, 6702-7, 10.1016/j.biomaterials.2009.08.055.1, 171-181, 10.1146/annurev.matsci.31.1.171.
- Stephan Benedikt; Jieping Wang; Marica Markovic; Norbert Moszner; Kurt Dietliker; Aleksandr Ovsianikov; Hansjörg Grützmacher; Robert Liska; Highly efficient water-soluble visible light photoinitiators. Biancamaria Baroli; Photopolymerization of biomaterials: issues and potentialities in drug delivery, tissue engineering, and cell encapsulation applications. Journal of PolyChemer Science Part A: Polymer Chemistrical Technology & Biotechnology 2015, 54, 473-479, 10.1002/pola.27903.06, 81, 491-499, 10.1002/jctb.1468.
- Georgina Müller; Michal Zalibera; Georg Gescheidt; Amos Rosenthal; Gustavo Santiso-Quiñones; Kurt Dietliker; Hansjörg Grützmacher; Simple One-Pot Syntheses of Water-Soluble Bis(acyl)phosphane Oxide Photoinitiators and Their Application in Surfactant-Free Emulsion Polymerization. Macr† Jeffrey W. Stansbury; Curing dental resins and composites by photopolymerization.. Jomolecular Rapid Communicationrnal of esthetic dentis try 20015, 36, 553-557, 10.1002/marc.201400743.0, 12, 300-308, 10.1111/j.1708-8240.2000.tb00239.x.
- Paola Occhetta; Roberta Visone; Laura Russo; Laura Cipolla; Matteo Moretti; Marco Rasponi; VA-086 methacrylate gelatine photopolymerizable hydrogels: A parametric study for highly biocompatible 3D cell embedding. JouM. E. Khosroshahi; Mohammad Atai; M. S. Nourbakhsh; Photopolymerization of dental resin as restorative material using an argon laser. Lasers inal of Biom Medical Materials ResSciencearch Part A 2014, 1007, 23, 2109-2117, 10.1002/jbm.a.35346., 399-406, 10.1007/s10103-007-0487-1.
- Seda Kızılel; Victor H. Perez-Luna; Fouad Teymour; Seda Kizilel; Photopolymerization of Poly(Ethylene Glycol) Diacrylate on Eosin-Functionalized Surfaces. LS. H. Dickens; J. W. Stansbury; K. M. Choi; C. J. E. Floyd; Photopolymerization Kinetics of Methacrylate Dental Resins. Mangcromuir olecules 2004, 20, 8652-8658, 10.1021/la0496744.3, 36, 6043-6053, 10.1021/ma021675k.
- Elbadawy A. Kamoun; Andreas Winkel; Michael Eisenburger; Henning Menzel; Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Nicholas A. Peppas; J. Z. Hilt; A. Khademhosseini; Robert Langer; Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Ardvabian Journal of Cheminced Materialstry 20016, 9, 745-754, 10.1016/j.arabjc.2014.03.008., 18, 1345-1360, 10.1002/adma.200501612.
- R. R. Batchelor; G. Kwandou; Patrick T. Spicer; Martina H. Stenzel; (-)-Riboflavin (vitamin B2) and flavin mononucleotide as visible light photo initiators in the thiol–ene polymerisation of PEG-based hydrogels. PolymJamie L. Ifkovits; Jason A. Burdick; Review: Photopolymerizable and Degradable Biomaterials for Tissue Engineering Applications. Tissuer Chemistry Engineering 20107, 8, 980-984, 10.1039/C6PY02034H., 13, 2369-2385, 10.1089/ten.2007.0093.
- Sajjad Dadashi-Silab; Cansu Aydogan; Yusuf Yagci; Shining a light on an adaptable photoinitiator: advances in photopolymerizations initiated by thioxanthones. PMaya Gonen-Wadmany; Liat Oss-Ronen; Dror Seliktar; Protein–polymer conjugates for forming photopolymerizable biomimetic hydrogels for tissue engineering. Biolymer Chemiaterialstry 20015, 6, 6595-6615, 10.1039/C5PY01004G.7, 28, 3876-3886, 10.1016/j.biomaterials.2007.05.005.
- Maximilian Tromayer; Ágnes Dobos; Peter Gruber; Aliasghar Ajami; Roman Dědic; Aleksandr Ovsianikov; Robert Liska; A biocompatible diazosulfonate initiator for direct encapsulation of human stem cells via two-photon polymerization. PoAmy E. Herr; Anup K. Singh; Photopolymerized Cross-Linked Polyacrylamide Gels for On-Chip Protein Sizing. Analymertical Chemistry 20018, 9, 3108-3117, 10.1039/c8py00278a.4, 76, 4727-4733, 10.1021/ac049686u.
- Han Young Woo; Janice W. Hong; Bin Liu; Alexander Mikhailovsky; Dmitry Korystov; Guillermo C. Bazan; Water-Soluble [2.2]Paracyclophane Chromophores with Large Two-Photon Action Cross Sections. JQian Cao; Tobias Heil; Baris Kumru; Markus Antonietti; Bernhard V. K. J. Schmidt; Visible-light induced emulsion photopolymerization with carbon nitride as a stabilizer and photoinitiator. Pournal of the Americanymer Chemical Societstry 2005, 19, 127, 820-821, 10.1021/ja0440811.0, 5315-5323, 10.1039/c9py01157a.
- Bruce A. Reinhardt; Lawrence L. Brott; Stephen J. Clarson; Ann G. Dillard; Jayprakash C. Bhatt; Ramamurthi Kannan; Lixiang Yuan; Guang S. He; Paras N. Prasad; Highly Active Two-Photon Dyes: Design, Synthesis, and Characterization toward Application. ChemistR. Censi; Tina Vermonden; M.J. Van Steenbergen; H. Deschout; K. Braeckmans; Stefaan C. De Smedt; Cornelus F. Van Nostrum; P. Di Martino; Wim E. Hennink; Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. Jourynal of MaterialControlled Releas 1e 200998, , 140, 1863-1874, 10.1021/cm980036e., 230-236, 10.1016/j.jconrel.2009.06.003.
- Thomas Weiß; Gerhard Hildebrand; Ronald Schade; Klaus Liefeith; Thomas Weiß; Two-Photon polymerization for microfabrication of three-dimensional scaffolds for tissue engineering application. EnginJ. Elisseeff; K. Anseth; D. Sims; W. McIntosh; M. Randolph; Robert Langer; Transdermal photopolymerization for minimally invasive implantation. Proceerding in Lifes of the National Academy of Sciences 200 1999, 9, 384-390, 10.1002/elsc.200900002.6, 3104-3107, 10.1073/pnas.96.6.3104.
- Aleksandr Ovsianikov; Andrea Deiwick; Sandra Van Vlierberghe; Peter Dubruel; Lena Möller; Gerald Dräger; Boris Chichkov; Laser Fabrication of Three-Dimensional CAD Scaffolds from Photosensitive Gelatin for Applications in Tissue Engineering. BiomQiang Ye; Jonggu Park; Elizabeth Topp; Paulette Spencer; Effect of photoinitiators on the in vitro performance of a dentin adhesive exposed to simulated oral environment. Dentacromolecule Materials 2011, 109, 2, 851-858, 10.1021/bm1015305.5, 452-458, 10.1016/j.dental.2008.09.011.
- Xiaojun Wan; Yuxia Zhao; Jianqiang Xue; Feipeng Wu; Xiangyun Fang; Water-soluble benzylidene cyclopentanone dye for two-photon photopolymerization. JourKunio Ikemura; Takeshi Endo; A review of the development of radical photopolymerization initiators used for designing light-curing dental adhesives and resin composites.. Dental of Photochemistry and PhotobioMaterials Journalogy A: Chemistry 20109, , 202, 74-79, 10.1016/j.jphotochem.2008.10.029.9, 481-501, 10.4012/dmj.2009-137.
- Zhiquan Li; Jan Torgersen; Aliasghar Ajami; Severin Mühleder; Xiao-Hua Qin; Wolfgang Husinsky; Wolfgang Holnthoner; Aleksandr Ovsianikov; Jürgen Stampfl; Robert Liska; et al. Initiation efficiency and cytotoxicity of novel water-soluble two-photon photoinitiators for direct 3D microfabrication of hydrogels. RSCVinícius Esteves Salgado; Diogo Cavassoni; Ana Paula R. Gonçalves; Carmem Pfeifer; Rafael R. Moraes; Luis Felipe Schneider; Photoinitiator system and water effects on C=C conversion and solubility of experimental etch-and-rinse dental adhesives. International Journal of Advhesion ancd Adhesives 2013, 3, 15939, 10.1039/c3ra42918k.7, 72, 6-9, 10.1016/j.ijadhadh.2016.09.001.
- Jason D. Clapper; Jessica M. Skeie; Robert F. Mullins; C. Allan Guymon; Development and characterization of photopolymerizable biodegradable materials from PEG–PLA–PEG block macromonomers. Polymer 2007, 48, 6554-6564, 10.1016/j.polymer.2007.08.023.
- Kemin Wang; Jian Lu; Ruixue Yin; Lu Chen; Shuang Du; Yan Jiang; Qiang Yu; Preparation and properties of cyclic acetal based biodegradable gel by thiol-ene photopolymerization. Materials Science and Engineering: C 2013, 33, 1261-1266, 10.1016/j.msec.2012.12.024.
- Frederik Claeyssens; Erol A. Hasan; Arune Gaidukeviciute; Demetra S. Achilleos; A. Ranella; Carsten Reinhardt; Aleksandr Ovsianikov; Xiao Shizhou; Costas Fotakis; Maria Vamvakaki; et al.Boris ChichkovMaria Farsari Three-Dimensional Biodegradable Structures Fabricated by Two-Photon Polymerization. Langmuir 2009, 25, 3219-3223, 10.1021/la803803m.
- Jennifer Elisseeff; Winnette McIntosh; Karen Fu; Torsten Blunk; Robert Langer; Controlled-release of IGF-I and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. Journal of Orthopaedic Research 2001, 19, 1098-1104, 10.1016/s0736-0266(01)00054-7.
- Stephanie J. Bryant; Garret D. Nicodemus; Idalis Villanueva; Designing 3D Photopolymer Hydrogels to Regulate Biomechanical Cues and Tissue Growth for Cartilage Tissue Engineering. Pharmaceutical Research 2008, 25, 2379-2386, 10.1007/s11095-008-9619-y.
- Heyun Wang; Yakai Feng; Bo An; Wencheng Zhang; Minglin Sun; Zichen Fang; Wenjie Yuan; Massuri Khan; Fabrication of PU/PEGMA crosslinked hybrid scaffolds by in situ UV photopolymerization favoring human endothelial cells growth for vascular tissue engineering. Journal of Materials Science: Materials in Electronics 2012, 23, 1499-1510, 10.1007/s10856-012-4613-7.
- Aleksander Skardal; Jianxing Zhang; Lindsi McCoard; Xiaoyu Xu; Siam Oottamasathien; Glenn D. Prestwich; Photocrosslinkable Hyaluronan-Gelatin Hydrogels for Two-Step Bioprinting. Tissue Engineering Part A 2010, 16, 2675-2685, 10.1089/ten.tea.2009.0798.
- Chao Zhong; Jun Wu; C.A. Reinhart-King; C. C. Chu; Cynthia A. Reinhart-King; Synthesis, characterization and cytotoxicity of photo-crosslinked maleic chitosan–polyethylene glycol diacrylate hybrid hydrogels. Acta Biomaterialia 2010, 6, 3908-3918, 10.1016/j.actbio.2010.04.011.
- Di Zhou; Yoshihiro Ito; Visible light-curable polymers for biomedical applications. Science China Chemistry 2014, 57, 510-521, 10.1007/s11426-014-5069-z.
- Jennifer L. West; Jeffrey A. Hubbell; Comparison of covalently and physically cross-linked polyethylene glycol-based hydrogels for the prevention of postoperative adhesions in a rat model. Biomaterials 1995, 16, 1153-1156, 10.1016/0142-9612(95)93579-3.
- Ilse De Paepe; Heidi Declercq; Maria Cornelissen; Etienne Schacht; Novel hydrogels based on methacrylate-modified agarose. Polymer International 2002, 51, 867-870, 10.1002/pi.945.
- Ine Van Nieuwenhove; Sandra Van Vlierberghe; Achim Salamon; Kirsten Peters; Hugo Thienpont; Peter Dubruel; Photo-crosslinkable biopolymers targeting stem cell adhesion and proliferation: the case study of gelatin and starch-based IPNs. Journal of Materials Science: Materials in Electronics 2015, 26, 1-8, 10.1007/s10856-015-5424-4.
- Jinho Hyun; Ashutosh Chilkoti; Surface-Initiated Free Radical Polymerization of Polystyrene Micropatterns on a Self-Assembled Monolayer on Gold. Macromolecules 2001, 34, 5644-5652, 10.1021/ma002125u.
- Christian Heller; Martin Schwentenwein; Günter Russmüller; Thomas Koch; Doris Moser; Christian Schopper; Franz Varga; Jürgen Stampfl; Robert Liska; Vinylcarbonates and vinylcarbamates: Biocompatible monomers for radical photopolymerization. Journal of Polymer Science Part A: Polymer Chemistry 2010, 49, 650-661, 10.1002/pola.24476.
- Mustafa Uygun; Muhammet Kahveci; Dilek Odaci; Suna Timur; Yusuf Yagci; Antibacterial Acrylamide Hydrogels Containing Silver Nanoparticles by Simultaneous Photoinduced Free Radical Polymerization and Electron Transfer Processes. Macromolecular Chemistry and Physics 2009, 210, 1867-1875, 10.1002/macp.200900296.
- Jennifer H. Ward; Nicholas A. Peppas; Preparation of controlled release systems by free-radical UV polymerizations in the presence of a drug.. Journal of Controlled Release 2001, 71, 183-192, 10.1016/s0168-3659(01)00213-9.
- Joanna Ortyl; Jarosław Wilamowski; Piotr Milart; Mariusz Galek; Roman Popielarz; Relative sensitization efficiency of fluorescent probes/sensitizers for monitoring and acceleration of cationic photopolymerization of monomers. Polymer Testing 2015, 48, 151-159, 10.1016/j.polymertesting.2015.10.006.
- Joanna Ortyl; Piotr Milart; Roman Popielarz; Applicability of aminophthalimide probes for monitoring and acceleration of cationic photopolymerization of epoxides. Polymer Testing 2013, 32, 708-715, 10.1016/j.polymertesting.2013.03.009.
- Kytai Truong Nguyen; Jennifer L. West; Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307-4314, 10.1016/s0142-9612(02)00175-8.
- R. A. A. Upul Ranaweera; Schuman P. Thomas; Rongpeng Wang; Bradley D. Miller; Kathleen V. Kilway; Effect of moisture on cationic polymerization of silicone epoxy monomers. Journal of Applied Polymer Science 2015, 132, 41831, 10.1002/app.41831.
- P. Barker; J. T. Guthrie; M. J. Davis; A. A. Godfrey; P. N. Green; Sensitized photoinitiated grafting of N-vinyl-2-prrolidone (NVP) to woolen substrates. Journal of Applied Polymer Science 1981, 26, 521-527, 10.1002/app.1981.070260212.
- Aleksandr Ovsianikov; Andrea Deiwick; Sandra Van Vlierberghe; Peter Dubruel; Lena Möller; Gerald Dräger; Boris Chichkov; Laser Fabrication of Three-Dimensional CAD Scaffolds from Photosensitive Gelatin for Applications in Tissue Engineering. Biomacromolecules 2011, 12, 851-858, 10.1021/bm1015305.
- Xiaojun Wan; Yuxia Zhao; Jianqiang Xue; Feipeng Wu; Xiangyun Fang; Water-soluble benzylidene cyclopentanone dye for two-photon photopolymerization. Journal of Photochemistry and Photobiology A: Chemistry 2009, 202, 74-79, 10.1016/j.jphotochem.2008.10.029.
- Zhiquan Li; Jan Torgersen; Aliasghar Ajami; Severin Mühleder; Xiao-Hua Qin; Wolfgang Husinsky; Wolfgang Holnthoner; Aleksandr Ovsianikov; Jürgen Stampfl; Robert Liska; et al. Initiation efficiency and cytotoxicity of novel water-soluble two-photon photoinitiators for direct 3D microfabrication of hydrogels. RSC Advances 2013, 3, 15939, 10.1039/c3ra42918k.
- Thomas Weiß; Gerhard Hildebrand; Ronald Schade; Klaus Liefeith; Thomas Weiß; Two-Photon polymerization for microfabrication of three-dimensional scaffolds for tissue engineering application. Engineering in Life Sciences 2009, 9, 384-390, 10.1002/elsc.200900002.
- Bruce A. Reinhardt; Lawrence L. Brott; Stephen J. Clarson; Ann G. Dillard; Jayprakash C. Bhatt; Ramamurthi Kannan; Lixiang Yuan; Guang S. He; Paras N. Prasad; Highly Active Two-Photon Dyes: Design, Synthesis, and Characterization toward Application. Chemistry of Materials 1998, 10, 1863-1874, 10.1021/cm980036e.
- Han Young Woo; Janice W. Hong; Bin Liu; Alexander Mikhailovsky; Dmitry Korystov; Guillermo C. Bazan; Water-Soluble [2.2]Paracyclophane Chromophores with Large Two-Photon Action Cross Sections. Journal of the American Chemical Society 2005, 127, 820-821, 10.1021/ja0440811.
- Maximilian Tromayer; Ágnes Dobos; Peter Gruber; Aliasghar Ajami; Roman Dědic; Aleksandr Ovsianikov; Robert Liska; A biocompatible diazosulfonate initiator for direct encapsulation of human stem cells via two-photon polymerization. Polymer Chemistry 2018, 9, 3108-3117, 10.1039/c8py00278a.
- Benjamin D. Fairbanks; Michael Schwartz; Christopher N. Bowman; Kristi S. Anseth; Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702-7, 10.1016/j.biomaterials.2009.08.055.
- Stephan Benedikt; Jieping Wang; Marica Markovic; Norbert Moszner; Kurt Dietliker; Aleksandr Ovsianikov; Hansjörg Grützmacher; Robert Liska; Highly efficient water-soluble visible light photoinitiators. Journal of Polymer Science Part A: Polymer Chemistry 2015, 54, 473-479, 10.1002/pola.27903.
- Georgina Müller; Michal Zalibera; Georg Gescheidt; Amos Rosenthal; Gustavo Santiso-Quiñones; Kurt Dietliker; Hansjörg Grützmacher; Simple One-Pot Syntheses of Water-Soluble Bis(acyl)phosphane Oxide Photoinitiators and Their Application in Surfactant-Free Emulsion Polymerization. Macromolecular Rapid Communications 2015, 36, 553-557, 10.1002/marc.201400743.
- Paola Occhetta; Roberta Visone; Laura Russo; Laura Cipolla; Matteo Moretti; Marco Rasponi; VA-086 methacrylate gelatine photopolymerizable hydrogels: A parametric study for highly biocompatible 3D cell embedding. Journal of Biomedical Materials Research Part A 2014, 103, 2109-2117, 10.1002/jbm.a.35346.
- Seda Kızılel; Victor H. Perez-Luna; Fouad Teymour; Seda Kizilel; Photopolymerization of Poly(Ethylene Glycol) Diacrylate on Eosin-Functionalized Surfaces. Langmuir 2004, 20, 8652-8658, 10.1021/la0496744.
- Elbadawy A. Kamoun; Andreas Winkel; Michael Eisenburger; Henning Menzel; Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arabian Journal of Chemistry 2016, 9, 745-754, 10.1016/j.arabjc.2014.03.008.
- R. R. Batchelor; G. Kwandou; Patrick T. Spicer; Martina H. Stenzel; (-)-Riboflavin (vitamin B2) and flavin mononucleotide as visible light photo initiators in the thiol–ene polymerisation of PEG-based hydrogels. Polymer Chemistry 2017, 8, 980-984, 10.1039/C6PY02034H.
- Sajjad Dadashi-Silab; Cansu Aydogan; Yusuf Yagci; Shining a light on an adaptable photoinitiator: advances in photopolymerizations initiated by thioxanthones. Polymer Chemistry 2015, 6, 6595-6615, 10.1039/C5PY01004G.
- Stephanie J. Bryant; Charles R. Nuttelman; Kristi S. Anseth; Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro.. Journal of Biomaterials Science, Polymer Edition 2000, 11, 439-457, 10.1163/156856200743805.
- Andrit Allushi; Ceren Kütahya; Cansu Aydogan; Johannes Kreutzer; Gorkem Yilmaz; Yusuf Yagci; Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polymer Chemistry 2017, 8, 1972-1977, 10.1039/C7PY00114B.
- Adina I. Ciuciu; Piotr J. Cywiński; Two-photon polymerization of hydrogels – versatile solutions to fabricate well-defined 3D structures. RSC Advances 2014, 4, 45504-45516, 10.1039/c4ra06892k.
- Anna Eibel; David E. Fast; Georg Gescheidt; Choosing the ideal photoinitiator for free radical photopolymerizations: predictions based on simulations using established data. Polymer Chemistry 2018, 9, 5107-5115, 10.1039/c8py01195h.
- Frederic Dumur; Recent advances on carbazole-based photoinitiators of polymerization. European Polymer Journal 2020, 125, 109503, 10.1016/j.eurpolymj.2020.109503.
- Junyi Zhou; Xavier Allonas; Ahmad Ibrahim; Xiaoxuan Liu; Progress in the development of polymeric and multifunctional photoinitiators. Progress in Polymer Science 2019, 99, 101165, 10.1016/j.progpolymsci.2019.101165.
- D. J. Lougnot; C. Turck; J. P. Fouassier; Water-soluble polymerization initiators based on the thioxanthone structure: a spectroscopic and laser photolysis study. Macromolecules 1989, 22, 108-116, 10.1021/ma00191a022.
- Itsuro Tomatsu; Ke Peng; Alexander Kros; Photoresponsive hydrogels for biomedical applications. Advanced Drug Delivery Reviews 2011, 63, 1257-1266, 10.1016/j.addr.2011.06.009.
- Xiao-Hua Qin; Aleksandr Ovsianikov; Jürgen Stampfl; Robert Liska; Additive manufacturing of photosensitive hydrogels for tissue engineering applications. BioNanoMaterials 2014, 15, 49-70, 10.1515/bnm-2014-0008.
- Andrea M. Kasko; Darice Y Wong; Two-photon lithography in the future of cell-based therapeutics and regenerative medicine: a review of techniques for hydrogel patterning and controlled release. Future Medicinal Chemistry 2010, 2, 1669-1680, 10.4155/fmc.10.253.
- Tugce Nur Eren; Jacques Lalevée; Duygu Avci; Water soluble polymeric photoinitiator for dual-curing of acrylates and methacrylates. Journal of Photochemistry and Photobiology A: Chemistry 2020, 389, 112288, 10.1016/j.jphotochem.2019.112288.
- Molamma P. Prabhakaran; J. Venugopal; Dan Kai; Seeram Ramakrishna; Biomimetic material strategies for cardiac tissue engineering. Materials Science and Engineering: C 2011, 31, 503-513, 10.1016/j.msec.2010.12.017.
- Jane Ru Choi; Kar Wey Yong; Jean Yu Choi; Alistair C Cowie; Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 2019, 66, 40-53, 10.2144/btn-2018-0083.
- Saburo Fukui; Kenji Sonomoto; Nobuya Itoh; Atsuo Tanaka; Several novel method for immobilization of enzymes, microbial cells and organelles. Biochimie 1980, 62, 381-386, 10.1016/s0300-9084(80)80169-6.
- J.F. Almeida; Paula Ferreira; A. Lopes; Maria Helena Gil; Photocrosslinkable biodegradable responsive hydrogels as drug delivery systems. International Journal of Biological Macromolecules 2011, 49, 948-954, 10.1016/j.ijbiomac.2011.08.010.
- E.B. Masurovsky; E.R. Peterson; Photo-reconstituted collagen gel for tissue culture substrates. Experimental Cell Research 1973, 76, 447-448, 10.1016/0014-4827(73)90399-6.
- Sien Lin; Wayne Yuk-Wai Lee; Qian Feng; Liangliang Xu; Bin Wang; Gene Chi Wai Man; Yuanfeng Chen; Xiaohua Jiang; Liming Bian; Liao Cui; et al.Bo WeiGang Li Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation.. Stem Cell Research & Therapy 2017, 8, 221, 10.1186/s13287-017-0672-5.
- Chaoliang He; Fengfu Li; Jae Il Ahn; Malcolm Latorre; May Griffith; Photo-induced in situ forming hydrogels based on collagen and a biocompatible macromolecular photoinitiator. Journal of Controlled Release 2011, 152, e207-e208, 10.1016/j.jconrel.2011.09.012.
- Jin Geng; Weishuo Li; Yichuan Zhang; Neelima Thottappillil; Jessica Clavadetscher; Annamaria Lilienkampf; Mark Bradley; Radical polymerization inside living cells. Nature Chemistry 2019, 11, 578-586, 10.1038/s41557-019-0240-y.
- Jianmin Yang; Jingchao Li; Xinlong Wang; Xiaomeng Li; Naoki Kawazoe; Guoping Chen; Single mammalian cell encapsulation by in situ polymerization.. Journal of Materials Chemistry B 2016, 4, 7662-7668, 10.1039/c6tb02491b.
- Stephanie K. Seidlits; Christine E. Schmidt; Jason B. Shear; High-Resolution Patterning of Hydrogels in Three Dimensions using Direct-Write Photofabrication for Cell Guidance. Advanced Functional Materials 2009, 19, 3543-3551, 10.1002/adfm.200901115.
- Elli Käpylä; Tomáš Sedlačík; Dogu Baran Aydogan; Jouko Viitanen; Frantisek Rypacek; Minna Kellomäki; Direct laser writing of synthetic poly(amino acid) hydrogels and poly(ethylene glycol) diacrylates by two-photon polymerization. Materials Science and Engineering: C 2014, 43, 280-289, 10.1016/j.msec.2014.07.027.
- Guifang Gao; Tomo Yonezawa; Karen Hubbell; Guohao Dai; Xiaofeng Cui; Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnology Journal 2015, 10, 1568-1577, 10.1002/biot.201400635.
- Zongjie Wang; Raafa Abdulla; Benjamin Parker; Roya Samanipour; Sanjoy Ghosh; Keekyoung Kim; A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009, 10.1088/1758-5090/7/4/045009.
- B. Husár; M. Hatzenbichler; V. Mironov; R. Liska; J. Stampfl; Aleksandr Ovsianikov; Photopolymerization-based additive manufacturing for the development of 3D porous scaffolds. Biomaterials for Bone Regeneration 2014, 6, 149-201, 10.1533/9780857098104.2.149.
- Seda Kızılel; Erin Sawardecker; Fouad Teymour; Victor Perez Luna; Seda Kizilel; Sequential formation of covalently bonded hydrogel multilayers through surface initiated photopolymerization. Biomaterials 2006, 27, 1209-1215, 10.1016/j.biomaterials.2005.08.025.