The TMS is a noninvasive method to investigate the CNS in the human. Transcranial magnetic stimulation, since its introduction in 1985, has brought important innovations to the study of cortical excitability as it is a non-invasive method and, therefore, can be used both in healthy and sick subjects. Since the introduction of this cortical stimulation technique, it has been possible to deepen the neurophysiological aspects of motor activation and control. Repetitive physical training is generally considered as a principal strategy for acquiring a motor skill, and this process can elicit cortical motor representational changes referred to as use-dependent plasticity. In training settings, physical practice combined with the observation of target movements can enhance cortical excitability and facilitate the process of learning. The data to date suggest that TMS is a valid technique to investigate the changes in motor cortex excitability in trained and untrained subjects.
- corticalexcitability
- transcranial magnetic stimulation
- motor cortex
- TMS
1. Transcranial Magnetic Stimulation (TMS)
The TMS is a noninvasive method to investigate the CNS in the human [1]. Since its introductions close [2], TMS, has been used to study intracortical, cortico-cortical, and cortico-subcortical interactions [3]. In 1982, Polson, Barker and Freeston produced the first magnetic stimulator capable of stimulating peripheral nerves and in 1985 Barker, Jalinous and Freeston were the first to describe magnetic stimulation of the human motor cortex [2]. The information described above led to the development of the TMS. With this device, through a coil held on the scalp, magnetic fields are generated capable of inducing weak currents able to excite the underlying neural tissue. These currents cause activity in specific parts of the brain, with minimal discomfort, allowing us to study neural functions and interconnections in the intact human being. Brain stimulation techniques, as well as those of the peripheral nerve, trigger a series of events which, by depolarizing the neuron membranes, trigger the action potential. Experience from invasive stimulation during neurosurgery or epilepsy monitoring shows that stimulation parameters for the CNS are similar to those needed for peripheral nerve: short pulses with a duration of less than 1 ms and with an amplitude of few milliamperes. TMS methods for brain stimulation face the problem of delivering such a stimulus across the high resistance barrier of the periencephalic ‘layers’, including scalp, skull, meninges and cerebrospinal fluid [4]. The first brain stimulation studies were conducted using high voltage electrical stimuli through the use of electrodes placed directly on the scalp. This technique is known as transcranial electrical stimulation (TES) [5]. The TES did have the huge merit of introducing a neurophysiological technique for studying for the first time excitability and propagation properties along CNS fibers in intact and cooperative human beings [4]. However, the fields of application declined rapidly with the introduction of TMS because high-voltage TES is uncomfortable [2]. The ability of TMS to stimulate deep neural structures, such as the motor cortex, has enabled researchers to investigate the integrity of the brain to muscle pathway and the functionality of cortical networks [6]. To appreciate the potential of TMS, it is necessary to characterize the neuromuscular responses to cortical stimulation (
). The MEPs were elicited by positioning the coil tangentially to the scalp with the handle of the coil pointing backward and 45° laterally from the interhemispheric line (
).
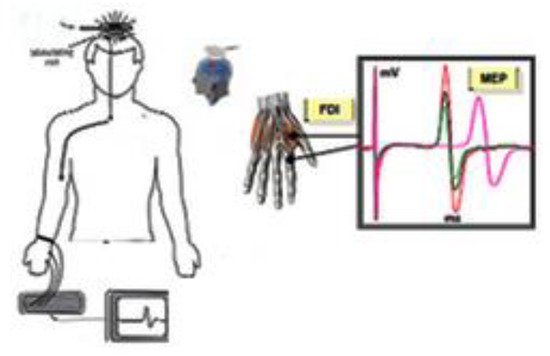
Motor Evoked Potential (MEP). Motor evoked potentials are the electrical signals recorded from the descending motor pathways or from muscles following stimulation of motor pathways within the brain.
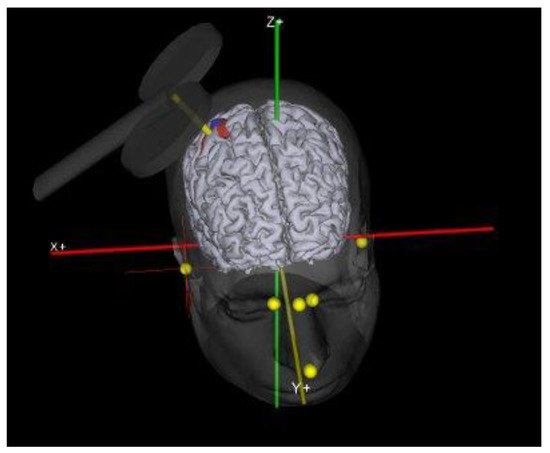
Coil Position. In this figure was show the exact coil location for motor cortex stimulation.
Since neurons connecting to muscles in distinct regions of the body have their own geographical location across the motor cortex [7], it is possible to deliver magnetic stimuli to discrete collections of neurons relating to specific muscle groups. The TMS has been used also to study the human nervous system within clinical populations [8][9][10]; mechanisms of fatigue in small, isolated muscle groups [11][12][13][14]; corticospinal contributions during human gaitand acute neural adaptations following strength training [15][16][17][18]. In neurostimulation studies using TMS, magnetic pulses are delivered directly to the motor cortex and their MEPS is recorded on the muscle by surface electromyography. The intensity of magnetic stimuli is typically given as a multiple or a percentage of the resting motor threshold, which is the intensity to evoke members of a certain amplitude in a specified fraction of a series of consecutive demonstrations in a hand muscle. However, several studies show that the resting motor threshold in humans varies from subject to subject, so to obtain significant results it would be advisable to have an adequate number of subjects involved in the studies. The resting motor threshold (rMT) is the intensity of the stimulus necessary to evoke a muscular response; the rMT is used as target intensity for the following stimulations [19]. TMS induces electrical currents in the brain via Faraday’s principle of electromagnetic induction [20]. Faraday has shown that an electrical impulse that runs through a wire wound in a coil generates a magnetic field, and the speed variation of this magnetic field causes the induction of a secondary current in a nearby conductor. This is what happens with TMS, where an electrical stimulus, which reaches peak strength and decreases to zero in a short period of time (<1ms), is sent through the conductive wiring inside the TMS coil. The rapid fluctuation of this current produces a magnetic field perpendicular to the plane of the coil that similarly rises (up to about 2.5 T) and falls rapidly in time. This rapidly fluctuating magnetic field passes unimpeded through the subject’s scalp and skull and induces a current in the brain in the opposite direction of the original current [20][21][22][23][24]. When TMS is performed with the target muscle steadily contracting, it shows different results than when the muscle is relaxed. Muscle contraction has three main effects [25]: The threshold for evoking the motor response is reduced, the latency of the MEP is shortened, and the amplitude of the MEP is markedly increased [26]. A subthreshold stimulus followed by a suprathreshold test stimulus (S1) at interstimulus interval (ISI) of 1–6 ms, the MEP generated by the S1 is inhibited and this is known as short interval intracortical inhibition (SICI). On the other hand, the MEP generated by S1 is facilitated at ISI of 8–30 ms and this is termed intracortical facilitation (ICF) [27]. The underlying mechanisms for facilitation are not entirely understood but likely include increased cortical and spinal excitability [28]. With voluntary contraction, the resting potential of the anterior horn cell (AHC) is closer to a threshold, requiring less temporal summation of descending volleys, which means that the discharge can occur at an earlier I or D wave, thus shortening the onset latency. The increase of the compound muscle action potential amplitude indicates the recruitment of a greater number of spinal motoneurons. This could also be due to increased spinal excitability, increased synchronization of spinal motoneuron firing, or an increasing number of I waves bringing more AHCs to the threshold. In the past years, the deep brain stimulation (DBS) technique has been used to investigate movement disorders. DBS involves the implantation of electrodes in certain areas of the brain. These electrodes produce electrical pulses that regulate abnormal pulses. The amount of stimulation in deep brain stimulation is controlled by a pacemaker-like device placed under the skin in the upper chest. A wire that travels under the skin connects this device to electrodes in the brain. Neuroscience investigations have revealed that DBS may be correlated to several mechanisms including functional changes with neuronal activation or inhibition, neurotransmitter release, and long-term plastic changes in target and remote areas [29]. The DBS technique is more invasive than tms; moreover, DBS is mainly indicated for the treatment of some pathologies. Future studies exploiting the combined use of TMS and DBS in patients with movement disorders could lead to new treatment strategies for these patients.
2. Cortical Excitability and Physical Exercise
In recent decades, in order to understand how the brain networks build and optimize motor programs, responsible for the different types of muscle activity and related coordination [30], numerous studies have been performed that included the use of neuroimaging and TMS [31][32]. The ability of TMS to stimulate deep neural structures, such as the motor cortex, has enabled researchers to investigate the integrity of the brain to muscle pathway and the functionality of cortical networks [33]. Since MEP are readily measurable by electromyographic recordings on peripheral muscles, the investigation of cortical excitability has become the focus of numerous studies. The brain reorganization in human is highly dependent on the specific behavioral demands of the training experience.
2.1. Skill Training
As showed by Pearce et al., highly skilled racket players show larger hand motor representation and also showed increase in MEP amplitudes compared with less proficient players and nonplaying controls [34]. Moreover, Tyc et al. show that highly skilled volleyball players showed significantly larger and more overlapping representations of medial deltoid and carpi radialis muscles, compared to runners [35]. Furthermore, TMS could be suitable for investigating the effect of acute motor exercise on the excitability of the motor pathway [36]. In fact, the augmented amplitudes of MEP have been reported as a result of acute exercise bouts, substantiating the increased neuronal excitability during fatigue.
2.2. Fatigue
In sport competition, fatigue has a large influence on performance. The term fatigue refers to any exercise inducing loss of ability to exert force or power with a muscle or a muscle group [35][36][37][38]. This phenomenon seems to be due to changes in the excitability of the motor pathway both at central and peripheral levels [39][40][41][42][43]. During the execution of maximal voluntary contractions, fatigue results from both peripheral and central factors, which play an important role in the decline of strength which results from a sub optimal output from the primary motor cortex, which ultimately leads to sub-optimal firing rates of motor neurons. On the other hand, when an incremental exhaustive exercise is performed, a rapid decrease in muscle phosphocreatine and ATP occurs and consequent accumulation of metabolites such as pyruvate and lactate [44][45][46]. There are few reports on TMS and fatigue in sports-specific motor activities.
2.3. Aerobic and Anaerobic Exercise
The first study to show the possible use of TMS in sports and various kinds of everyday exercises was undertaken by Hollge et al. [47]. This authors investigated the changes in muscle response and in central motor conduction times after aerobic (climbing stairs and jogging), and anaerobic (press-ups, dumb-bell holding, and 400 m run) exercises. Exhausting strength exercises resulted in an important decrement in muscle response measured by electromyography with an relative improvement in cortical excitability, while no significant changes were elicited by aerobic exercises [48][49][50][51][52]. Other authors [53] investigated the fatigue-induced change in the corticospinal drive to back muscles in elite rowers compared to an untrained subject. These authors found an improvement in cortical excitability in elite athletes. Recently, in different investigations, were reported that, the excitability in the primary hand motor cortex investigated with TMS, is enhanced at the end of a maximal incremental test and that this improvement strongly correlates with the increase in the blood lactate concentration [54][55]. However, recently study shows that an increase of blood lactate is correlated to an enhancement of the cortical excitability evaluated with TMS. In fact, after fatiguing hand-grip exercise, there was an increase in blood lactate with a significant decrease in rMT and MEP amplitude in a trained subject (taekwondo athletes) and in an untrained subject (non-athletes). Compared to pre-exercise values, blood lactate strongly increased at the end of exercise in each group, decline after 3’ min, and recovered to the pre-exercise value within 10 min. However, as expected, in non-athletes’ blood lactate increase strongly compared to athletes. In this investigation was showed that a voluntary sub-maximal tonic contraction is associated with a significant increase in blood lactate level. This increase in blood lactate was a consequence of the relatively small muscle mass involved in the exercise coupled to the low-level work done during grip [55][56][57][58][59][60]. Regarding the relationship between excitability and blood lactate, it has been suggested that when lactate increases due to strenuous exercise, the brain absorbs a similar amount to that of glucose. In this investigation, the reduction of rMT is maximal at the end of maximal exercise in parallel with the increase of blood lactate. Furthermore, also at the end of maximal exercise, and in parallel however non-athletes show higher depression of MEP amplitude compared to athletes at the end of exercise (−22.97% vs. −71.15%). Furthermore, in non-athletes, significant decrease emerged after 3 min of the end of exercise, while in athletes this differences disappeared. Therefore, it seems that, besides a possible role of exercise-elicited reduction of the blood flow in the cortex, the exercise-induced increase of blood lactate could be capable, in the frontal lobe, of worsening the performance in the prefrontal cortex and improving the excitability of motor cortex [61][62].
3. The Use of TMS in Sport Science
The use of the TMS for research purposes in the motor and sports field is of great interest as it is applied to investigate post-exercise facilitation, central fatigue, sensorimotor integration, motor coordination, and neuronal plasticity. For example, with TMS, it was possible to demonstrate that when a subject performs a voluntary non-maximal muscle contraction, the corticospinal path to the muscle is facilitated [63]. Additionally, other neurostimulation studies with TMS have shown greater improvement in MEP for precision movement than for general gripping tasks, and this seems to be due to greater recruitment of pyramidal neurons [64]. Instead, there are conflicting arguments regarding the facilitating effects during a voluntary contraction of the ipsilateral orneighboring homonyms muscles [32]. However, in addition to the acute effects of motor activity, long-term effects of MEP enhancement can also be appreciated. In fact, Brasi-Neto et al. show that 10-s activation could lead to post-exercise facilitation, which decayed to the baseline over 2 to 4 min [65]. Since these effects were not present after the magnetic stimulations, the researchers hypothesized that these are the changes in the intracorticular plastics. Hollge et al. (1997) were the first to apply TMS to the study of dynamic exercise [47]. Those authors found significant decreases in MEP amplitude evoked in the primary muscles associated with exhaustive 400 m running, press-ups and dumbbell holding. This decrease were described as a central failure because responses to peripheral nerve stimulation were unchanged [47]. Confirming this CNS impairment, reduced intracortical facilitation was found after pull-ups to task failure, reflecting a decreased excitability of interneuronal circuits within the motor cortex [47]. Others authors shoed reduced MEP amplitudes of both the quadriceps and diaphragm after maximal incremental treadmill exercise, with no change in the response to peripheral nerve stimulation [66]. Transcranial magnetic stimulation has also been used to assess supraspinal fatigue of small muscle groups working in isolation. Goodall et al. (2012) used TMS to evaluate supraspinal fatigue of the knee-extensor muscles in response to sustained, high-intensity cycling in normoxia and acute severe hypoxia. Cortical voluntary activation declined after exercise in both conditions, but the decline was two-fold greater in hypoxia. Recently, Moscatelli et al. (2016), investigated the relationship between blood lactate and cortical excitability in taekwondo athletes. In this study, the authors show that blood lactate seems to have a greater influence in athletes compared to untrained subjects. It seems that, during extremely intensive exercise in athletes, lactate may the onset of fatigue not only by maintaining the excitability of muscle but also by increasing the primary motor cortex excitability more than in non-athletes [30]. Collectively, these findings suggest that TMS has the potential to quantify the contribution of central processes to fatigue of limb locomotor muscles. A recent investigation showed that, after 8 weeks of aerobic training, there was a significant increase of distance covered during Cooper’s test and a significant increase of VO2max; there was also an improvement in resting motor threshold, MEP latency and ME amplitude improvement [67]. Transcranial magnetic stimulation can be used to investigate physiological states other than fatigue. For example, it is well established that neuromuscular adaptation readily occurs as a result of resistance exercise training [6]. The M1 is heavily involved in voluntary contraction of skeletal muscle and shows a high degree of plasticity, or capacity to change quickly, with motor practice [31][32][33]. In a classic example, Muellbacher et al. (2002) showed that 20 min practice of a ballistic pinching task elicited a significant improvement in task performance [68]. The improvement in task performance was accompanied by an immediate increase in the corticospinal response, demonstrating that M1 has an adaptive role in the consolidation of motor tasks (
).
TMS in physical exercise. In this table are sreported the research performed to investigated the relationship between cortical excitability and physical exercise.
| Authors | Type of Sport | Type of Exercise | Main Findings |
|---|
| Jensen et al., 2005 [28][17] | Original research | Strength training | The results of this investigation show that increased corticospinal excitability may develop over several weeks of skill training and indicate that these changes may be of importance for task acquisition. |
| Moscatelli et al., 2016 [41][30] | Original research | Karate | Karate athletes show higher corticospinal excitability compared to non athletes indicating the presence of an activity-dependent alteration in the balance and interactions between inhibitory and facilitatory circuits determining the final output from the M1 |
| Moscatelli et al., 2016 [42][31] | Original research | Karate | The practice of competitive sports affects central/peripheral nervous system. Subjects that showed higher cortical excitability showed also higher velocity at which the neural signal is propagated from the motor cortex to the muscle and consequently better reaction time. |
| Moscatelli et al., 2016 [44][33] | Original research | Taekwondo | The results of this study show that blood lactate seems to have the greater effect in taekwondo athletes compared to untrained subjects. During extremely intensive exercise in taekwondo athletes, lactate may delay the onset of fatigue not only by maintaining the excitability of muscle, but also by increasing the excitability of the primary motor cortex more than in non-athletes. |
| Tergau et al., 2000 [47][36] | Original research | Lifting | Double-pulse TMS gives access to the motor cortex independently of spinal or peripheral mechanisms, reduced Intra Cortical Facilitation reflects decreased excitability of interneuronal circuits within the motor cortex. |
| Coco et al., 2014 [52][41] | Original research | Intensive isometric exercises | The relation between blood lactate and the amplitudes of motor-evoked potentials showed a significant direct proportionality. |
| Höllgeet al., 1997 [58][47] | Original research | Aerobic and anaerobic exercise | This investigation show the possible use of TMS in sports medicine, indicating that only exhaustive or strength exercises result in reduced MEPs. |
| Ljubisavljević et al., 1996 [59][48] | Original research | submaximal isometric voluntary contraction | The increase in MEP magnitude after the sustained 60% maximal voluntary contraction may indicate residual changes in cortical activity after fatiguing contraction. |
| MaKay et al., 1996 [60][49] | Original research | Isometric maximal contraction | These results of this investigation suggest that MEP and SP might have common sources of facilitation during maximal voluntary contraction and that inhibitory mechanisms remain focally augmented following a fatiguing maximal voluntary contraction. |
| Fulton et al., 2002 [64][53] | Original research | Rowers | There were no differences in MEP depression or latency between elite rowers and non-rowers after intense exercise. The authors conclude that the smaller degree of MEP depression in the elite rowers after light exercise reflects less central fatigue within corticospinal control pathways than that seen in the non-rowers. |
| Coco et al., 2010 [70][59] | Original research | Cycling | In this study was observed that an increase of blood lactate is associated with a decrease of motor threshold, that is, an enhancement of motor cortex excitability. The authors conclude by hypothesizing that in the motor cortex the lactate could have a protective role against fatigue. |
| Moscatelli et al., 2020 [78][67] | Original resea | Aerobic exercise | This study shows that aerobic activity seems to induce changes in cortical excitability if performed for a period longer than 4 weeks, in addition to typical cardiorespiratory benefits in previously untrained males |
| Percivalle et al., 2010 [68][57] | Original research | Maximal exhausting exercise | The authors observed a similar enhancement of excitability of primary motor cortex, concomitantly with an increase of blood lactate, in both young male and female athletes. However, the improvement was significantly higher in women than in men, suggesting a greater sensitiveness of female cerebral cortex to blood lactate. |
| Cros et al., 2007 [75][64] | Original research | Isometric contraction | The timing of central conduction was different depending on functional role of the target muscle, as either agonist or joint fixator. These results indicate that the architecture of motor plans remain grossly undisrupted by cortical stimulation applied during voluntary motor behavior. |
| Brasil-Neto et al., 1994 [76][65] | Original research | Isometric and isotonic exercise | The results are similar to those found at the neuromuscular junction in myasthenia gravis and are consistent with a reduced safety factor of cortical synaptic transmission in central nervous system fatigue. |
| Verin et al., 1985 [77][66] | Original research | Incremental treadmill exercise | The results of this study confirm significant depression of both diaphragm and quadriceps MEPs after incremental treadmill exercise. |
Therefore, TMS enables a greater understanding of the behavior of the corticospinal tract in ‘top-down’ paradigms, where the effect of motor skills on corticospinal plasticity and neuromuscular adaptation can be examined. The remainder of this section will explore some potential applications of TMS for the investigation of M1 plasticity during and following different experimental paradigms, including task-specific contractions and resistance exercise training.
An interesting study was recently published that shows the effects of tDCS using the Halo Sport device on repeated sprint cycling ability and cognitive performance. The authors found that by using this device, the power delivered by repeated sprint cycles was improved. Interest in the possible ergogenic effect of noninvasive brain stimulation is growing and therefore in the future it could be useful to conduct new experiments to evaluate the impact on learning and motor performance [69][70][71].
References
- Chen, R. Intracortical Circuits and Their Interactions. In Cortical Connectivity; Springer: Heidelberg, Germany, 2012; p. 65. ISBN 9783642327667.
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107.
- Rotenberg, A. Prospects for clinical applications of transcranial magnetic stimulation and real-time EEG in epilepsy. Brain Topogr. 2010, 22, 257–266.
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di, I.R.; Di, V.L.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee 3. Clin.Neurophysiol. 2015, 126, 1071–1107.
- Merton, P.A.; Morton, H.B. Stimulation of the cerebral cortex in the intact human subject. Nature 1980, 285, 227.
- Goodall, S.; Howatson, G.; Romer, L.; Ross, E. Transcranial magnetic stimulation in sport science: A commentary. Eur. J. Sport Sci. 2014, 14 (Suppl. S1), S332–S340.
- Penfield, W.; Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937, 60, 389–443.
- Hallett, M. Transcranial magnetic stimulation: A useful tool for clinical neurophysiology. Ann. Neurol. 1996, 40, 344–345.
- Hallett, M.; Rothwell, J. Milestones in clinical neurophysiology. Mov. Disord. 2011, 26, 958–967.
- Rothwell, J.C. Using transcranial magnetic stimulation methods to probe connectivity between motor areas of the brain. Hum. Mov. Sci. 2011, 30, 906–915.
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789.
- Taylor, J.L.; Gandevia, S.C. Transcranial magnetic stimulation and human muscle fatigue. Muscle Nerve 2001, 24, 18–29.
- Taylor, J.L.; Gandevia, S.C. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J. Appl. Physiol. 2008, 104, 542–550.
- Viggiano, A.; Chieffi, S.; Tafuri, D.; Messina, G.; Monda, M.; De Luca, B. Laterality of a second player position affects lateral deviation of basketball shooting. J. Sports Sci. 2014, 32, 46–52.
- Carroll, T.J.; Riek, S.; Carson, R.G. Corticospinal responses to motor training revealed by transcranial magnetic stimulation. Exerc. Sport Sci. Rev. 2001, 29, 54–59.
- Gruber, M.; Linnamo, V.; Strojnik, V.; Rantalainen, T.; Avela, J. Excitability at the motoneuron pool and motor cortex is specifically modulated in lengthening compared to isometric contractions. J. Neurophysiol. 2009, 101, 2030–2040.
- Jensen, J.L.; Marstrand, P.C.D.; Nielsen, J.B. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J. Appl. Physiol. 2005, 99, 1558–1568.
- Messina, G.; Monda, V.; Moscatelli, F.; Valenzano, A.A.; Monda, G.; Esposito, T.; Blasio, S.D.; Messina, A.; Tafuri, D.; Rosaria, M.; et al. Role of Orexin System in Obesity. Biol. Med. 2015, 7, 1–6.
- Wassermann, E.M. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, 5–7 June 1996. Electroencephalogr. Clin. Neurophysiol. 1998, 108, 1–16.
- Rotenberg, A.; Horvath, J.C.; Pascual-Leone, A. The Transcranial Magnetic Stimulation (TMS) Device and Foundational Techniques. In Transcranial Magnetic Stimulation; Humana Press: New York, NY, USA, 2014; pp. 3–13.
- Kobayashi, M.; Pascual-Leone, A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003, 2, 145–156.
- Hallett, M. Transcranial magnetic stimulation and the human brain. Nature 2000, 406, 147–150.
- Triggiani, A.I.; Valenzano, A.; Ciliberti, M.A.P.; Moscatelli, F.; Villani, S.; Monda, M.; Messina, G.; Federici, A.; Babiloni, C.; Cibelli, G. Heart rate variability is reduced in underweight and overweight healthy adult women. Clin. Physiol. Funct. Imaging 2015, 37, 162–167.
- Messina, A.; De Fusco, C.; Monda, V.; Esposito, M.; Moscatelli, F.; Valenzano, A.; Carotenuto, M.; Viggiano, E.; Chieffi, S.; De Luca, V.; et al. Role of the Orexin System on the Hypothalamus-Pituitary-Thyroid Axis. Front. Neural Circuits 2016, 10, 66.
- Kernell, D.; Chien-Ping, W. Post-synaptic effects of cortical stimulation on forelimb motoneurones in the baboon. J. Physiol. 1967, 191, 673–690.
- Thompson, P.D.; Day, B.L.; Rothwell, J.C.; Dressler, D.; de Noordhout, A.M.; Marsden, C.D. Further observations on the facilitation of muscle responses to cortical stimulation by voluntary contraction. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials 1991, 81, 397–402.
- Kiers, L.; Fernando, B.; Tomkins, D. Facilitatory effect of thinking about movement on magnetic motor-evoked potentials. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control 1997, 105, 262–268.
- Wilson, S.A.; Thickbroom, G.W.; Mastaglia, F.L. An investigation of the late excitatory potential in the hand following magnetic stimulation of the motor cortex. Electroencephalogr. Clin. Neurophysiol. Electromyogr. 1995, 97, 55–62.
- Udupa, K.; Chen, R. The mechanisms of action of deep brain stimulation and ideas for the future development. Prog. Neurobiol. 2015, 133, 27–49.
- Moscatelli, F.; Messina, G.; Valenzano, A.; Monda, V.; Viggiano, A.; Messina, A.; Petito, A.; Triggiani, A.I.; Ciliberti, M.A.P.; Monda, M.; et al. Functional Assessment of Corticospinal System Excitability in Karate Athletes. PLoS ONE 2016, 11, e0155998.
- Moscatelli, F.; Messina, G.; Valenzano, A.; Petito, A.; Triggiani, A.I.; Messina, A.; Monda, V.; Viggiano, A.; De Luca, V.; Capranica, L.; et al. Differences in corticospinal system activity and reaction response between karate athletes and non-athletes. Neurol. Sci. 2016, 37, 1947–1953.
- Slovenica, K. Transcranial magnetic stimulation offers new possibilities for the study of motor control transkranialnamagnetna. 2004, 104, 78–104.
- Moscatelli, F.; Valenzano, A.; Petito, A.; IvanoTriggiani, A.; Anna Pia Ciliberti, M.; Luongo, L.; Carotenuto, M.; Esposito, M.; Messina, A.; Monda, V.; et al. Relationship between blood lactate and cortical excitability between taekwondo athletes and non-athletes after hand-grip exercise. Somatosens. Mot. Res. 2016, 33, 137–144.
- Pearce, A.J.; Thickbroom, G.W.; Byrnes, M.L.; Mastaglia, F.L. Functional reorganisation of the corticomotor projection to the hand in skilled racquet players. Exp. Brain Res. 2000, 130, 238–243.
- Tyc, F.; Boyadjian, A.; Devanne, H. Motor cortex plasticity induced by extensive training revealed by transcranial magnetic stimulation in human. Eur. J. Neurosci. 2005, 21, 259–266.
- Tergau, F.; Geese, R.; Bauer, A.; Baur, S.; Paulus, W.; Reimers, C.D. Motor cortex fatigue in sports measured by transcranial magnetic double stimulation. Med. Sci. Sports Exerc. 2000, 32, 1942–1948.
- Valenzano, A.; Moscatelli, F.; Triggiani, A.I.; Capranica, L.; De Ioannon, G.; Piacentini, M.F.; Mignardi, S.; Messina, G.; Villani, S.; Cibelli, G. Heart-Rate Changes After an Ultraendurance Swim From Italy to Albania: A Case Report. Int. J. Sports Physiol. Perform. 2016, 11, 407–409.
- Messina, G.; AValenzano, A.; Moscatelli, F.; Triggiani, A.I.; Capranica, L.; Messina, A.; Piombino, L.; Tafuri, D.; Cibelli, G.; Monda, M. Effects of Emotional Stress on Neuroendocrine and Autonomic Functions in Skydiving. J. Psychiatry 2015, 18, 1–7.
- Messina, G.; Palmieri, F.; Monda, V.; Messina, A.; Dalia, C.; De Luca, V. Exercise Causes Muscle GLUT4 Translocation in an Insulin-Independent Manner. Biol. Med. 2015, 1, 7.
- Moscatelli, F.; Messina, G.; Valenzano, A.; Petito, A.; Triggiani, A.I.; Ciliberti, M.A.P.; Monda, V.; Messina, A.; Tafuri, D.; Capranica, L.; et al. Relationship between RPE and Blood Lactate after Fatiguing Handgrip Exercise in Taekwondo and Sedentary Subjects. Biol. Med. 2015.
- Coco, M.; Alagona, G.; Perciavalle, V.; Perciavalle, V.; Cavallari, P.; Caronni, A. Changes in cortical excitability and blood lactate after a fatiguing hand-grip exercise. Somatosens. Mot. Res. 2014, 31, 35–39.
- Coaccioli, S.; Varrassi, G.; del Giorno, R.; Pace, M.C.; Sansone, P.; Angelucci, D.; Paladini, A.; Moscatelli, F.; Messina, A.; Monda, V.; et al. Meditation as a Useful Chance for Chronic Pain Decrease. J. Psychiatry 2016, 19.
- Chieffi, S.; Iachini, T.; Iavarone, A.; Messina, G.; Viggiano, A.; Monda, M. Flanker interference effects in a line bisection task. Exp. Brain Res. 2014, 232, 1327–1334.
- Bangsbo, J. Quantification of anaerobic energy production during intense exercise. Med. Sci. Sports Exerc. 1998, 30, 47–52.
- Chieffi, S.; Iavarone, A.; Iaccarino, L.; La Marra, M.; Messina, G.; De Luca, V.; Monda, M. Age-related differences in distractor interference on line bisection. Exp. Brain Res. 2014, 232, 3659–3664.
- Viggiano, E.; Monda, V.; Messina, A.; Moscatelli, F.; Valenzano, A.; Tafuri, D.; Cibelli, G.; De Luca, B.; Messina, G.; Monda, M. Cortical spreading depression produces a neuroprotective effect activating mitochondrial uncoupling protein-5. Neuropsychiatr. Dis. Treat. 2016, 12, 1705–1710.
- Höllge, J.; Kunkel, M.; Ziemann, U.; Tergau, F.; Geese, R.; Reimers, C.D. Central fatigue in sports and daily exercises. A magnetic stimulation study. Int. J. Sports Med. 1997, 18, 614–617.
- Ljubisavljević, M.; Milanović, S.; Radovanović, S.; Vukčević, I.; Kostić, V.; Anastasijević, R. Central changes in muscle fatigue during sustained submaximal isometric voluntary contraction as revealed by transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control 1996, 101, 281–288.
- McKay, W.B.; Stokic, D.S.; Sherwood, A.M.; Vrbova, G.; Dimitrijevic, M.R. Effect of fatiguing maximal voluntary contraction on excitatory and inhibitory responses elicited by transcranial magnetic motor cortex stimulation. Muscle Nerve. 1996, 19, 1017–1024.
- Messina, G.; De Luca, V.; Viggiano, A.; Ascione, A.; Iannaccone, T.; Chieffi, S.; Monda, M. Autonomic nervous system in the control of energy balance and body weight: Personal contributions. Neurol. Res. Int. 2013, 1–5.
- Monda, M.; Messina, G.; Scognamiglio, I.; Lombardi, A.; Martin, G.A.; Sperlongano, P.; Porcelli, M.; Caraglia, M.; Stiuso, P. Short-term diet and moderate exercise in young overweight men modulate cardiocyte and hepatocarcinoma survival by oxidative stress. Oxid. Med. Cell. Longev. 2014, 2014.
- Piombino, L.; Messina, A. An Assessment of Body Composition and Lifestyle in Children Aged from 8 to 10 years. Biol. Med. 2016, 8.
- Fulton, R.C.; Strutton, P.H.; McGregor, A.H.; Davey, N.J. Fatigue-induced change in corticospinal drive to back muscles in elite rowers. Exp. Physiol. 2002, 87, 593–600.
- Moscatelli, F.; Valenzano, A.; Monda, V.; Ruberto, M.; Monda, G.; Triggiani, A.I.; Monda, E.; Chieffi, S.; Villano, I.; Parisi, L.; et al. Transcranial Magnetic Stimulation (TMS) application in sport medicine: A brief review. Acta Med. Mediterr. 2017, 33, 423–430.
- Viggiano, A.; Nicodemo, U.; Viggiano, E.; Messina, G.; Viggiano, A.; Monda, M.; De Luca, B. Mastication overload causes an increase in O 2—Production into the subnucleusoralis of the spinal trigeminal nucleus. Neuroscience 2010, 166, 416–421.
- Monda, V.; Valenzano, A.; Moscatelli, F.; Messina, A.; Piombino, L.; Zannella, C.; Viggiano, E.; Monda, G.; De Luca, V.; Chieffi, S.; et al. Modifications of Activity of Autonomic Nervous System, and Resting Energy Expenditure in Women Using Hormone-Replacement Therapy. Biol. Med. 2016, 8.
- Perciavalle, V.; Coco, M.; Alagona, G.; Maci, T. Gender differences in changes of motor cortex excitability during elevated blood lactate levels. Somatosens. Mot. Res. 2010, 27, 106–110.
- Alagona, G.; Coco, M.; Rapisarda, G.; Costanzo, E.; Maci, T.; Restivo, D.; Maugeri, A.; Perciavalle, V. Changes of blood lactate levels after repetitive transcranial magnetic stimulation. Neurosci. Lett. 2009, 450, 111–113.
- Coco, M.; Alagona, G.; Rapisarda, G.; Costanzo, E.; Calogero, R.A.; Perciaevalle, V.; Perciavalle, V. Elevated blood lactate is associated with increased motor cortex excitability. Somatosens. Mot. Res. 2010, 27, 1–8.
- Di Bernardo, G.; Messina, G.; Capasso, S.; Del Gaudio, S.; Cipollaro, M.; Peluso, G.; Casale, F.; Monda, M.; Galderisi, U. Sera of overweight people promote in vitro adipocyte differentiation of bone marrow stromal cells. Stem Cell Res. Ther. 2014, 5, 4.
- Messina, G.; Dalia, C.; Tafuri, D.; Monda, V.; Palmieri, F.; Dato, A.; Russo, A.; De Blasio, S.; Messina, A.; De Luca, V.; et al. Orexin-A controls sympathetic activity and eating behavior. Front. Psychol. 2014, 5, 997.
- Rinaldi, B.; Guida, F.; Furiano, A.; Donniacuo, M.; Luongo, L.; Gritti, G.; Urbanek, K.; Messina, G.; Maione, S.; Rossi, F.; et al. Effect of Prolonged Moderate Exercise on the Changes of Nonneuronal Cells in Early Myocardial Infarction. Neural Plast. 2015, 2015, 265967.
- Rothwell, J.C.; Thompson, P.D.; Day, B.L.; Boyd, S.; Marsden, C.D. Stimulation of the human motor cortex through the scalp. Exp. Physiol. 1991, 76, 159–200.
- Cros, D.; Soto, O.; Chiappa, K.H. Transcranial magnetic stimulation during voluntary action: Directional facilitation of outputs and relationships to force generation. Brain Res. 2007, 1185, 103–116.
- Brasil-Neto, J.P.; Cohen, L.G.; Hallett, M. Central fatigue as revealed by postexercise decrement of motor evoked potentials. Muscle Nerve 1994, 17, 713–719.
- Verin, E.; Ross, E.; Demoule, A.; Hopkinson, N.; Nickol, A.; Fauroux, B.; Moxham, J.; Similowski, T.; Polkey, M.I. Effects of exhaustive incremental treadmill exercise on diaphragm and quadriceps motor potentials evoked by transcranial magnetic stimulation. J. Appl. Physiol. 2004, 96, 253–259.
- Moscatelli, F.; Messina, G.; Valenzano, A.; Triggiani, A.I.; Sessa, F.; Carotenuto, M.; Tartaglia, N.; Ambrosi, A.; Cibelli, G.; Monda, V. Effects of 12 weeks’ aerobic training on motor cortex excitability. J. Sports Med. Phys. Fit. 2020, 60, 1383–1389.
- Muellbacher, W.; Ziemann, U.; Wissel, J.; Dang, N.; Kofler, M.; Facchini, S.; Boroojerdi, B.; Poewe, W.; Hallett, M. Early consolidation in human primary motor cortex. Nature 2002, 415, 640–644.
- Bocci, T.; Caleo, M.; Tognazzi, S.; Francini, N.; Briscese, L.; Maffei, L.; Rossi, S.; Priori, A.; Sartucci, F. Evidence for metaplasticity in the humanvisualcortex. J. Neural Transm. 2014, 121, 221–231.
- Ziemann, U.; Paulus, W.; Nitsche, M.A.; Pascual-Leone, A.; Byblow, W.D.; Berardelli, A.; Siebner, H.R.; Classen, J.; Cohen, L.G.; Rothwell, J.C. Consensus: Motorcortexplasticityprotocols. Brain Stimul. 2008, 1, 164–182.
- Lingyan, H.; Yuqin, D.; Xinyan, Z.; Yu, L. Transcranial direct current stimulation with halo sport enhances repeated sprint cycling and cognitive performance. Front. Physiol. 2019, 10, 118.
