Carbohydrate quantity was shown to affect sleep architecture, and especially N3 and REM sleep stages. Alterations in both quantity and quality of carbohydrate intake showed a significant effect on sleep initiation. Variations in carbohydrate quality significantly affected measures of sleep continuation. Further studies are needed to assess the effect of long-term carbohydrate interventions on sleep.
- glycemic index
- glycemic load
- nutrition
- sleep
- polysomnography
- actigraphy
1. Introduction
Fundamental and one of the very first studies for sleep has shown a link high-carb meals with a big reduction in deep sleep and an increase in REM sleep (for both, excessively high- and low- carb meals) [1]. REM percentage was reduced in another research while deep sleep increased with a restriction in carbohydrates. These changes were observed in a short-term study and they may be linked to the sudden change in diet [2]. In obese people, a low-carb diet has shown an improvement in sleep disturbances and a normalization of sleep architecture [3].
Sleep constitutes a lifelong element of human existence. It is defined as a reversible state of decreased or absent consciousness that initiates from wakefulness and evolves to light, deep, and rapid eye movement (REM) sleep stages [14]. Alterations in sleep-related parameters are often part of physiological responses that may be induced by nutrition interventions and might be translated into modifications in sleep architecture [25], quantity, or continuation [36].
The required sleep quantity varies among individuals and its adequacy is reflected by the absence of sleep-induced or sleep-related health issues, daytime dysfunction, or sleepiness [47]. Insufficient sleep traits have been increased over the last years [58][69]. Its association with numerous diseases [710] underlined the necessity to examine practical and effective approaches, including manipulation of nutritional aspects to optimize sleep [811]. Various nutrition interventions have been studied for their effects on sleep-related parameters. Interestingly, both dietary supplements (e.g., tryptophan [912]) and consumption of whole foods (e.g., kiwifruit [1013], tart cherry juice [1114]) have shown promising effects on improving sleep quality.
Animal models revealed that hormones [1215][1316] and peptides [1417] entrain the body’s circadian system and affect sleep. Since the circadian system facilitates most human behavioral and physiological processes, observational studies in humans have tried to investigate the effect of macronutrient intake on sleep quality and stated that alterations in the distribution and periodization of macronutrients are associated with sleep optimization [1518]. Towards this direction, the findings of nutrient–sleep interactions show to be positive but are yet unverified, with unsettled biological mechanism explanations [1619].
The acute manipulation of dietary carbohydrates (CHO) has been highlighted over the years with regard to its effect on sleep-related parameters. Carbohydrates are considered to be a critical macronutrient for sleep, not only because they serve as a primary source of energy for all human cells, but due to their relationship with brain function and sleep-related hormonal regulation [1720]. Glucose metabolism is highly interrelated with sleep [1821] by modifying the plasma tryptophan concentration [1922], a precursor of serotonin and melatonin, which, in turn, has a significant effect on sleep initiation and continuation [2023]. Two recent reviews suggest that dietary melatonin intake (either from fruits and vegetables [2124], or milk and cherry juice [2225]) could have sleep-promoting effects. Research shows that carbohydrates are associated with alterations in sleep onset latency [2326], sleep time [36], sleep continuity [36], and sleep stages [2427]. Nonetheless, the effect of CHO intake in sleep has not been systematically reviewed yet. Furthermore, since dietary CHO intake from individual studies may vary in quantity, quality, or timing/duration of intervention, it is of utmost importance for these factors to be taken into account and analyzed distinctively.
2. Effects of Carbohydrates on Sleep
Over the last decades, there has been a great interest in the relationship between diet on sleep. The current meta-analysis of clinical trials showed that CHO intake could significantly affect both sleep architecture, sleep initiation, and continuation. In particular, a lower quantity of CHO intake significantly lengthens N3 stage sleep proportion and duration compared to higher CHO consumption. Increased dietary CHO intake significantly prolonged REM stage sleep compared to lower CHO intake. Small effects were also observed for increased CHO quantity and REM attainment and sleep depth compared to lower CHO intake. The quality of CHO intake did not show any significant effect on sleep stages. Sleep onset latency showed to be affected by both carbohydrate quantity and quality. Alterations in the quality of carbohydrate intake showed a significant effect on measures of sleep continuation.
Diet-induced alternations in CHO quality and quantity have shown some promising results with regards to sleep-related parameters, based on the limited data. Replacing a typical diet, one that is mainly composed of CHO as the main source of energy intake, with an LCI diet showed increases in both duration and proportion of deep sleep [25][2427][2528][2629][2730]. Evidence from genetic studies has shown that sleep duration is associated with rs2031573 and rs1037079 alleles, with relevance to shorter sleep [2831]; however, it is not established yet whether diet modifications could affect the epigenetics of those genes and consequently the quality of sleep. The effect of CHO intake in deep sleep could be attributable to various biological mechanisms related to diet-dependent hormonal regulation (Figure 1). It is proposed that dietary fat and protein (or their digestion products) stimulate cholecystokinin (CCK) release to a greater extent than CHO [2932][3033]. CCK is produced in a number of tissues in humans, including enteroendocrine cells of the duodenum, and is distributed in the brain as well [3134]. In healthy male and female volunteers, an LCHO meal led to higher postprandial CCK concentrations and increased subjective feelings of sleepiness [2932]. Nonetheless, the relationship between CCK and sleep was first demonstrated in animal experiments [1215][1316]. In both rats and rabbits, intraperitoneal injection of cholecystokinin showed a dose–response relationship between circulating CCK levels and N3 sleep stage (referred to as SWS or “deep sleep”). Following the same line, both dietary fat and CCK may result in higher levels of peptide-tyrosine-tyrosine (PYY) [3033]. PYY is a peptide released in the gastrointestinal tract and it is usually studied for its role in appetite regulation, and especially its anorexigenic effect [3033]. In animal models, nocturnal intraperitoneal administration of PYY decreased wakefulness and enhanced NREM sleep [1417]. In contrast to PYY, ghrelin is an appetite-stimulating hormone that is linked to sleep-wake behavior [3235]. The magnitude of the effect of ghrelin on sleep is differentiated according to sex, being greater in males than females [3336]. It is proposed that ghrelin stimulates the activity of the hypothalamic-pituitary-adrenal and hypothalamic-somatotrophic axes, altering growth hormone (GH) and cortisol levels [3235], both hormones that are involved in sleep regulation. N3 sleep stage coincides with approximately 70% of GH pulses, and the amount of N3 sleep stage is positively related to the amount of GH secretion during these pulses [3437]. Moreover, cortisol administration in both young [3538] and elder adults [3639] is positively linked with endogenous GH secretion and N3 sleep stage. In this meta-analysis, all individual studies that modified CHO quantity acutely or in the short term showed increases in SWS [25][2427][2528][2629]. The pooled results suggest that an LCHO pre-bed meal ranging between 0–47 g of CHO or an LCHO diet ranging between 2–100 g of CHO increased the N3 sleep stage by 8.5 min or by 3.2% compared to an HCHO pre-bed meal ranging between 130–196 g of CHO or an HCHO diet ranging from 240–600 g of CHO. As N3 occupies approximately 20% of TST [14], an increase of 3.2% is translated to 16% of the time that is spent in this specific sleep stage.
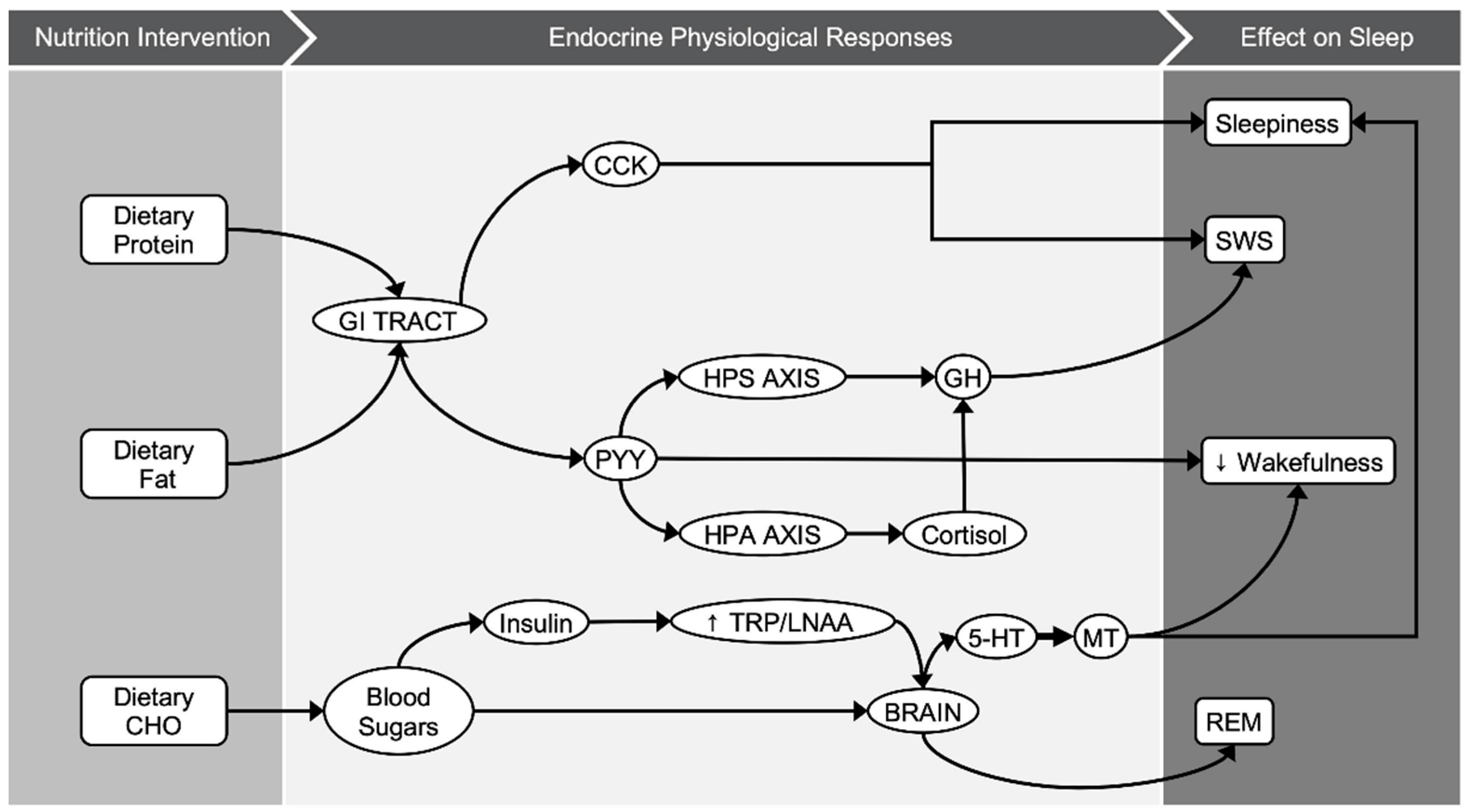
Figure 1. Graphical illustration of potential biological pathways behind macronutrients–sleep interactions.
3. Conclusions
The current results highlight the effect of nutrition, and especially carbohydrates, on sleep. It was observed that a lower quantity of CHO intake significantly increases N3 stage sleep and higher dietary CHO intake significantly prolongs REM stage sleep. The quality of CHO intake did not show any significant effect on sleep stages. The effectiveness of carbohydrate quantity and quality in sleep onset latency was significantly explained by alterations of carbohydrate intake as a percentage of daily energy intake and alterations in the glycemic load, respectively. Changes in glycemic load partially explained the variance of the effectiveness of sleep quality in sleep efficiency and wake after sleep onset. To date, there is no clear interpretation of the relevant biological mechanisms. Results for CHO quantity and sleep stages are promising and need to be further addressed in future studies with long-term interventions in different age groups for both genders.
References
- Berry, R.B.; Gamaldo, C.E.; Harding, S.M.; Brooks, R.; Lloyd, R.M.; Vaughn, B.V.; Marcus, C.L. AASM Scoring Manual Version 2.2 Updates: New Chapters for Scoring Infant Sleep Staging and Home Sleep Apnea Testing. J. Clin. Sleep Med. 2015, 11, 1253–1254.F Phillips, C N Chen, A H Crisp, J Koval, B McGuinness, R S Kalucy, E C Kalucy, J H Lacey (1975). Isocaloric diet changes and electroencephalographic sleep. Lancet.18;2(7938):723-5, doi: 10.1016/s0140-6736(75)90718-7.
- Afaghi, A.; O’Connor, H.; Chow, C.M. Acute effects of the very low carbohydrate diet on sleep indices. Nutr. Neurosci. 2008, 11, 146–154.Ahmad Afaghi 1, Helen O'Connor, Chin Moi Chow (2008).Acute effects of the very low carbohydrate diet on sleep indices. Nutr Neurosci. 2008 Aug;11(4):146-54. doi: 0.1179/147683008X301540.
- Vlahoyiannis, A.; Aphamis, G.; Andreou, E.; Samoutis, G.; Sakkas, G.K.; Giannaki, C.D. Effects of High vs. Low Glycemic Index of Post-Exercise Meals on Sleep and Exercise Performance: A Randomized, Double-Blind, Counterbalanced Polysomnographic Study. Nutrients 2018, 10, 1795.Ana I. Castro,1,2,† Diego Gomez-Arbelaez,1,3,† Ana B. Crujeiras,1,2,† Roser Granero,2,4 Zaida Aguera,2,5 Susana Jimenez-Murcia,2,5 Ignacio Sajoux,6 Patricio Lopez-Jaramillo,7 Fernando Fernandez-Aranda,2,5,‡ and Felipe F. Casanueva (2018).Effect of A Very Low-Calorie Ketogenic Diet on Food and Alcohol Cravings, Physical and Sexual Activity, Sleep Disturbances, and Quality of Life in Obese PatientsNutrients. 2018 Oct; 10(10): 1348.
- Dickinson, D.L.; Wolkow, A.P.; Rajaratnam, S.M.W.; Drummond, S.P.A. Personal sleep debt and daytime sleepiness mediate the relationship between sleep and mental health outcomes in young adults. Depression Anxiety 2018, 35, 775–783.Berry, R.B.; Gamaldo, C.E.; Harding, S.M.; Brooks, R.; Lloyd, R.M.; Vaughn, B.V.; Marcus, C.L. AASM Scoring Manual Version 2.2 Updates: New Chapters for Scoring Infant Sleep Staging and Home Sleep Apnea Testing. J. Clin. Sleep Med. 2015, 11, 1253–1254.
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults—United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 137–141.Afaghi, A.; O’Connor, H.; Chow, C.M. Acute effects of the very low carbohydrate diet on sleep indices. Nutr. Neurosci. 2008, 11, 146–154.
- Vlahoyiannis, A.; Aphamis, G.; Bogdanis, G.C.; Sakkas, G.K.; Andreou, E.; Giannaki, C.D. Deconstructing athletes’ sleep: A systematic review of the influence of age, sex, athletic expertise, sport type, and season on sleep characteristics. J. Sport Heal. Sci. 2020, 25, S2095–S2546.Vlahoyiannis, A.; Aphamis, G.; Andreou, E.; Samoutis, G.; Sakkas, G.K.; Giannaki, C.D. Effects of High vs. Low Glycemic Index of Post-Exercise Meals on Sleep and Exercise Performance: A Randomized, Double-Blind, Counterbalanced Polysomnographic Study. Nutrients 2018, 10, 1795.
- Ham, O.K.; Kim, J.; Lee, B.G.; Choi, E. Behavioral Characteristics and Cardiovascular Disease Risks Associated with Insomnia and Sleep Quality Among Middle-Aged Women in South Korea. Res. Nurs. Health 2017, 40, 206–217.Dickinson, D.L.; Wolkow, A.P.; Rajaratnam, S.M.W.; Drummond, S.P.A. Personal sleep debt and daytime sleepiness mediate the relationship between sleep and mental health outcomes in young adults. Depression Anxiety 2018, 35, 775–783.
- Vlahoyiannis, A.; Sakkas, G.K.; Manconi, M.; Aphamis, G.; Giannaki, C.D. Athletes’ sleep assessment: From lifestyle to pharmacological interventions and vice versa. Sleep Med. 2021, 78, 49–50.Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults—United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 137–141.
- Hudson, C.; Hudson, S.P.; Hecht, T.; MacKenzie, J. Protein source tryptophan versus pharmaceutical grade tryptophan as an efficacious treatment for chronic insomnia. Nutr. Neurosci. 2005, 8, 121–127.Vlahoyiannis, A.; Aphamis, G.; Bogdanis, G.C.; Sakkas, G.K.; Andreou, E.; Giannaki, C.D. Deconstructing athletes’ sleep: A systematic review of the influence of age, sex, athletic expertise, sport type, and season on sleep characteristics. J. Sport Heal. Sci. 2020, 25, S2095–S2546.
- Lin, H.-H.; Tsai, P.-S.; Fang, S.-C.; Liu, J.-F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011, 20, 169–174.Ham, O.K.; Kim, J.; Lee, B.G.; Choi, E. Behavioral Characteristics and Cardiovascular Disease Risks Associated with Insomnia and Sleep Quality Among Middle-Aged Women in South Korea. Res. Nurs. Health 2017, 40, 206–217.
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916.Vlahoyiannis, A.; Sakkas, G.K.; Manconi, M.; Aphamis, G.; Giannaki, C.D. Athletes’ sleep assessment: From lifestyle to pharmacological interventions and vice versa. Sleep Med. 2021, 78, 49–50.
- Kapás, L.; Obál, F., Jr.; Opp, M.R.; Johannsen, L.; Krueger, J.M. Intraperitoneal injection of cholecystokinin elicits sleep in rabbits. Physiol. Behav. 1991, 50, 1241–1244.Hudson, C.; Hudson, S.P.; Hecht, T.; MacKenzie, J. Protein source tryptophan versus pharmaceutical grade tryptophan as an efficacious treatment for chronic insomnia. Nutr. Neurosci. 2005, 8, 121–127.
- Kapás, L.; Obál, F.; Alföldi, P.; Rubicsek, G.; Penke, B. Effects of nocturnal intraperitoneal administration of cholecystokinin in rats: Simultaneous increase in sleep, increase in EEG slow-wave activity, reduction of motor activity, suppression of eating, and decrease in brain temperature. Brain Res. 1988, 438, 155–164.Lin, H.-H.; Tsai, P.-S.; Fang, S.-C.; Liu, J.-F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011, 20, 169–174.
- Akanmu, M.A.; Ukponmwan, O.E.; Katayama, Y.; Honda, K. Neuropeptide-Y Y2-receptor agonist, PYY3–36 promotes non-rapid eye movement sleep in rat. Neurosci. Res. 2006, 54, 165–170.Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916.
- Grandner, M.A.; Kripke, D.F.; Naidoo, N.; Langer, R.D. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010, 11, 180–184.Kapás, L.; Obál, F., Jr.; Opp, M.R.; Johannsen, L.; Krueger, J.M. Intraperitoneal injection of cholecystokinin elicits sleep in rabbits. Physiol. Behav. 1991, 50, 1241–1244.
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319.Kapás, L.; Obál, F.; Alföldi, P.; Rubicsek, G.; Penke, B. Effects of nocturnal intraperitoneal administration of cholecystokinin in rats: Simultaneous increase in sleep, increase in EEG slow-wave activity, reduction of motor activity, suppression of eating, and decrease in brain temperature. Brain Res. 1988, 438, 155–164.
- Fernstrom, J.D.; Wurtman, R.J. Brain Serotonin Content: Physiological Dependence on Plasma Tryptophan Levels. Science 1971, 173, 149–152.Akanmu, M.A.; Ukponmwan, O.E.; Katayama, Y.; Honda, K. Neuropeptide-Y Y2-receptor agonist, PYY3–36 promotes non-rapid eye movement sleep in rat. Neurosci. Res. 2006, 54, 165–170.
- Stamatakis, K.A.; Punjabi, N.M. Effects of Sleep Fragmentation on Glucose Metabolism in Normal Subjects. Chest 2010, 137, 95–101.Grandner, M.A.; Kripke, D.F.; Naidoo, N.; Langer, R.D. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010, 11, 180–184.
- Herrera, C.P.; Smith, K.; Atkinson, F.; Ruell, P.; Chow, C.M.; O’Connor, H.; Brand-Miller, J. High-glycaemic index and -glycaemic load meals increase the availability of tryptophan in healthy volunteers. Br. J. Nutr. 2011, 105, 1601–1606.Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319.
- Brzezinski, A.; Vangel, M.G.; Wurtman, R.J.; Norrie, G.; Zhdanova, I.; Ben-Shushan, A.; Ford, I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med. Rev. 2005, 9, 41–50.Fernstrom, J.D.; Wurtman, R.J. Brain Serotonin Content: Physiological Dependence on Plasma Tryptophan Levels. Science 1971, 173, 149–152.
- Noorwali, E.; Hardie, L.; Cade, J. Bridging the Reciprocal Gap between Sleep and Fruit and Vegetable Consumption: A Review of the Evidence, Potential Mechanisms, Implications, and Directions for Future Work. Nutrients 2019, 11, 1382.Stamatakis, K.A.; Punjabi, N.M. Effects of Sleep Fragmentation on Glucose Metabolism in Normal Subjects. Chest 2010, 137, 95–101.
- Pereira, N.; Naufel, M.F.; Ribeiro, E.B.; Tufik, S.; Hachul, H. Influence of Dietary Sources of Melatonin on Sleep Quality: A Review. J. Food Sci. 2019, 85, 5–13.Herrera, C.P.; Smith, K.; Atkinson, F.; Ruell, P.; Chow, C.M.; O’Connor, H.; Brand-Miller, J. High-glycaemic index and -glycaemic load meals increase the availability of tryptophan in healthy volunteers. Br. J. Nutr. 2011, 105, 1601–1606.
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-glycemic-index carbohydrate meals shorten sleep onset. Am. J. Clin. Nutr. 2007, 85, 426–430.Brzezinski, A.; Vangel, M.G.; Wurtman, R.J.; Norrie, G.; Zhdanova, I.; Ben-Shushan, A.; Ford, I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med. Rev. 2005, 9, 41–50.
- Phillips, F.; Chen, C.N.; Crisp, A.H.; Koval, J.; McGuinness, B.; Kalucy, R.S.; Kalucy, E.C.; Lacey, J.H. Isocaloric diet changes and electroencephalographic sleep. Lancet 1975, 2, 723–725.Noorwali, E.; Hardie, L.; Cade, J. Bridging the Reciprocal Gap between Sleep and Fruit and Vegetable Consumption: A Review of the Evidence, Potential Mechanisms, Implications, and Directions for Future Work. Nutrients 2019, 11, 1382.
- Porter, J.; Horne, J. Bed-time food supplements and sleep: Effects of different carbohydrate levels. Electroencephalogr. Clin. Neurophysiol. 1981, 51, 426–433.Pereira, N.; Naufel, M.F.; Ribeiro, E.B.; Tufik, S.; Hachul, H. Influence of Dietary Sources of Melatonin on Sleep Quality: A Review. J. Food Sci. 2019, 85, 5–13.
- Kwan, R.M.F.; Thomas, S.; Mir, M.A. Effects of a Low Carbohydrate Isoenergetic Diet on Sleep Behavior and Pulmonary Functions in Healthy Female Adult Humans. J. Nutr. 1986, 116, 2393–2402.Afaghi, A.; O’Connor, H.; Chow, C.M. High-glycemic-index carbohydrate meals shorten sleep onset. Am. J. Clin. Nutr. 2007, 85, 426–430.
- St-Onge, M.-P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. 2016, 12, 19–24.Phillips, F.; Chen, C.N.; Crisp, A.H.; Koval, J.; McGuinness, B.; Kalucy, R.S.; Kalucy, E.C.; Lacey, J.H. Isocaloric diet changes and electroencephalographic sleep. Lancet 1975, 2, 723–725.
- Ollila, H.M.; Kettunen, J.; Pietiläinen, O.; Aho, V.; Silander, K.; Kronholm, E.; Perola, M.; Lahti, J.; Räikkönen, K.; Widen, E.; et al. Genome-wide association study of sleep duration in the Finnish population. J. Sleep Res. 2014, 23, 609–618.Porter, J.; Horne, J. Bed-time food supplements and sleep: Effects of different carbohydrate levels. Electroencephalogr. Clin. Neurophysiol. 1981, 51, 426–433.
- Wells, A.S.; Read, N.; Uvnas-Moberg, K.; Alster, P. Influences of Fat and Carbohydrate on Postprandial Sleepiness, Mood, and Hormones. Physiol. Behav. 1997, 61, 679–686.Kwan, R.M.F.; Thomas, S.; Mir, M.A. Effects of a Low Carbohydrate Isoenergetic Diet on Sleep Behavior and Pulmonary Functions in Healthy Female Adult Humans. J. Nutr. 1986, 116, 2393–2402.
- Chaudhri, O.; Small, C.; Bloom, S. Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1187–1209.St-Onge, M.-P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. 2016, 12, 19–24.
- Dockray, G.J. Cholecystokinin and gut–brain signalling. Regul. Pept. 2009, 155, 6–10.Ollila, H.M.; Kettunen, J.; Pietiläinen, O.; Aho, V.; Silander, K.; Kronholm, E.; Perola, M.; Lahti, J.; Räikkönen, K.; Widen, E.; et al. Genome-wide association study of sleep duration in the Finnish population. J. Sleep Res. 2014, 23, 609–618.
- Steiger, A.; Dresler, M.; Schüssler, P.; Kluge, M. Ghrelin in mental health, sleep, memory. Mol. Cell. Endocrinol. 2011, 340, 88–96.Wells, A.S.; Read, N.; Uvnas-Moberg, K.; Alster, P. Influences of Fat and Carbohydrate on Postprandial Sleepiness, Mood, and Hormones. Physiol. Behav. 1997, 61, 679–686.
- Schuessler, P.; Uhr, M.; Ising, M.; Schmid, D.; Weikel, J.; Steiger, A. Nocturnal ghrelin levels-relationship to sleep EEG, the levels of growth hormone, ACTH and cortisol—and gender differences. J. Sleep Res. 2005, 14, 329–336.Chaudhri, O.; Small, C.; Bloom, S. Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1187–1209.
- Van Cauter, E.; Plat, L. Physiology of growth hormone secretion during sleep. J. Pediatr. 1996, 128, S32–S37.Dockray, G.J. Cholecystokinin and gut–brain signalling. Regul. Pept. 2009, 155, 6–10.
- Friess, E.; Tagaya, H.; Grethe, C.; Trachsel, L.; Holsboer, F. Acute Cortisol Administration Promotes Sleep Intensity in Man. Neuropsychopharmacology 2003, 29, 598–604.Steiger, A.; Dresler, M.; Schüssler, P.; Kluge, M. Ghrelin in mental health, sleep, memory. Mol. Cell. Endocrinol. 2011, 340, 88–96.
- Bohlhalter, S.; Murck, H.; Holsboer, F.; Steiger, A. Cortisol Enhances non-REM Sleep and Growth Hormone Secretion in Elderly Subjects. Neurobiol. Aging 1997, 18, 423–429.Schuessler, P.; Uhr, M.; Ising, M.; Schmid, D.; Weikel, J.; Steiger, A. Nocturnal ghrelin levels-relationship to sleep EEG, the levels of growth hormone, ACTH and cortisol—and gender differences. J. Sleep Res. 2005, 14, 329–336.
- Van Cauter, E.; Plat, L. Physiology of growth hormone secretion during sleep. J. Pediatr. 1996, 128, S32–S37.
- Friess, E.; Tagaya, H.; Grethe, C.; Trachsel, L.; Holsboer, F. Acute Cortisol Administration Promotes Sleep Intensity in Man. Neuropsychopharmacology 2003, 29, 598–604.
- Bohlhalter, S.; Murck, H.; Holsboer, F.; Steiger, A. Cortisol Enhances non-REM Sleep and Growth Hormone Secretion in Elderly Subjects. Neurobiol. Aging 1997, 18, 423–429.
