Methanol is a natural ingredient with major occurrence in fruit spirits, such as apple, pear, plum or cherry spirits, but also in spirits made from coffee pulp. The compound is formed during fermentation and the following mash storage by enzymatic hydrolysis of naturally present pectins. Methanol is toxic above certain threshold levels and legal limits have been set in most jurisdictions. Therefore, the methanol content needs to be mitigated and its level must be controlled. This article will review the several factors that influence the methanol content including the pH value of the mash, the addition of various yeast and enzyme preparations, fermentation temperature, mash storage, and most importantly the raw material quality and hygiene
- alcoholic beverages
- spirits
- methanol
- risk mitigation
- legal limits
- quality control
1. Introduction
Methanol is an alcohol that is typically found in almost all kinds of alcoholic beverages and some other fermented food products [1][2][3][4][5]. Methanol may occur in alcoholic beverages through two major pathways: a natural one (pectin degradation), as well as an artificial one (adulteration by illegal addition of the pure compound). Only the latter pathway (adulteration) is typically associated with major morbidity and mortality due to methanol poisoning [6][7][8][9]. While adulteration is still prevalent and incidences have increased due to alcohol shortages during the COVID-19 pandemic [10], this article will exclusively focus on the first pathway, the natural content of methanol in spirits and its mitigation. Regarding the mitigation of problems related to methanol addition, we have recently provided a separate review [11].
In the human body, methanol may be endogenously present in low concentrations [12][13], while in most alcoholic beverages such as beer and wine, the natural content of methanol is also quite low. This differs with fruit spirits, so that the major focus on methanol reduction measures lies on this kind of beverage.
Prunus
Malus
Pyrus
Coffea
Methanol concentrations in spirits are closely linked to enzymatic activities in the fruits and during the alcoholic fermentation process. Pectin methylesterase activity (1) may derive endogenously from the fruits themselves but also during alcoholic fermentation by pectin methylesterase formed from yeast metabolism or from other microorganisms [16][17][18]. Pectin methylesterase activity may also be exogenously introduced by addition of certain pectolytic enzyme preparations. A negligible pathway may be thermic demethylation of pectins [19].
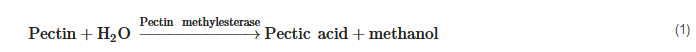
When methanol has been released from the fruits’ pectin, it inevitably becomes part of the mash [20]. Its level is dependent on the degree of esterification of the pectin inside the fruits and the fruit-dependent ratio between sugar and pectin [5][21]. Another pathway suggested for methanol formation in protein-rich fruits such as jejube (Chinese date,
Ziziphus jujube
The European Union (EU) regulates maximum methanol contents in spirits dependent on the utilized raw materials [4][23][24]. For ethyl alcohol of agricultural origin, the maximum level of methanol is 30 g/hL of 100% vol alcohol (pure alcohol, pa), while for vodka it is 10 g/hL pa and the lowest level is defined for London gin with 5 g/hL pa. The limits are higher for fruit-based materials: for wine spirit 200 g/hL pa, for grape marc and cider 1000 g/hL pa, for fruit marc 1500 g/hL pa, for fruit spirits in general 1000 g/hL pa, except 1200 g/hL pa for apples, apricots, plum, mirabelle, peach, pear, blackberry and raspberry, and 1350 g/hL pa for quince, Williams pear and some other berries [23]. While these EU limits are set to reduce toxic effects on the human body, they were also judged as being rather low and, for some types of fruit, as challenging to be upheld by small artisanal distillers [25]. Lower limits in other countries such as the USA may also prohibit export of fruit spirits to these countries [26].
2. Factors Influencing the Methanol Content of Fruit Spirits
Table 1.
| Method | Methanol Reduction Potential | 1 | Authors’ Judgment about Applicability |
References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Improvement of quality of raw material | up to 40% | Raw material is extremely important and the type and quality highly affects the methanol content. Removal of pectin-rich fruit parts such as skins may reduce methanol content. | [4][16][25][27][28] | [4,16,25,30,31] | ||||||||||
| Acidification of mash | up to 50% | Acidification of mash inhibits the activity of pectin methylesterase. It also inhibits spoilage microorganisms, which may produce pectin methylesterase. | [25][29][30][31][32][33] | [25,32,33,34,35,36] | ||||||||||
| Sterilization of mash | 40–90% | Temperature treatment efficiently denaturizes pectin methylesterase enzymes. High energy requirement and not feasible for artisanal distillers. | [18] | 18 | [22][28][34][35][ | ,22 | 36 | ,31 | ] | ,37 | [37 | ,38 | ][38] | [,39,40,41] |
| Decreased storage time of fermented mash before distillation | up to 50% | Storage time should be avoided or being minimized as far as possible, because sharp methanol increases were reported during storage. | [26][29][30] | [26,32,33] | ||||||||||
| Selection of appropriate yeast strains | up to 25% | Yeasts with low capacity of producing pectin methylesterase to be preferred. | [4][27][39][40] | [4,30,42,43] | ||||||||||
| Decreased fermentation temperature | up to 25% | Lower temperatures and the use of cold fermentation yeast is recommended. | [26] | |||||||||||
| Improvement in distillation method and conditions | up to 80% | Methanol is enriched in tailings. Earlier cut (not below 50% vol). No recycling of tailings. | [4] | 4 | [14][20][22][27][29][ | ,14 | 30 | ,20 | ] | ,22 | [31 | ,30 | ][41] | [,32,33,34,44] |
| Demethanolization following distillation | 50–90% | Effective in industry but not feasible for small artisanal distillers, high expenditure | [36][37][39][41][42][43] | [39,40,42,44,45,46] | ||||||||||
| Avoidance of liquefaction enzymes | up to 20% | Avoid pectin methylesterase enzymes which release methanol. | [4]36][39][44] | [4 | [22][26][ | ,22 | 31 | ,26 | ] | ,34 | [ | ,39,42,47] | ||
| Application of alternative liquefaction enzymes | up to 88% | Substitute pectin methylesterase enzymes by pectin lyase enzymes to reduce the release of methanol | [45][46] | [48,49] |
2.1. Raw Materials, Mash Preparation and Fermentation
Prior to sensitization of industry regarding the methanol problem and the implementation of maximum limits by the EU in the first spirits regulation in 1989 [47], so-called liquefaction enzymes were often applied during mash preparation. In addition to the desired pectin hydrolysis activity, these enzymes also had pectin esterase activity, resulting in methanol formation of up to five to six times higher than in untreated fruit mash [17][48][49]. Such conventional, unspecific enzymes should only be used with caution—if at all—and only if methanol monitoring is implemented [48]. The use of commercial mash enzymes (i.e., pectolytic enzymes such as pectin methylesterase) always resulted in very high methanol contents (similar to the maximum methanol release potential) [25][50][51]. In the case of Rubinette apples, methanol increases between 5.5% and 12% occur after addition of various pectin enzymes, which are used to liquefy the mashes without adding water, compared to the untreated sample [31]. In quince, the lowest methanol contents were measured in the mashes blended with 33% water [25]. The avoidance of conventional liquefaction enzymes alone can lead to a 20% reduction in methanol content [44]. However, thick fruit mashes usually require a more or less high addition of water for fermentation and distillation, which means time and increased energy input during distillation, and at the same time leads to lower alcohol yields [31]. If pectinolytic enzymes have to be applied, pure lyases should be preferred. Besides the scrutiny in use of enzymes the raw material quality, mash preparation and fermentation conditions have potential to mitigate the methanol release.
2.1.1. Quality and Treatment of Raw Materials
The methanol content is directly related to the fruit type or types used in the fermentation process (mainly dependent on the sugar/pectin ratio) but there are also differences between cultivars and harvest years [18][19][26][27]. For example, in studying distillates of Bartlett pear between 1978 and 1995, the 1993 vintage was the year with a strikingly lower methanol content [41]. In addition to the fruit type, it is very evident that the fruit quality used affects the quantity of the methanol formation [4][25]. At what stage of fruit development and how it is harvested also effects the methanol content [27].
Early harvest or hard pears led to higher methanol levels [31]. For pears and apricots, other researchers corroborated this finding showing that overripe fruit led to the lowest methanol contents [16]. In deviation of this finding, Adam reported an increase of methanol through advancing maturity of Williams Christ pears [41][44].
Utilization of plum juice leads to lower methanol contents than plum mashes [27]. On the other hand, destoned cherry mashes showed higher methanol contents than mashes with complete fruits including stones [52]. However, in another investigation of the same research group, destoned cherry mashes showed consistently lower methanol contents [53]. The conflicting results currently cannot be explained, other than confounding factors not controlled in the studies.
As pectins have a major occurrence in the skin layer, the removal of the fruit skins before fermentation may also reduce the methanol level by about 50% during production of wine spirits [28]. Cores and stems were also described to contain high levels of pectins [34]. Peeling and coring of pears, therefore, led to a methanol reduction of up to 42% [16]. However, this method is judged as not economically feasible for most spirits.
2.1.2. Inhibition of Pectin Methylesterase by Acidification of Mash
pH is one of the most important factors which highly affects the activity of enzymes. Pectin methylesterase showed an optimum at pH 8 and 50 °C [54]. Other authors suggested pH 5–6 as optimum for pectin methylesterase [34][35]. Pectin methylesterases from yeast may have optimal pH values ranging from 3.75 to 6 [55].
Therefore, the proposed pH for fermentations to avoid pectin methylesterase activity is 2.5 [29][31] (
Figure 1). No large differences were reported between pH 2.8 and 3.3, however [44]. Denes et al. [56] stated a decrease to 1% of the enzyme activity by decreasing the pH to 4.5 (pectin methylesterase from apples).
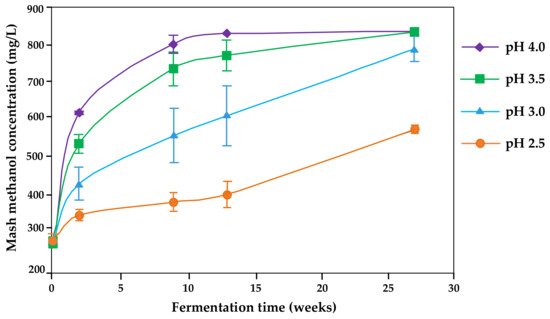
Figure 1. Kinetics of methanol formation in Bartlett pear mashes affected by the initial mash pH and fermentation time (redrawn from [29]).
There is a clear indication from several studies of an up to 50% reduction in methanol by acidification of fruit mashes [4][22][25][26][30][31][32].
There is not a clear preference about the kind of acid to be used. Gössinger et al. suggest ortho-phosphoric acid (85%) [26][50] while Pieper et al. suggested sulfuric acid [32]. Commercially available products for acidification often contain mixtures of several acids such as malic acid/hydroxypropionic acid or phosphoric acid/lactic acid.
Gerogiannaki-Christopoulou used citric acid resulting in a decrease of about 15% methanol in grape pomace distillate [33]. However, while some organic acids such as citric acid might be depleted during fermentation by their inclusion in metabolic pathways, inorganic acids appear to be more appropriate. Buffer systems ensuring a long-term stability of mash pH might be an interesting option for future investigation.
2.1.3. Inhibition of Pectin Methylesterase by Sterilization of Mash
A significant reduction of methanol by 40–90% [34][35] can be achieved by thermal deactivation of pectin methylesterase (often referred to as “mash heating”). There are various suggestions for temperature/time combinations to achieve the enzyme’s denaturation.
Sterilization at temperatures higher than 70 °C was generally suggested to effectively prevent the production of methanol by inactivation of pectin methylesterase [54][57]. Methanol can be reduced by targeted thermal deactivation of pectin methylesterase by heating the mash to 80 °C up to 85 °C for a holding time of 30 min or to 60 °C for 45 min [28][34][35]. Pasteurization at 72 °C for 15 s prevented the production of methanol in fermented plant beverages containing
Morinda citrifolia (noni fruit) [57]. In cider spirit, the pasteurization (30 min at 50 °C, then heated to about 85 °C) of the apple juice prior to fermentation reduced the methanol content by 34–46% [18]. Lower methanol levels were obtained in Williams and plums by heating the mash to 65 °C for 5 min, followed by re-cooling for fermentation [31].
Further technological approaches for inactivation of methylesterase are thermosonication (ultrasound plus temperature at 70° led to 30% methanol reduction in plum wine) or use of microwaves (70 °C for 1 min led to 70% methanol reduction in plum wine). The authors indicated an additional nonthermal effect of both ultrasonication and microwaving with improved sensory properties of the product [38].
2.1.4. Inhibition and Substitution of Pectin Methylesterase by Certain Additives
Pectinolytic enzymes (pectinase) are classified into esterase and depolymerase (lyase and hydrolase). Lyase produces oligo- or mono-galacturonate, while esterase produces pectic acid and methanol [58]. The addition of pectin lyase significantly (α = 0.01) reduced the resulting methanol contents in the mash of apricot and quince by 40–71% [25][26]. Lyase appears to inhibit the activity of the naturally contained pectolytic enzymes. The mechanism was speculated as being a cleavage of the pectin chains by the pectin lyase in such a fashion that the pectin fragments are not accessible as substrate for the pectin methylesterase [26]. The effectiveness of lyase enzymes can be increased by dilution of the mashes with water [26]. Similarly, the addition of certain detergents (anionic surfactants) as well as polyphenols (tannins) has a reducing effect on the release of methanol by full or partial inhibition of pectin methylesterases [19][31][32][38]. However, a large amount of agents is needed, which are rather expensive so that these methods were not widely implemented in practice [36].
Substituting the application of liquefying pectin methylesterase enzymes by pectinlysase reduced the methanol concentrations in apple distillates by 40–88%. The combination of mash sterilization and pectinlyase liquefaction resulted in an average methanol reduction of 94 ± 4% in the same distillates [45].
2.1.5. Selection of Yeast Strains and Fermentation
Microbiological control of the process could also be used to prevent methanol formation in fermented beverages. For instance, pure culture inoculation using commercial yeast in contrast to spontaneous inoculation by wild yeasts should be practiced [40]. Mashes fermented without pure yeast cultures generally lead to higher methanol levels [31]. Yeast culture selection can reduce methanol contents in the distillates by up to 20% [31].
However, the reason why there are significant differences from yeast breed to yeast breed is hypothetically due to the fact that the individual breeds apparently differ in their ability to inhibit pectin esterase and thus the release of methanol from pectin [31]. Strains of
Saccharomyces yeasts may produce all three types of pectinolytic enzymes[58]. Selection of yeasts which do not form pectin methylesterase was suggested to contribute to reduction of methanol occurrence [30]. Selected mutant
Saccharomyces cerevisiae S12 exhibited a methanol content during wine fermentations decreased by 73% compared to that of the wild-type strain [40]. On the other hand, Rodríguez Madrera et al. reported lower methanol concentrations in apple pomace spirits fermented with indigenous yeast than with commercial wine yeast [51].
In a comparison of three different yeast types (one newly developed strain with improved genetic and physiological performances and two commercial distillers’ yeasts), the new yeast showed higher methanol contents in plum and pear mashes, but not in cherry mashes [59]. In another investigation with the same yeast types, the new yeast showed lower methanol contents in plum mashes but higher in cherry mashes [52]. In a third study with these yeast types, the new yeast showed consistently lower methanol values than the commercial yeast in cherry spirits [53]. These conflicting results were interpreted by other influences on methanol content rather than a yeast influence. Similarly, different strains of yeast were used in fermentations but no significant change in the quality or quantity was noticed over time [4].
Pichia methanolica [60] and
Candida boidinii [61] which have the capacity of utilizing pectin or the methyl ester moiety of pectin and methanol, thus preventing the accumulation of methanol in fermented products [58]. However, the application of these microorganisms for fermentation of spirits has not been demonstrated so far.
2.1.6. Fermentation Conditions
The activity of the pectin methylesterase enzyme is directly linked with the temperature [62]. Increasing the temperature of the mash increases the speed of reaction until the temperature reaches a very high level where the enzyme starts denaturizing. Lowering the fermentation temperature from 20 °C to 12 °C with use of cold fermentation yeast may result in a 10–24% reduction in methanol release in the mash [26], but not in all cases [25][26].
2.2. Storage of Fermented Mash before Distillation
Figure 1) [29]. Depending on the pH level, an almost 100% release can be expected after only some weeks of storage. During mash storage of 4 weeks, methanol contents increased, in some cases sharply by 15–50% [25][26]. Therefore, the optimal practice would be to conduct the distillation as soon as fermentation has been complete or at least to minimize storage time as far as possible [30].
2.3. Distillation Method and Conditions
2.3.1. Methanol Reduction during Pot Still Distillation
Figure 2) [4][5][14][20][29][34][37][44]. However, even today many professional distillers believe that methanol concentrates preferably in the first fractions (heads fractions). And that methanol is the reason that heads fractions smell and taste bad (which is caused by acetaldehyde and ethyl acetate but not by methanol). It is of note that single studies that suggested that methanol may be enriched in the first distillation fractions were not plausible and potentially erroneous (e.g., compare the abstract with the conclusion section in Xia et al. [22], which report completely conflicting information—from the data presented in the work it can be assumed that the study from China is in fact corroborating the studies from Europe and the United States that methanol is enriched in the tailings while the information in the abstract that it is enriched in the heads fractions is most probably a translation mistake).
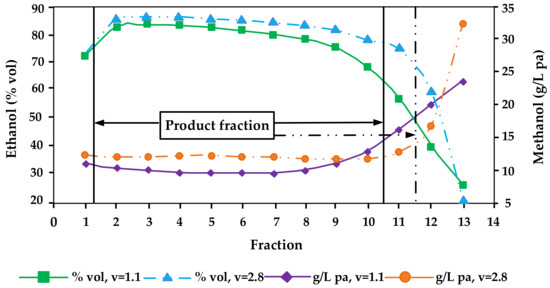
Figure 2. Distillation characteristics of ethanol and methanol affected by different reflux ratios (v) during distillation of Bartlett pear mashes (redrawn from [29]).
Various distillation tests carried out show that the methanol content in the product (hearts) fractions can hardly be influenced by different distillation techniques. Even in experiments with various “catalysts”, no groundbreaking findings have yet emerged. Only relatively expensive silver wool as adsorbent led to methanol reductions of up to 20% [31].
Therefore, the separation of tailings, which also has to be done for sensorial reasons, is so far the only option for a reduction of methanol during pot still distillation. The reduction of methanol contents of the product fractions in g/hL pa compared to mash may be between 20 and 30%. On the other hand, an extremely late separation of tailings can cause an increase of methanol contents of about 20% in the product fractions [36].
In general, it can be seen that the methanol content in the spirit increases with reflux ratio increases. That means the higher the reinforcement and the slower the distillation is, the higher the methanol content in the distillate [29][63] (
- Perform double distillation: it is always advisable to carry out two subsequent distillations with regard to methanol separation
- Increase separation efficiency: The methanol separation can be increased by a simple optional parallel connection of a conventional spirits tube and a more separation-efficient column. If possible, this column should be at least partially cooled at the top to increase internal reflux and thus separation efficiency.
-
Cooling at the head: When use of an additional column is not feasible, partial cooling of the spirits tube at the beginning of the second distillation can also increase the internal reflux and thus increase the separation efficiency.
-
Perform double distillation: it is always advisable to carry out two subsequent distillations with regard to methanol separation
-
Increase separation efficiency: The methanol separation can be increased by a simple optional parallel connection of a conventional spirits tube and a more separation-efficient column. If possible, this column should be at least partially cooled at the top to increase internal reflux and thus separation efficiency.
-
Cooling at the head: When use of an additional column is not feasible, partial cooling of the spirits tube at the beginning of the second distillation can also increase the internal reflux and thus increase the separation efficiency.
2.3.2. Methanol Reduction during Large-Scale Distillation
In contrast to pot stills that typically consist of a small column (three or four plates), industrial-scale distilleries with 15 to 30 plates provide the possibility of continuous distillation and advanced regulation of distillation including processes of demethylation [36].
Methanol content can be decreased during the rectification by using demethanolization columns [30][37]. This process is efficient and successfully reduces the methanol content up to 40–90% in comparison to the starting amount. However, investment is only viable for rather big businesses with high capacity utilization [36].
A combined evaporation/condensation method to reduce methanol from distillates was patented by Capovilla [43]. The application of the method was found to reduce methanol in fruit spirits by 58–190 g/hL pa [39]. However, such methods may not be economically viable as they considerably reduce the alcohol content along with the methanol content [26]. The promised results of the evaporation/condensation method were also criticized as implausible with independent investigations showing lesser methanol reduction (9–92 g/hL pa) always connected with inacceptable losses of ethanol (up to 10% vol) [42]. All in all evaporation/condensation methods for demethanolization were judged as economically unviable specifically for smaller businesses.
2.4. Storage of Distillate after Fermentation
Not much evidence is available regarding the methanol evolution during the distillates’ storage and aging process. Botelho et al. [4] suggested a tendency for low amounts of methanol in advanced wood-cask aged spirits, attributable to methanol oxidation and subsequent acetalization reaction with the formation of diethoxymethane. On the other hand, methanol is expected to be quite stable in inert containers without the presence of oxygen. This is also in line with the authors’ experience from validating methods for methanol determination, which suggested that methanol is a stable compound in bottled hydroalcoholic solutions [64].
