Awns are extending structures from lemmas in grasses and are very active in photosynthesis, contributing directly to the filling of the developing grain. Barley (Hordeum vulgare L.) awns are highly diverse in shape and length and are known to be controlled by multiple awn-related genes. The genetic effects of these genes on awn diversity and development in barley are multiplexed and include complementary effect, cumulative effect, duplicate effect, recessive epistasis, dominant epistasis, and inhibiting effect, each giving a unique modified Mendelian ratio of segregation. The complexity of gene interactions contributes to the awn diversity in barley. Excessive gene interactions create a challenging task for genetic mapping and specific strategies have to be developed for mapping genes with specific interactive effects. Awn gene interactions can occur at different levels of gene expression, from the transcription factor-mediated gene transcription to the regulation of enzymes and metabolic pathways. A better understanding of gene interactions will greatly facilitate deciphering the genetic mechanisms underlying barley awn diversity and development.
- barley
- awn
- gene interaction
- transcription factor
- gene mapping
1. Introduction
The development of grass awns has become a topic of intense research largely owing to its important function in grain filling [1]. Awn in barley (Hordeum vulgare L.) is more diverse than in rice and wheat [2]. The awnness trait can be distinguished into different phenotypes, including awnless, straight (long, longer than the length of spike axis; short, shorter than the length of spike axis; and awnlet, shorter than 1 cm), hooded, leafy, and crooked at the end of a lemma (Figure 1). They are controlled by different awnness loci that interact with each other. Analysis of the interactions among these loci can potentially reveal the mechanisms by which awn development is regulated. This topic has not been systematically studied, although some historic and recent investigations may have provided interesting hints [3][4][5].
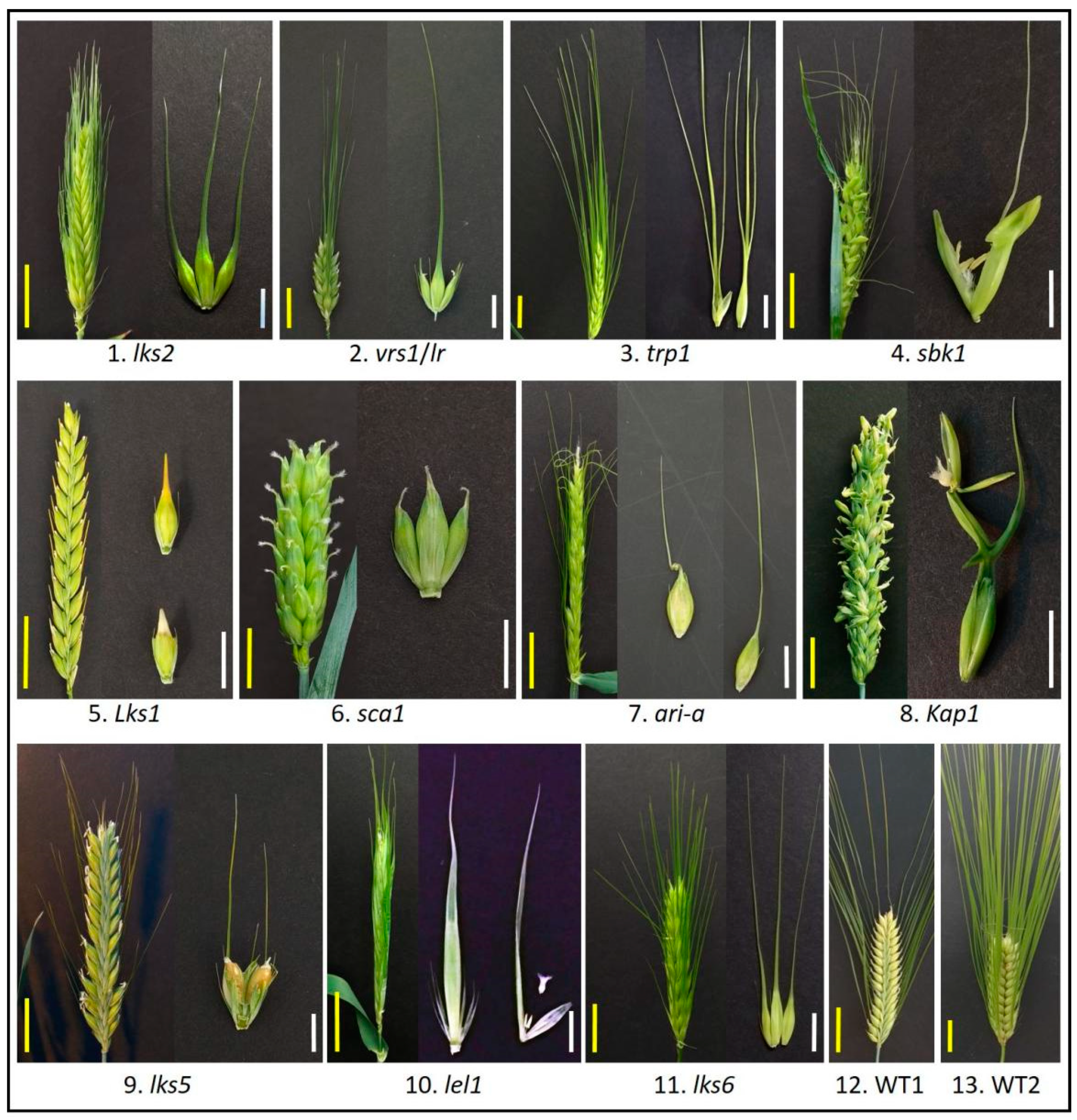
Figure 1. Barley awn diversity. Barley awn mutants obtained from the National Small Grains Collection, USDA-ARS (Agricultural Research Service, United States Department of Agriculture), Aberdeen Idaho. 1-lks2, short awn; 2-vrs1(lr), six-rowed with awnless lateral spikelet; 3-trp1, triple awned lemma; 4-sbk1, subjacent hooded lemma; 5-Lks1, awnless; 6-sca1, short crooked awn; 7-ari-a, short awn; 8-Kap1, hooded lemma; 9-lks5, short awn; 10-lel1, leafy lemma; 11-lks6, short awn; 12-WT1, wild type, two-rowed with long awn; 13-WT2, wild type, six-rowed with long awn. Yellow scale bar = 3 cm; White scale bar = 1 cm.
The genetic control of awn development in barley is very complicated, involving all sorts of gene interactions. Part of the genes and interactions for awn development found in barley are summarized in Table 1.
| Interaction | Genes | Segregation in F2 generation | Reference |
|---|---|---|---|
| Comp. | N/n and H/h | 9 HA (N_H_): 7 NA (N_hh + nnH_ + nnhh) | [6] |
| Dup. | Lel1/lel1 and Lel2/lel2 | 15 NA (Lel1_Lel2_ + Lel1_lel2lel2 + lel1lel1Lel2_): 1 LF (lel1lel1lel2lel2) | [7] |
| Cum. | Lks2/lks2 and Lks5/lks5 | 9 LA (Lks2_ Lks5_): 6 SA (Lks2_ lks5lks5 +lks2lks2 Lks5_): 1 AL (lks2lks2 lks5lks5) | [8] |
| Rec. epi. | Lks2/lls2 and Kap1/kap1 | 9 HA (Lls2_Kap1_): 3 LA (Lks2_kap1kap1): 4 SA (lks2lks2Kap1_ + lks2lks2kap1kap1) | [5] |
| Dom. epi. | Lsa1/lsa1 and Kap1/kap1 | 12 AL (Lsa1_Kap1_ + Lsa1_kap1kap1): 3 HA (lsa1 lsa1Kap1_): 1 ST (lsa1 lsa1kap1kap1) | [3] |
| Inh. | S/s and A/a | 13 AL (S_A_ + S_aa + ssaa): 3 LA (ssA_) | [9] |
Note: Comp., complementary; Dup., duplicate; Cum., cumulative; Rec. epi., recessive epistasis; Dom. epi., dominant epistasis; Inh., inhibiting; HA, hooded awn; NA, normal awn; LF, leafy awn; LA, long awn; SA, short awn; AL, awnless; ST, straight awn.
2. Molecular Mechanisms of Awnness Gene Interactions in Barley
There has so far been a limited understanding of molecular mechanisms of awn gene interactions. Analysis of the expression patterns of awn genes An-1 and An-2 in rice suggests that An-1 regulates the formation of awn primordia, while An-2 promotes awn elongation [10]. The upstream genes often regulate the expression of downstream genes. In barley, the mutant gene prbs (poly-rowed-and-branched spike) is recessive epistatic over the row type gene Vrs1/vrs1, and prbs may function upstream of vrs1 [11][12]. Two awnness-specific genes have so far been cloned in barley and they each encode a type of transcription factor. One is Kap1 for hooded lemma 1, while the other is lks2 for short awn 2. Barley lks2 is recessive epistatic over the hooded-awn gene Kap1, and may function upstream of Kap1 [13][14]. Lks2 encodes a SHORT INTERNODES (SHI)-type transcription factor [14]. The SHI proteins contain two conserved regions, the RING-finger motif and the IGGH domain—the former being implicated in zinc-binding and the latter being required for dimerization and transcription activation [15]. Kap1 encodes KNOX-type transcription factor. KNOX family is known to regulate the maintenance of the shoot apical meristem and the initiation of lateral organs in plants [16]. Awnless gene B1 in wheat encodes C2H2 zinc finger proteins [17], which may act as transcriptional repressors to regulate gene expression in developmental processes such as the formation of flower, seed, and rudimentary glume [18]. Barley HvFT3, as the counterpart of awnless gene B1 in wheat, functions upstream of the row-type genes (Vrs1, Vrs4, and Int-c) [17][19].
Genomics studies could be helpful to reveal molecular mechanisms of gene interactions in the future. CRISPR (clustered regularly interspaced short palindromic repeats) knocking out could be a method to prove gene interaction at molecular level. By knocking out the upstream genes, the downstream genes will be expressed and produce corresponding phenotypes. Time-series transcriptomics data provided abundant proof of metabolic processes [20], then gene interactions at molecular and metabolic level could be proved. The yeast two-hybrid technique is also a good system to find the interactive protein, and then provides direct proof for gene interaction.
3. Concluding Remarks
Barley awns are highly diverse in morphology, varying from long, short, awnlet, to awnless in length, and from straight to hooded or crooked in shape. A set of genetic loci associated with the diversity of awns have been identified. Interactions among awn genes contribute to this diversity. Further research on awn gene interactions at the genetic, metabolic, and molecular levels will provide insights into the essence of the interactions and elucidate the mechanisms of awn initiation and development in barley.
References
- Ntakirutimana, F.; Xie, W. Morphological and genetic mechanisms underlying awn development in monocotyledonous grasses. Genes 2019, 10, 573.
- Terzi, V.; Tumino, G.; Pagani, D.; Rizza, F.; Ghizzoni, R.; Morcia, C.; Stanca, A.M. Barley developmental mutants: The high road to understand the cereal spike morphology. Diversity 2017, 9, 21.
- Huang, B.; Huang, D.; Hong, Z.; Owie, S.O.; Wu, W. Genetic analysis reveals four interacting loci underlying awn trait diversity in barley (Hordeum vulgare). Sci. Rep. 2020, 10, 1–8.
- Myler, J.L. Awn inheritance in barley. J. Agric. Res. 1942, 65, 405–412.
- Woodward, R.W.; Rasmussen, D.C. Hood and awn development in barley determined by two gene pairs. Agron. J. 1957, 49, 92–94.
- Miyake, K.; Imai, Y. Genetic studies in barley. I. Bot. Mag. Tokyo 1922, 36, 25–38.
- Pozzi, C.; Faccioli, P.; Terzi, V.; Stanca, A.M.; Cerioli, S.; Castiglioni, P.; Fink, R.; Capone, R.; Müller, K.J.; Bossinger, G.; et al. Genetics of mutations affecting the development of a barley floral bract. Genetics 2000, 154, 1335–1346.
- Litzenberger, S.C.; Green, J.M. Inheritance of awns in barley. Agron. J. 1951, 43, 117–123.
- Grunewaldt, J. The transmission of awn-length in barley: I. Factor analysis of a short awned mutant and an awnless primitive form. Theor. Appl. Genet. 1974, 44, 211–215.
- Luo, J.; Liu, H.; Zhou, T.; Gu, B.; Huang, X.; Shangguan, Y.; Zhu, J.; Li, Y.; Zhao, Y.; Wang, Y.; et al. An-1 encodes a basic helix-loop-helix protein that regulates awn development, grain size, and grain number in rice. Plant Cell 2013, 25, 3360–3376.
- Wu, J.Y.; Ding, J.Q.; Du, Y.X.; Chen, W.C. Identification and molecular tagging of two complementary dominance resistant genes to maize dwarf mosaic virus. Acta Genet. Sin. 2002, 29, 1095–1099.
- Huang, B.; Wu, W.; Liu, S.; Huang, Z. Genetic analysis on poly-row-and-branched spike mutant in barley. Hereditas (Beijing) 2004, 26, 903–906.
- McCoy, S.B. Understanding Epistasis in Linkage Analysis: The Kap and lks2 Loci in the Oregon Wolfe Barley Population. Bachelor’s Thesis, Oregon State University, Corvallis, OR, USA, 2000.
- Yuo, T.; Yamashita, Y.; Kanamori, H.; Matsumoto, T.; Lundqvist, U.; Sato, K.; Ichii, M.; Jobling, S.A.; Taketa, S. A SHORT INTERNODES (SHI) family transcription factor gene regulates awn elongation and pistil morphology in barley. J. Exp. Bot. 2012, 63, 5223–5232.
- Kuusk, S.; Sohlberg, J.J.; Eklund, D.M.; Sundberg, E. Functionally redundant SHI family genes regulate Arabidopsis gynoecium development in a dose-dependent manner. Plant J. 2006, 47, 99–111.
- Druka, A.; Franckowiak, J.; Lundqvist, U.; Bonar, N.; Alexander, J.; Houston, K.; Radovic, S.; Shahinnia, F.; Vendramin, V.; Morgante, M.; et al. Genetic dissection of barley morphology and development. Plant Physiol. 2011, 155, 617–627.
- Huang, D.; Zheng, Q.; Melchkart, T.; Bekkaoui, Y.; Konkin, D.J.F.; Kagale, S.; Martucci, M.; You, F.M.; Clarke, M.; Adamski, N.M.; et al. Dominant inhibition of awn development by a putative zinc-finger transcriptional repressor expressed at the B1 locus in wheat. New Phytol. 2020, 225, 340–355.
- Lyu, T.; Liu, W.; Hu, Z.; Xiang, X.; Liu, T.; Xiong, X.; Cao, J. Molecular characterization and expression analysis reveal the roles of Cys2/His2 zinc-finger transcription factors during flower development of Brassica rapa subsp. chinensis. Plant Mol. Biol. 2020, 102, 123–141.
- Mulki, M.A.; Bi, X.; von Korff, M. FLOWERING LOCUS T3 controls spikelet initiation but not floral development. Plant Physiol. 2018, 178, 1170–1186.
- Bechtold, U.; Penfold, C.A.; Jenkins, D.J.; Legaie, R.; Moore, J.D.; Lawson, T.; Matthews, J.S.A.; Vialet-Chabrand, S.R.M.; Baxter, L.; Subramaniam, S.; et al. Time-series transcriptomics reveals that AGAMOUS-LIKE22 affects primary metabolism and developmental processes in drought-stressed Arabidopsis. Plant Cell 2016, 28, 345–366.
