High-grade glioma (HGG)'s treatment development is hampered by (1) the blood–brain barrier (BBB), (2) an infiltrative growth pattern, (3) rapid development of therapeutic resistance, and, in many cases, (4) dose-limiting toxicity due to systemic exposure. Convection-enhanced delivery (CED) has the potential to significantly limit systemic toxicity and increase therapeutic index by directly delivering homogenous drug concentrations to the site of disease.
- glioblastoma
- high-grade glioma
- refractory glioma
- convection enhanced delivery
- neuro-oncology
- refractory glioblastoma
1. Introduction
Glioblastoma (GBM) is the commonest malignant brain tumor of primary origin. Despite an annual incidence of less than 10 per 100,000 people worldwide, this aggressive and invasive glioma has a devastating prognosis with a current 1-year survival rate of 42.8% and 5-year survival rate at an abysmal 7.2% [1]. Often characterized as molecularly heterogenous tumors, the recent c-IMPACT-now consensus classified GBM as isocitrate dehydrogenase (IDH)-wild type WHO grade IV glioma with microvascular proliferation, necrotic lesions, or possessing > 1 of the following genetic alterations: TERT promoter mutation, EGFR gene amplification, +7/−10 chromosome copy number changes [2][3]. The current standard of care consists of maximally safe surgical resection with concurrent radiochemotherapy followed by at least 6 months of chemotherapy [4]. Complete resection of malignant tissue is limited by the highly invasive nature of this malignancy, thereby creating a reliance to chemotherapeutic options such as temozolomide, which have traditionally been delivered systemically. The restrictive blood–brain barrier (BBB) and toxicities from systemic delivery have limited therapeutic options.
2. Convection Enhanced Delivery
Developed in the 1990s by Bobo et al., CED is a unique infusion technique whereby pressure-driven catheters are utilized to drive infusate directly into a localized area of parenchymal tissue within the central nervous system (CNS) [5]. This approach focuses on establishing a pressure rather than concentration gradient to enhance therapeutic delivery. Traditional infusion techniques rely on diffusion primarily, necessitating large concentration gradients to maximize molar flow as per Fick’s law. Alternatively, generating a pressure gradient allows infusate to be distributed via bulk flow, where molar velocity is directly proportional to the pressure gradient as per Darcy’s law. Clinical application involves stereotactically siting catheters via burr holes into malignant CNS tissues under magnetic resonance imaging (MRI) guidance. Catheters are then connected to extra-cranial infusion pumps to establish a pressure gradient at the tips, distributing infusate into the tumor bulk by convective transport regardless of molecular size. Thus, therapeutic agents can penetrate tissues in the order of centimeters within a pseudo-spherical distribution from the catheter tip as opposed to only a few millimeters in diffusion-dependent infusion modalities [6]. Due to the steep pressure gradient generated, CED also increases peri-tumoral interstitial fluid flow (IFF), which has been associated with altered molecular transport and extracellular matrix (ECM) rearrangement. Computational modeling of IFF has thus been explored with varying success in light of its notable implications in predicting molecular transport and maximizing therapeutic delivery [7].
By avoiding systemic exposure, the maximum tolerated dose is greater if delivered by CED compared to systemic delivery [8][9][10]. In spite of this, CED usually does not require as high a concentration as stereotactic injection to attain therapeutic levels in affected parenchyma [6]. However, this procedure is not without its caveats. The most common limitations undermining previous CED trials consist of infusate reflux around the perimeter of catheters, suboptimal catheter placement, use of non-specialized catheters, an inability to trace drug substances, and risks relating to placing catheters (~1% risk of hemorrhage/catheter placed; ~5% risk of infection). Recent modifications have addressed these issues and led to considerable improvements in pharmacokinetic and infusion parameters. Notable adaptations involve co-administering drug products with contrast-enhancing agents, utilizing computer software to optimize catheter placement, and design of specialized CED catheters to prevent infusate reflux and leakage. An example of an infusion performed by CED visualized on MRI with co-convected contrast is shown in Figure 1.
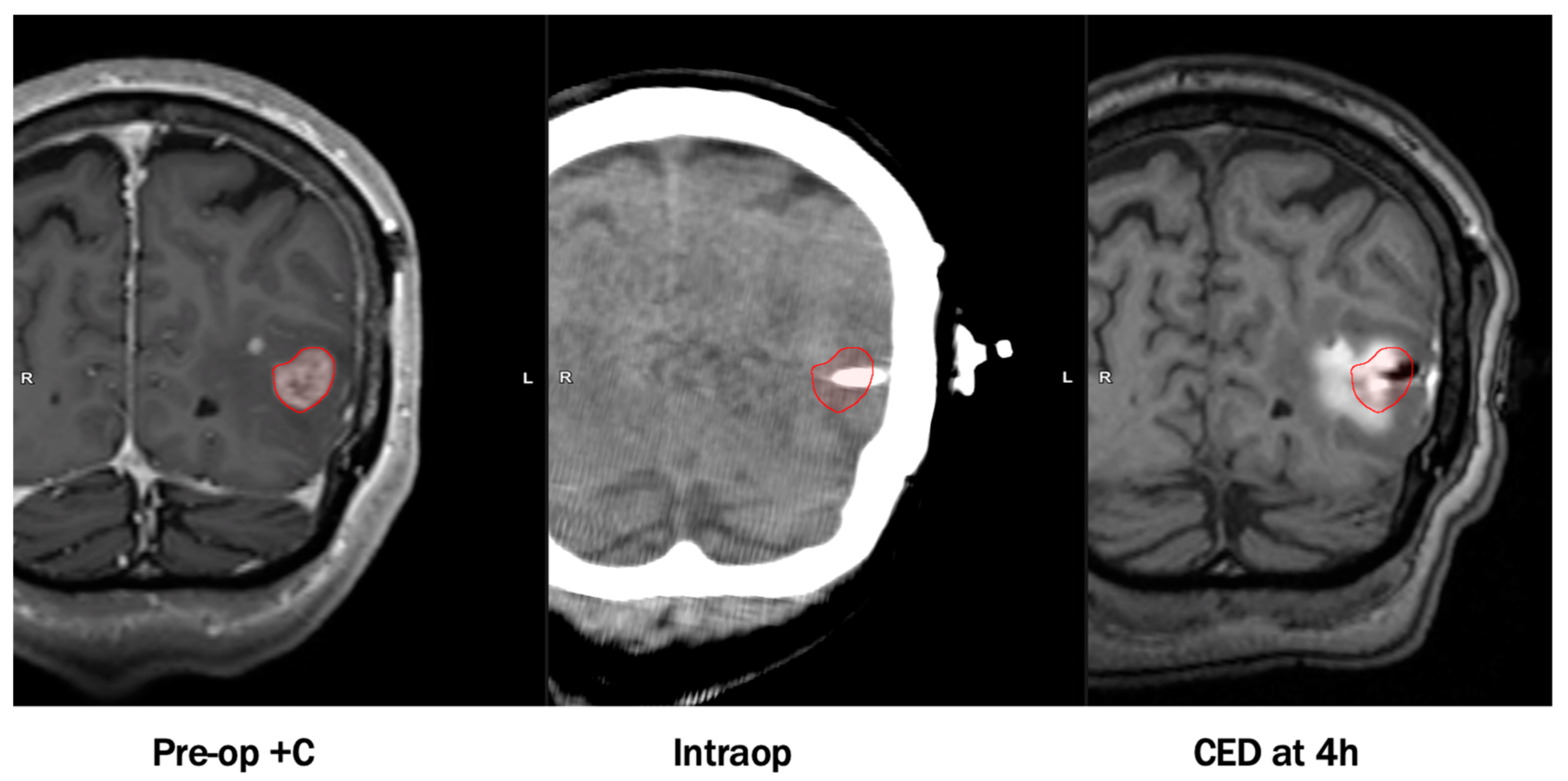
An example of an infusion performed by convection-enhanced delivery (CED) visualized on magnetic resonance imaging (MRI) with co-convected contrast. Images were obtained with permission from Dr. Michael Vogelbaum and Moffitt Cancer Center.
CED trial results have provided further insight into the efficacy and adverse effects possibly associated with this delivery method.
References
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro. Oncol. 2020, 22, iv1–iv96.
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820.
- Louis, D.N.; Wesseling, P.; Aldape, K.; Brat, D.J.; Capper, D.; Cree, I.A.; Eberhart, C.; Figarella-Branger, D.; Fouladi, M.; Fuller, G.N.; et al. cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020, 30, 844–856.
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996.
- Hunt Bobo, R.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080.
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371.
- Stine, C.A.; Munson, J.M. Convection-Enhanced Delivery: Connection to and Impact of Interstitial Fluid Flow. Front. Oncol. 2019, 9, 966.
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200.
- Stukel, J.M.; Caplan, M.R. Targeted drug delivery for treatment and imaging of glioblastoma multiforme. Expert Opin. Drug Deliv. 2009, 6, 705–718.
- Kawakami, K.; Kawakami, M.; Kioi, M.; Husain, S.R.; Puri, R.K. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J. Neurosurg. 2004, 101, 1004–1011.
