Paraprobiotics are defined as “inactivated microbial cells (non-viable) that confer a health benefit to the consumer” and hold the ability to regulate the adaptive and innate immune systems, to exhibit anti-inflammatory, antiproliferative and antioxidant properties, and to exert antagonistic effect against pathogens, thus confirming that viability of probiotics is not an absolute pre-requisite for promoting health effects.
The use of paraprobiotics allows to overcome several drawbacks related to the use of probiotics. In fact, paraprobiotics can exhibit technological and practical benefits, such as longer shelf life, since the cold chain is not required for microbial viability and stability. In addition, products containing paraprobiotics may show enhanced safety, i.e., a reduced risk of sepsis and antibiotic resistance, making them suitable also for vulnerable people, such as elderly and immunocompromised individuals.
Due to these important features, paraprobiotics open new perspectives in the design of novel functional foods and nutraceuticals that are safer and significantly simplify industrial handling and marketing.
Paraprobiotics are defined as “inactivated microbial cells (non-viable) that confer a health benefit to the consumer” and hold the ability to regulate the adaptive and innate immune systems, to exhibit anti-inflammatory, antiproliferative and antioxidant properties, and to exert antagonistic effect against pathogens, thus confirming that viability of probiotics is not an absolute pre-requisite for promoting health effects.
- paraprobiotics
- probiotics
- dairy foods
- non-dairy food
- health benefits
- microbial inactivation
- immunomodulation
1. Introduction
Since the first observation by Metchnikoff more than 100 years ago, the popularity of probiotics boosted substantially. In the last decades, particularly in the last five years, a large body of experimental and clinical evidence on the health benefits of probiotics has appeared [1]. Their biological effects include disease treatment (i.e., restoration of health), disease prevention (i.e., preservation of health) and health “optimization” [2]. The ongoing interest in probiotic bacteria goes hand in hand with a rapid and lucrative expansion of the sector of functional foods and supplements containing these bacteria. However, a rigorous evaluation and validation of health and/or functionality claims along with safety and practical use aspects remains a critical issue for the field of probiotic and functional food [3].
According to the earlier revised definition by the International Scientific Association for Probiotics and Prebiotics (ISAPP), probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [4]. Thus, probiotic cultures should be formulated in such a way that they can reach the target site in the host after surviving throughout processing, storage and gastrointestinal transit while remaining highly viable and in sufficient numbers. Nevertheless, there is still no consensus what an adequate intake of live microorganisms is [1,5]. Moreover, concerns about probiotic adverse effects, especially for at-risk groups, such as immunocompromised individuals, people with an abnormal gastrointestinal mucosal barrier, patients following surgical treatments or premature newborns, have been raised. If present in high concentration, probiotics can negatively influence the balance between anti- and proinflammatory cytokines as well as other cellular functions, causing altered long-term immune responses in subjects with immune disorders [6,7]. The European Food Safety Authority (EFSA) has registered cases of
According to the earlier revised definition by the International Scientific Association for Probiotics and Prebiotics (ISAPP), probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [4]. Thus, probiotic cultures should be formulated in such a way that they can reach the target site in the host after surviving throughout processing, storage and gastrointestinal transit while remaining highly viable and in sufficient numbers. Nevertheless, there is still no consensus what an adequate intake of live microorganisms is [1][5]. Moreover, concerns about probiotic adverse effects, especially for at-risk groups, such as immunocompromised individuals, people with an abnormal gastrointestinal mucosal barrier, patients following surgical treatments or premature newborns, have been raised. If present in high concentration, probiotics can negatively influence the balance between anti- and proinflammatory cytokines as well as other cellular functions, causing altered long-term immune responses in subjects with immune disorders [6][7]. The European Food Safety Authority (EFSA) has registered cases of
Lactobacillus rhamnosus
sepsis associated with probiotic therapy. A study regarding 89 patients with
Lactobacillus
bacteremia reported the mortality rate of 26% within 1 month and 48% within 1 year following infection onset. Even if rare, endocarditis due to
Lactobacillus
infection results in a high mortality rate, averaging 30%. A randomized controlled trial Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA) highlighted a significantly increased mortality (16% vs. 6%) due to bowel ischemia (9 vs. 0) among severe acute pancreatitis patients subjected to probiotic administration [8]. A systematic review of articles published between 1976 and 2018 pointed out 93 cases of patients who developed infections as a consequence of probiotic ingestion.
Saccharomyces
was the most frequent genus with 47 cases, followed by
Lactobacillus
(26 cases),
Bifidobacterium
(12 cases),
Bacillus
(5 cases),
Pediococcus
(2 cases) and
Escherichia (1 cases), respectively [9]. Bacteremia and fungemia represent the most frequently reported ailments, but the list is set to expand in the near future. The possible horizontal transfer of genes from pathogenic bacteria in the gut is another critical issue due to the risk of development and spread of virulence traits and antibiotic resistance [1,10,11,12]. Other doubts could be raised about the probiotic mechanism of action, their strain-specific properties and their being in competition with commensal gut microflora for colonization [13]. Viability and safety are relevant challenges for the probiotic industry. The scientific community and regulators ought to clear up doubts surrounding probiotic preparations, especially considering that the next generation of probiotics comprising new species being used for this intended purpose without a long history of use (i.e.
(1 cases), respectively [9]. Bacteremia and fungemia represent the most frequently reported ailments, but the list is set to expand in the near future. The possible horizontal transfer of genes from pathogenic bacteria in the gut is another critical issue due to the risk of development and spread of virulence traits and antibiotic resistance [1][10][11][12]. Other doubts could be raised about the probiotic mechanism of action, their strain-specific properties and their being in competition with commensal gut microflora for colonization [13]. Viability and safety are relevant challenges for the probiotic industry. The scientific community and regulators ought to clear up doubts surrounding probiotic preparations, especially considering that the next generation of probiotics comprising new species being used for this intended purpose without a long history of use (i.e.
Akkermansia muciniphila
,
Faecalibacterium
and
Bacteroides species) will keep being launched more and more often [1,14].
species) will keep being launched more and more often [1][14].
All these drawbacks related to the administration of viable microorganisms led to the interest in non-viable probiotic preparations. Since 2004, increasing evidence has been suggesting that some health benefits of physiologically active bacteria are not strictly associated with their viability. In fact, probiotic products also contain dead cells, which can produce a biological response as effectively as their live equivalents, highlighting the fact that probiotic products may be further used beyond their expiry. This is called the “probiotic paradox” (or, as some authors have suggested, the “probiotic advantage”), i.e., both live and dead cells can produce a biological response. Though there may be a potential benefit from the consumption of dead microorganisms, they cannot be classified as probiotic [15]. Hence, the term “paraprobiotic” together with a wide range of synonyms has been coined. According to the most recurrent definition, paraprobiotics, also known as non-viable probiotics, inactivated probiotics, tyndallized probiotics or ghost probiotics, are “non-viable microbial cells (either intact or broken), or crude cell extracts, which, when administered (orally or topically) in adequate amounts, confer a benefit on the human or animal consumer” [13,16].
All these drawbacks related to the administration of viable microorganisms led to the interest in non-viable probiotic preparations. Since 2004, increasing evidence has been suggesting that some health benefits of physiologically active bacteria are not strictly associated with their viability. In fact, probiotic products also contain dead cells, which can produce a biological response as effectively as their live equivalents, highlighting the fact that probiotic products may be further used beyond their expiry. This is called the “probiotic paradox” (or, as some authors have suggested, the “probiotic advantage”), i.e., both live and dead cells can produce a biological response. Though there may be a potential benefit from the consumption of dead microorganisms, they cannot be classified as probiotic [15]. Hence, the term “paraprobiotic” together with a wide range of synonyms has been coined. According to the most recurrent definition, paraprobiotics, also known as non-viable probiotics, inactivated probiotics, tyndallized probiotics or ghost probiotics, are “non-viable microbial cells (either intact or broken), or crude cell extracts, which, when administered (orally or topically) in adequate amounts, confer a benefit on the human or animal consumer” [13][16].
Although the molecular mechanisms underlying paraprobiotic action still need a thorough investigation, scientific evidence has shown that, similarly to probiotics, molecules present on the cell surface (peptidoglycan, teichoic acid, cell wall polysaccharides, cell surface-associated proteins, etc.) could constitute the first line of interaction between paraprobiotics and the host, thus mediating the beneficial effects [17].
Paraprobiotics have been proven to modulate anti-inflammatory and positive immune responses in animals and humans, with some advantages if compared to probiotics. Non-viable microbial cells may exhibit enhanced safety, i.e., reduced risk of sepsis and antibiotic resistance, as well as technological and practical benefits, i.e., longer shelf life, since the cold chain is not required for microorganism viability and stability. These features also enable their application in underdeveloped regions [7,18].
Paraprobiotics have been proven to modulate anti-inflammatory and positive immune responses in animals and humans, with some advantages if compared to probiotics. Non-viable microbial cells may exhibit enhanced safety, i.e., reduced risk of sepsis and antibiotic resistance, as well as technological and practical benefits, i.e., longer shelf life, since the cold chain is not required for microorganism viability and stability. These features also enable their application in underdeveloped regions [7][18].
Another great advantage is no loss of bioactivity when administered in combination with antibiotics or antifungal agents [19]. Killed probiotics also offer an attractive solution to overcome problems correlated to formulation of the food matrix [20]. Furthermore, research on gut microbiota brought about newly recognized bacteria from the gastrointestinal tract providing beneficial effects for human physiology, as mentioned above, and paraprobiotic preparations could be useful for solving complications related to stability during commercialization and safety of these next-generation probiotics since they are often strictly anaerobic bacteria; thus, their production and stability represent major challenges. Interestingly, besides the most studied probiotic genera
Lactobacillus
and
Bifidobacteria
that have been awarded the GRAS (generally recognized as safe) and QPS (qualified presumption of safety) status for intentional addition to food and feed by the FDA and EFSA respectively [11], other probiotic agents (e.g.
Escherichia coli
,
Bacillus
,
Saccharomyces
) and next-generation probiotics that need to be studied for their safety profile (
Faecalibacterium prausnitzii
and other members of Ruminococcaceae,
Bacteroides
,
Clostridium
XIVa cluster bacteria, and
Akkermansia spp.) are emerging [13,20]. There is also evidence about anti-inflammatory and anti-allergic activity exerted by acetic acid bacteria in foods (e.g., nata de coco, kombucha and fermented milk), but it is not clear whether the live or dead cells are responsible for these beneficial effects [21].
spp.) are emerging [13][20]. There is also evidence about anti-inflammatory and anti-allergic activity exerted by acetic acid bacteria in foods (e.g., nata de coco, kombucha and fermented milk), but it is not clear whether the live or dead cells are responsible for these beneficial effects [21].
An overview of the current state of the scientific literature setting “paraprobiotics” or “inactivated probiotics” as search terms, which revealed a hike in the number of articles published in the last ten years in different research areas, helps us to realize the growing interest in the inactivated microbial cells (
).
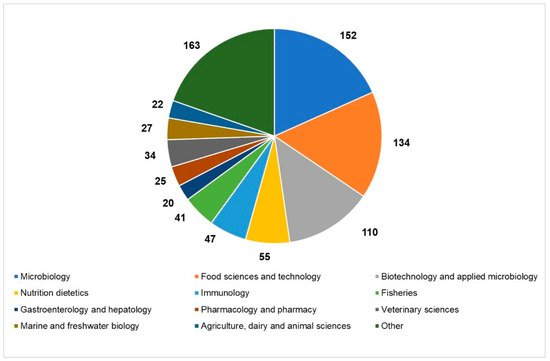
Current state of the scientific literature on paraprobiotics: distribution of papers published in the last 20 years in the main research areas (source: Web of Science; 2011–2020; updated to 22 December 2020).
2. Technological Features in the Production of Paraprobiotics
Industrial processing and storage of probiotic products still represent technological challenges as these could severely impair probiotic cell viability, putatively a key requisite for the probiotic effects. As a matter of fact, probiotic products actually contain viable and non-viable cells that could both contribute to the beneficial effects on human health [22][23][24]. On the other hand, several concerns have been raised for the functionality and safety of live microorganisms in foods, especially when administered to vulnerable people, such as the elderly and immunodeficient individuals [25].
The use of paraprobiotics allows overcoming several of these drawbacks and opens up new perspectives in the design of novel functional foods, significantly simplifying industrial handling and marketing. In fact, paraprobiotics could be added to several foods that, due to their chemical or physical properties, do not offer a suitable environment for the survival of probiotics (such as fruit juice). Furthermore, paraprobiotics do not directly interact with food matrices and do not modify their organoleptic features [26]. In addition, they could be added before thermal processing of food without completely impairing health-promoting features while assuring food safety and are not affected by antibiotic treatments [27].
On the other hand, it should be taken into consideration that inactivated cells should be unable to produce secreted metabolites (such as bacteriocins, lactic acid, vitamins, etc.) and enzymes which could have a relevant role in the probiotic health effects.
Due to these features, novel foods containing paraprobiotics are less affected by storage and transport conditions, thus guaranteeing a prolonged shelf life and assuring economic advantages. These points could offer an important opportunity to prompt the functional food market with safer and more stable products.
Inactivation of probiotic cells can be achieved using physical or chemical treatments capable of modifying microbial cell components (cell membranes and envelopes, proteins, DNA, etc.) and physiological functions (enzyme activities and membrane selectivity) without completely destroying the cell structure.
Conventional and emerging technologies for the production of paraprobiotics comprehensively reviewed by de Almada et al. [27] include those already applied for bacterial inactivation for safety purposes such as thermal processes [28][29], irradiation [30], UV rays [31], high pressure [32] and ultrasound [33]. Furthermore, a combination of techniques could result in more effective inactivation protocols [34]. These processes could specifically target different cell components and/or functions or generally damage the entire cell structure.
In particular, thermal treatments that are still the most widely used processes for producing paraprobiotics at laboratory and industrial levels [35] damage the cell membrane thus provoking leakage of nutrients and ions and cause ribosome aggregation, protein denaturation and DNA breakage [27]. As an alternative to conventional heat processes, ohmic heating has been very recently proposed for paraprobiotic production. This emerging technology involves the passage of alternating electric current through the sample, thus leading to a fast and uniform heating. Bacterial inactivation is thus caused both by thermal and non-thermal damage, such as electroporation caused by the electric field that increases the membrane permeability, inducing cell death. This technology has been applied and optimized for the inactivation of probiotic
,
, and
[36].
Similarly, high hydrostatic pressure and high-pressure homogenization treatments also used in combination with thermal processes can cause membrane rupture due to shear stress, as well as alteration of ribosomes and irreversible protein denaturation and coagulation leading to the inactivation of biological functions mediated by enzymes, extensive loss of solute and reduction of intracellular pH [37][38].
More recently, the application of high-intensity ultrasound (HIUS) in the inactivation of probiotics has been reviewed [39]. The effect of this technology on microorganisms is associated with physical forces generated by acoustic cavitation that cause cell wall shearing, free radicals, DNA damage and, eventually, membrane breakdown and cell lysis [34][39][40].
On the contrary, other inactivation methods more specifically target particular cell components. Microbial inactivation by ionizing radiation (gamma rays or X-rays) is mainly due to the damage of nucleic acids caused by oxidative radicals originating from the radiolysis of water [41]. Similarly, UV irradiation of microbial cells induces formation of DNA photoproducts such as the pyrimidine dimer, thus interrupting both DNA transcription and translation [42]. These inactivation methods are currently suitable for producing paraprobiotics at the laboratory scale; however, further studies are needed to develop technological processes for the industrial scale up of paraprobiotics production that would preserve the beneficial effects while being time- and cost-effective [43] (
).
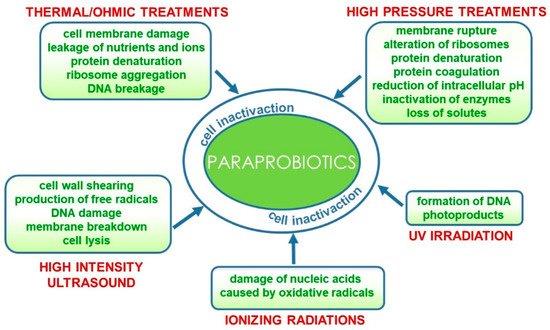
Technological processes for the production of paraprobiotics and their effects on bacterial cells.
A sound body of scientific evidence shows the beneficial effects of paraprobiotics on human and animal health, thus definitively assessing that cell vitality is not an absolute prerequisite for the health effects [44]. However, methods and process parameters for paraprobiotic production should be carefully tailored taking into consideration the characteristics of both target microbial species and food matrices to assure that products retain their efficacy. In fact, inactivation methods could affect the beneficial effects, and paraprobiotics obtained with different technologies could exhibit different functional features.
The ability to modulate the adaptive and innate immune systems represents the key feature of the paraprobiotic action [16][22]. Intriguingly, probiotic and paraprobiotic cells of the same species can induce similar immunological responses by triggering the same pathways or different mechanisms of action. For instance, UV-inactivated and live
GG cells were equally effective in decreasing IL-8 production in the intestinal epithelium cells (Caco-2 cells), but their mechanisms of action involved different pathways [31].
Aggregation and adhesion are also important properties of probiotics, being involved in gut colonization and antagonistic effect against pathogens. These features could be affected by experimental conditions used for cell inactivation. Ostad et al. demonstrated that both live and heat-inactivated forms of fecal
(treated at 60 °C for 30 min) were able to inhibit the attachment of pathogenic bacteria (
and
) to Caco-2 cells [45]. Similarly, Tareb et al. showed that heat-killed
CNCM I-3698 and
CNCM I-3699 (autoclaved at 120 °C for 15 min) exhibited co-aggregative abilities toward
and higher exclusion potential against binding of this pathogen to mucin compared to the live counterparts. The ability of inactivated cells to adhere to different intestinal matrix models (as evaluated by qPCR) was also reported [46].
More recently, Singh et al. investigated the adhesion and antagonistic activity of several probiotic
strains (live, heat-inactivated and treated with 5 M LiCl) toward select pathogens. The reported data highlighted that these properties were strictly strain-specific. The inactivated cells (80 °C for 10 min) adhered to Caco-2 cells, although to a slightly lesser extent compared to the live counterparts, while the pathogen inhibition abilities were significantly reduced. The adhesion and antagonistic potential of the probiotic strains were lost upon exposure to 5 M LiCl, thus indicating the involvement of surface proteins [47].
As a matter of fact, morphological changes on several heat-treated lactic acid bacteria strains were observed by field-emission scanning electron microscope (FE-SEM). In particular, resulting cell surfaces of all the heat-treated bacteria were rougher and more uneven than those of viable untreated cells. This finding was accompanied by a decrease of the adhesive ability of heat-killed bacteria with increasing temperature. Interestingly, heat treatment decreased the adhesion ability but did not affect the immunostimulatory activity [28].
However, further studies are required to definitively assess whether paraprobiotics retain their adhesion and pathogen exclusion abilities and clarify how these features are affected by the inactivation processes, applying the most up-to-date methodologies. It should also be kept in mind that models used to assess cell adhesion in vitro only represent simplifications of in vivo conditions and the counting of truly adherent paraprobiotic cells could still present several experimental difficulties [48].
Another interesting feature of probiotics is their ability to remove cholesterol from media via several possible mechanisms including assimilation during growth and incorporation into the cell membrane [49][50]. It has been reported that sonication-killed cells of
SPM1207 isolated from healthy adults and orally administrated to rats retained the ability of lowering cholesterol, blocking the body weight increase and relieving or eliminating constipation in rats, as also shown for the viable probiotic cells [33]. However, Lye et al. showed that, although low-intensity ultrasound treatment increased viability and cholesterol removal ability of lactobacilli, a decrease in both these features was observed for higher-intensity ultrasound treatment (100 W for 3 min), thus suggesting that the ability of lactobacilli cells to assimilate cholesterol could be partly associated with the growth ability [51].
3.Detailed studies aimed at Ainalytical Techniques for the Quality Control of Pvestigating the molecular mechanisms at the basis of the exhibited health effects of paraprobiotic-Containing Products and Regulatory Aspectss and performing comparisons with viable counterparts are still required and crucial to set up inactivation protocols that preserve their beneficial action.
3. Analytical Techniques for the Quality Control of Paraprobiotic-Containing Products and Regulatory Aspects
3.1. Paraprobiotics Detection and Quantification
Paraprobiotics are defined as “inactivated microbial cells (non-viable) that confer a health benefit to the consumer” [27]. Considering this definition, it is easy to understand that is not possible to enumerate the paraprobiotics using classical microbiology methods based on the ability of the single cells to grow and form colonies. The cultivation technique also does not provide information about cell integrity and metabolic activity, but damaged and injured cells can still retain some metabolic activity that contributes to health promotion [27]. On the other hand, culture-independent methodologies, such as polymerase chain reaction (PCR) techniques, are able to detect viable cells or the overall microbiota of different matrices (living and dead cells), but fail in the selective quantification of non-viable cells. Moreover, the inactivation methods to produce paraprobiotics could cause DNA damage that would negatively affect PCR results.
Flow cytometry is a potential analytical technique that holds the potential to quantify non-viable cells in a matrix. Over the last 20 years, flow cytometry has gained increased popularity in microbiological research since it allows the determination of viable bacteria but also the enumeration of damaged/dead cells [52]. This technique was initially developed for studying eukaryotic cells but is currently used to detect and explore the physiological state of prokaryotic cells in foods and probiotic products [53][54].
The flow cytometry principle is based on dual nucleic acid staining with a cell-permeant dye (thiazole orange, SYTO 9 or SYTO 24) and a cell-impermeant dye (propidium iodide). Thiazole orange or equivalents permeates membranes of total cells and stains the nucleic acids with green fluorescence. Propidium iodide penetrates only bacteria with damaged membranes, causing a reduction in thiazole orange fluorescence when both dyes are present. Thus, live cells with intact cell membranes fluoresce bright green, bacteria with slightly damaged membranes exhibit both green and red fluorescence, whereas bacteria with broken membranes fluoresce red [52][53]. The main advantages of this technique are as follows: short assay and data generation times (1–2 min), minimum sample volume (from 5 μL), detection of live and dead cells and less labor compared with conventional plating techniques (Wilkinson, 2020). Recently, flow cytometry was applied to characterize a multi-strain probiotic product. This technique allowed the authors to quantify non-viable cells in the overall population of analyzed samples, highlighting that flow cytometry could be a powerful tool to enumerate paraprobiotics cells in food matrices [53].
However, it has to be considered that by using flow cytometry, it is possible to assess the content of cells and their viability, but no taxonomical information on the microorganisms that are present can be obtained. Since the concept of “probiotic” also requires the identification of probiotic microorganisms in the final product, flow cytometry should be used as a complementary method along with PCR methods to allow paraprobiotics detection, count and identification. In addition, a study of Klein et al. showed the suitability of a proteomic approach based on capillary electrophoresis coupled to mass spectrometry (CE/MS) for quality control of inactivated probiotics preparations [55].
Soejima et al. have recently launched digital PCR as a robust tool for the routine analysis of heat-killed lactobacilli-supplemented foods [56]. This assay targets multiple copies of the 16S rDNA without being affected by several DNA recovery rates in the same sample. Hence, it seems to be useful to guarantee accurate cell supplementation of nutritional foods, thereby also avoiding a cell content higher by tenfold and, consequently, excessively high production costs.
3.2. Regulatory Aspects
Despite favorable perspective on the use of paraprobiotics, several aspects of this concept are not fully understood yet. The term “paraprobiotic” is misleading by itself, for it suggests, if literally taken, that it is effective only if administered in the presence (“para”, i.e., “side by side”) of a probiotic. The proliferation of overlapping terminology and the absence of a universally recognized definition induce vagueness, which makes challenging communication both between researchers and of the concept to the consumer. The current situation requires a consensus panel in order to draw attention to the confusion that reigns within the probiotic glossary and to address the emerging terms in the “biotics” field with the objective of establishing a generally agreed terminology. Some confusion also derives from the definition of paraprobiotics that includes non-viable intact or broken cells (i.e., cell lysates or fragments, cell membrane or cell wall components) or the cellular extract. This entails a partial overlap with the term “postbiotics”, defined as extracts of non-viable probiotics and comprising cell membrane components such as surface proteins, lipopolysaccharides, teichoic acids, etc. [16,57].
Despite favorable perspective on the use of paraprobiotics, several aspects of this concept are not fully understood yet. The term “paraprobiotic” is misleading by itself, for it suggests, if literally taken, that it is effective only if administered in the presence (“para”, i.e., “side by side”) of a probiotic. The proliferation of overlapping terminology and the absence of a universally recognized definition induce vagueness, which makes challenging communication both between researchers and of the concept to the consumer. The current situation requires a consensus panel in order to draw attention to the confusion that reigns within the probiotic glossary and to address the emerging terms in the “biotics” field with the objective of establishing a generally agreed terminology. Some confusion also derives from the definition of paraprobiotics that includes non-viable intact or broken cells (i.e., cell lysates or fragments, cell membrane or cell wall components) or the cellular extract. This entails a partial overlap with the term “postbiotics”, defined as extracts of non-viable probiotics and comprising cell membrane components such as surface proteins, lipopolysaccharides, teichoic acids, etc. [16][57].
We propose defining the term “paraprobiotic” as “inactivated microbial cells (non-viable), specifically, cells as a whole, including both structural components and synthesized or excreted metabolites that confer a health benefit to the consumer.” In addition, we recommend defining the term “postbiotics” as “compounds derived from microbial metabolism synthesized by cells or produced in the matrix by enzymatic action.” Postbiotics can be single metabolites or even very complex mixtures. A detailed definition of the different terms and the cell components involved in the biological activities are reported in
.
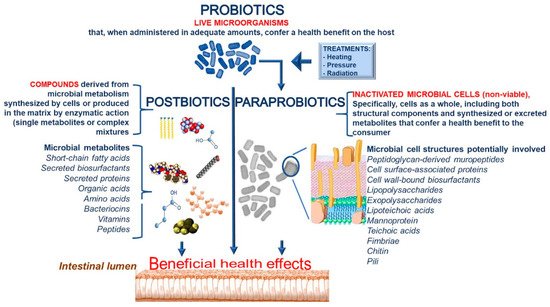
Definitions of probiotics, paraprobiotics and postbiotics together with cell components involved in biological activities.
There are several key points to be clarified in order to support regulatory authorities for defining the requirements for the registration and approval of foods and dietary supplements containing paraprobiotics. In addition to a punctual and unequivocal definition recognized at the international level, specific attention is required with regard to paraprobiotics production methods, quality control criteria, how to detect and quantify their presence and how to assess their safety and efficacy.
Means of inactivation may affect the physiological activity of the resulting dead cells and the stability of their beneficial effects during shelf life anyway. This is another aspect to deepen and clarify to make the best possible use of paraprobiotic opportunities [27].
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20]
Means of inactivation may affect the physiological activity of the resulting dead cells and the stability of their beneficial effects during shelf life anyway. This is another aspect to deepen and clarify to make the best possible use of paraprobiotic opportunities
[21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114]
.
References
- Zucko, J.; Starcevic, A.; Diminic, J.; Oros, D.; Mortazavian, A. M.; Putnik, P. Probiotic–friend or foe? Curr. Opin. Food Sci. 2020, 32, 45-49. doi: 10.1016/j.cofs.2020.01.007.Zucko, J.; Starcevic, A.; Diminic, J.; Oros, D.; Mortazavian, A.M.; Putnik, P. Probiotic–friend or foe? Curr. Opin. Food Sci. 2020, 32, 45–49.
- Chambers, L.; Avery, A.; Dalrymple, J.; Farrell, L.; Gibson, G.; Harrington, J.; et al. Translating probiotic science into practice. Nutr. Bull. 2019, 44, 165-173. doi: 10.1111/nbu.12385.Chambers, L.; Avery, A.; Dalrymple, J.; Farrell, L.; Gibson, G.; Harrington, J.; Rijkers, G.; Rowland, I.; Spiro, A.; Varela-Moreiras, G.; et al. Translating probiotic science into practice. Nutr. Bull. 2019, 44, 165–173.
- Rolim, F.R.; Neto, O.C.F.; Oliveira, M.E.G.; Oliveira, C.J.; Queiroga, R.C. Cheeses as food matrixes for probiotics: In vitro and in vivo tests. Trends Food Sci. Technol. 2020, 100, 138-154. doi: 10.1016/j.tifs.2020.04.008.Rolim, F.R.; Neto, O.C.F.; Oliveira, M.E.G.; Oliveira, C.J.; Queiroga, R.C. Cheeses as food matrixes for probiotics: In vitro and in vivo tests. Trends Food Sci. Technol. 2020, 100, 138–154.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. doi: 10.1038/ nrgastro.2014.66.Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.; Ross, R.P. Life under stress: the probiotic stress response and how it may be manipulated. Curr. Pharm. Des. 2008, 14, 1382-1399. doi: 10.2174/138161208784480225.Corcoran, B.M.; Stanton, C.; Fitzgerald, G.; Ross, R.P. Life under stress: The probiotic stress response and how it may be manipulated. Curr. Pharm. Des. 2008, 14, 1382–1399.
- de Simone, C. The unregulated probiotic market. Clin. Gastroenterol. Hepatol. 2019, 17(5), 809-817. doi: 10.1016/j.cgh.2018.01.018De Simone, C. The unregulated probiotic market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817.
- Shripada, R.; Gayatri, A.J.; Sanjay, P. Paraprobiotics. In Precision Medicine for Investigators, Practitioners and Providers, Faintuch, J., Faintuch S., Eds.; Academic Press. 2020, pp. 39-49. doi: 10.1016/C2018-0-03029-7.Shripada, R.; Gayatri, A.J.; Sanjay, P. Paraprobiotics. In Precision Medicine for Investigators, Practitioners and Providers; Faintuch, J., Faintuch, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 39–49.
- Kothari, D.; Patel, S.; Kim, S. K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Phar-macother. 2019, 111, 537-547. doi: 10.1016/j.biopha.2018.12.104.Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547.
- Costa, R. L.; Moreira, J.; Lorenzo, A.; Lamas, C. C. Infectious complications following probiotic ingestion: a potentially un-derestimated problem? A systematic review of reports and case series. BMC Complement. Altern. Med. 2018, 18(1), 1-8). doi: 10.1186/s12906-018-2394-3.Costa, R.L.; Moreira, J.; Lorenzo, A.; Lamas, C.C. Infectious complications following probiotic ingestion: A potentially un-derestimated problem? A systematic review of reports and case series. BMC Complement. Altern. Med. 2018, 18, 1–8.
- Akter, S.; Park, J.H.; Jung, H.K. Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. J. Microbiol. Biotechnol. 2020, 30, 477-481. doi: 10.4014/jmb.1911.11019.Akter, S.; Park, J.H.; Jung, H.K. Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. J. Microbiol. Biotechnol. 2020, 30, 477–481.
- EFSA Panel on Biological Hazards (BIOHAZ), Koutsoumanis, K.; Allende, A.; Alvarez‐Ordóñez, A.; Bolton, D.; Bover‐Cid, S.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 2020, 18, e06174. doi: 10.2903/j.efsa.2020.6174.EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: Suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 2020, 18, e06174.
- Seifert, A.; Kashi, Y.; Livney, Y.D. Delivery to the gut microbiota: A rapidly proliferating research field. Adv. Colloid Interface Sci. 2019, 274, 102038. doi: 10.1016/j.cis.2019.102038.Seifert, A.; Kashi, Y.; Livney, Y.D. Delivery to the gut microbiota: A rapidly proliferating research field. Adv. Colloid Interface Sci. 2019, 274, 102038.
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. doi: 10.1186/s12934-020-01426-w.Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168.
- Lin, T. L.; Shu, C. C.; Lai, W. F.; Tzeng, C. M.; Lai, H. C. & Lu, C. C. Investiture of next generation probiotics on amelioration of diseases–Strains do matter. Med. Microecol. 2019 1, 100002 doi: 10.1016/j.medmic.2019.100002.Lin, T.L.; Shu, C.C.; Lai, W.F.; Tzeng, C.M.; Lai, H.C.; Lu, C.C. Investiture of next generation probiotics on amelioration of diseases–Strains do matter. Med. Microecol. 2019, 1, 100002.
- Wilcox, H.; Carr, C.; Seney, S.; Reid, G.; Burton, J. Expired probiotics: What is really in your cabinet? FEMS Microbes, 2020, 1, xtaa007. doi: 10.1093/femsmc/xtaa007.Wilcox, H.; Carr, C.; Seney, S.; Reid, G.; Burton, J. Expired probiotics: What is really in your cabinet? FEMS Microbes 2020, 1, xtaa007.
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept. Genes Nutr. 2011, 6, 261-274. doi: 10.1007/s12263-011-0218-x.Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept. Genes Nutr. 2011, 6, 261–274.
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; et al. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 2020, 7, 570344. doi: 10.3389/fnut.2020.57034.Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 2020, 7, 570344.
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105-114. doi:10.1016/j.tifs.2018.03.009.Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114.
- Warda, A.K.; Rea, K.; Fitzgerald, P.; Hueston, C.; Gonzalez-Tortuero, E.; Dinan, T.G.; Hill, C. Heat-killed lactobacilli alter both microbiota composition and behaviour. Behav. Brain Res. 2019, 362, 213–223. doi: 10.1016/j.bbr.2018.12.047.Warda, A.K.; Rea, K.; Fitzgerald, P.; Hueston, C.; Gonzalez-Tortuero, E.; Dinan, T.G.; Hill, C. Heat-killed lactobacilli alter both microbiota composition and behaviour. Behav. Brain Res. 2019, 362, 213–223.
- Majhenic, A.C.; Lorbeg, P.M.; Treven, P. Enumeration and identification of mixed probiotic and lactic acid bacteria starter cultures. In Probiotic Dairy Products, 2nd ed.; Tamime, A.Y., Thomas L.V., Eds.; John Wiley & Sons Ltd. 2017, pp 207-251. doi: 10.1002/9781119214137.ch6.Majhenic, A.C.; Lorbeg, P.M.; Treven, P. Enumeration and identification of mixed probiotic and lactic acid bacteria starter cultures. In Probiotic Dairy Products, 2nd ed.; Tamime, A.Y., Thomas, L.V., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2017; pp. 207–251.
- Nakamura, S.; Mitsunaga, F. Anti-allergic effect of para-probiotics from non-viable acetic acid bacteria in ovalbumin-sensitized mice. Food Nutr. Sci. 2018, 9, 1376-1385. doi: 10.4236/fns.2018.912099.Nakamura, S.; Mitsunaga, F. Anti-allergic effect of para-probiotics from non-viable acetic acid bacteria in ovalbumin-sensitized mice. Food Nutr. Sci. 2018, 9, 1376–1385.
- Adams, C.A. The probiotic paradox: live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. doi: 10.1017/S0954422410000090.Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46.
- Fiore, W.; Arioli, S.; Guglielmetti, S. The neglected microbial components of commercial probiotic formulations. Microorganisms 2020, 8, 1177. doi: 10.3390/microorganisms8081177.Fiore, W.; Arioli, S.; Guglielmetti, S. The neglected microbial components of commercial probiotic formulations. Microorganisms 2020, 8, 1177.
- Lahtinen, S.J. Probiotic viability - Does it matter? Microb. Ecol. Health Dis. 2012, 23, 18567. doi: 10.3402/mehd.v23i0.18567.Lahtinen, S.J. Probiotic viability - Does it matter? Microb. Ecol. Health Dis. 2012, 23, 18567.
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. doi: 10.1093/cid/civ085.Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60, S129–S134.
- Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Graça, J.S.; Esmerino, E.A.; et al. Impact of probiotics and prebiotics on food texture. Curr. Opin. Food Sci. 2020, 33, 38-44. doi: 10.1016/j.cofs.2019.12.002.Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Graça, J.S.; Esmerino, E.A.; Silva, M.C.; Sant’Ana, A.S.; Duarte, M.C.K.; Freitas, M.Q.; et al. Impact of probiotics and prebiotics on food texture. Curr. Opin. Food Sci. 2020, 33, 38–44.
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant'Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. doi: 10.1016/j.tifs.2016.09.011.De Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114.
- Ou, C.C.; Lin, S.L.; Tsai, J.J.; Lin, M.Y. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. J. Food Sci. 2011, 76, M260-M267. doi: 10.1111/j.1750-3841.2011.02161.x.Ou, C.C.; Lin, S.L.; Tsai, J.J.; Lin, M.Y. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. J. Food Sci. 2011, 76, M260–M267.
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-α–induced interleukin-8 production in Caco-2 cells. J. Nutr. 2005, 135, 1752-1756. doi: 10.1093/jn/135.7.1752.Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-α–induced interleukin-8 production in Caco-2 cells. J. Nutr. 2005, 135, 1752–1756.
- Kamiya, T.; Wang, L.; Forsythe, P.; Goettsche, G.; Mao, Y.; Wang, Y.; et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 2006, 55, 191–196. doi: 10.1136/gut.2005.070987.Kamiya, T.; Wang, L.; Forsythe, P.; Goettsche, G.; Mao, Y.; Wang, Y.; Tougas, G.; Bienenstock, J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 2006, 55, 191–196.
- Lopez, M.; Li, N.; Kataria, J.; Russell, M.; Neu, J. Live and ultraviolet-inactivated Lactobacillus rhamnosus GG decrease flagellin-induced interleukin-8 production in Caco-2 cells. J. Nutr. 2008, 138, 2264–2268. doi: 10.3945/jn.108.093658.Lopez, M.; Li, N.; Kataria, J.; Russell, M.; Neu, J. Live and ultraviolet-inactivated Lactobacillus rhamnosus GG decrease flagellin-induced interleukin-8 production in Caco-2 cells. J. Nutr. 2008, 138, 2264–2268.
- Ananta, E.; Knorr, D. Comparison of inactivation pathways of thermal or high pressure inactivated Lactobacillus rhamnosus ATCC 53103 by flow cytometry analysis. Food Microbiol. 2009, 26, 542-546. doi: 10.1016/j.fm.2009.01.008.Ananta, E.; Knorr, D. Comparison of inactivation pathways of thermal or high pressure inactivated Lactobacillus rhamnosus ATCC 53103 by flow cytometry analysis. Food Microbiol. 2009, 26, 542–546.
- Shin, H.S.; Park, S.Y.; Lee, D.K.; Kim, S.A.; An, H.M.; Kim, J.R.; et al. Hypocholesterolemic effect of sonication-killed Bifidobacterium longum isolated from healthy adult Koreans in high cholesterol fed rats. Arch. Pharm. Res. 2010, 33, 1425-1431. doi: 10.1007/s12272-010-0917-7.Shin, H.S.; Park, S.Y.; Lee, D.K.; Kim, S.A.; An, H.M.; Kim, J.R.; Kim, M.J.; Cha, M.G.; Lee, S.W.; Kim, K.J.; et al. Hypocholesterolemic effect of sonication-killed Bifidobacterium longum isolated from healthy adult Koreans in high cholesterol fed rats. Arch. Pharm. Res. 2010, 33, 1425–1431.
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing – food quality assurance and food safety. Trends Food Sci. Technol. 2012, 26, 88–98. doi: 10.1016/j.tifs.2012.01.010.Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in food processing–food quality assurance and food safety. Trends Food Sci. Technol. 2012, 26, 88–98.
- Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.K.; Silva, M.C.; Silva, R.; et al. Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020, 32, 1-8. doi: 10.1016/j.cofs.2019.12.003.Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.K.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020, 32, 1–8.
- Barros, C.P.; Pires R.P.S.; Guimarães, J.T.; Abud, Y.K.D.; Almada C.N.; Pimentel T.C.; et al. Ohmic heating as a method of obtaining paraprobiotics: Impacts on cell structure and viability by flow cytometry. Food Res. Int. 2021, 140, 110061. doi: 10.1016/j.foodres.2020.110061.Barros, C.P.; Pires, R.P.S.; Guimarães, J.T.; Abud, Y.K.D.; Almada, C.N.; Pimentel, T.C.; Sant’Anna, C.; De-Melo, L.D.B.; Duarte, M.C.K.; Silva, M.C.; et al. Ohmic heating as a method of obtaining paraprobiotics: Impacts on cell structure and viability by flow cytometry. Food Res. Int. 2021, 140, 110061.
- Patrignani, F.; Lanciotti, R. Applications of high and ultra high pressure homogenization for food safety. Front. Microbiol. 2016, 7, 1132. doi: 10.3389/fmicb.2016.01132.Patrignani, F.; Lanciotti, R. Applications of high and ultra high pressure homogenization for food safety. Front. Microbiol. 2016, 7, 1132.
- Rendueles, E.; Omer, M.K.; Alvseike, O.; Alonso-Calleja, C.; Capita, R.; Prieto, M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT-Food Sci. Technol. 2011, 44, 1251-1260. doi: 10.1016/j.lwt.2010.11.001.Rendueles, E.; Omer, M.K.; Alvseike, O.; Alonso-Calleja, C.; Capita, R.; Prieto, M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT Food Sci. Technol. 2011, 44, 1251–1260.
- Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; Ashokkumar, M.; et al. High-intensity ultrasound: A novel technology for the development of probiotic and prebiotic dairy products. Ultrason. Sonochem. 2019, 57, 12-21. doi: 10.1016/j.ultsonch.2019.05.004.Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; Ashokkumar, M.; Freitas, M.Q.; Cruz, A.G. High-intensity ultrasound: A novel technology for the development of probiotic and prebiotic dairy products. Ultrason. Sonochem. 2019, 57, 12–21.
- Pagnossa, J.P.; Rocchetti, G.; Ribeiro, A.C.; Piccoli, R.H.; Lucini, L. Ultrasound: beneficial biotechnological aspects on microorganisms-mediated processes. Curr. Opin. Food Sci. 2020, 31, 24-30. doi: 10.1016/j.cofs.2019.10.006.Pagnossa, J.P.; Rocchetti, G.; Ribeiro, A.C.; Piccoli, R.H.; Lucini, L. Ultrasound: Beneficial biotechnological aspects on microorganisms-mediated processes. Curr. Opin. Food Sci. 2020, 31, 24–30.
- Farkas, J. Irradiation for better foods. Trends Food Sci. Technol. 2006, 17, 148-152. doi: 10.1016/j.tifs.2005.12.003.Farkas, J. Irradiation for better foods. Trends Food Sci. Technol. 2006, 17, 148–152.
- Franz, C.; Specht, I.; Cho, G.-S.; Graef, V.; Stahl, M. UV-C inactivation of microorganisms in naturally cloudy apple juice using novel inactivation equipment based on Dean vortex technology. Food Control 2009, 20, 1103-1107. doi: 10.1016/j.foodcont.2009.02.010.Franz, C.; Specht, I.; Cho, G.-S.; Graef, V.; Stahl, M. UV-C inactivation of microorganisms in naturally cloudy apple juice using novel inactivation equipment based on Dean vortex technology. Food Control 2009, 20, 1103–1107.
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. doi: 10.1016/j.foodres.2020.109502.Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502.
- Zorzela, L.; Ardestani, S.K.; McFarland, L.V.; Vohra, S. Is there a role for modified probiotics as beneficial microbes: A systematic review of the literature. Benef. Microbes 2017, 8, 739-754. doi: 10.3920/BM2017.0032.Zorzela, L.; Ardestani, S.K.; McFarland, L.V.; Vohra, S. Is there a role for modified probiotics as beneficial microbes: A systematic review of the literature. Benef. Microbes 2017, 8, 739–754.
- Ostad, S.N.; Salarian, A.A.; Ghahramani, M.H.; Fazeli, M.R.; Samadi, N.; Jamalifar, H. Live and heat-inactivated lactobacilli from feces inhibit Salmonella typhi and Escherichia coli adherence to Caco-2 cells. Folia Microbiol. 2009, 54, 157-160. doi: 10.1007/s12223-009-0024-7.Ostad, S.N.; Salarian, A.A.; Ghahramani, M.H.; Fazeli, M.R.; Samadi, N.; Jamalifar, H. Live and heat-inactivated lactobacilli from feces inhibit Salmonella typhi and Escherichia coli adherence to Caco-2 cells. Folia Microbiol. 2009, 54, 157–160.
- Tareb, R.; Bernardeau, M.; Gueguen, M.; Vernoux, J.P. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J. Med. Microbiol. 2013, 62, 637-649. doi: 10.1099/jmm.0.049965-0.Tareb, R.; Bernardeau, M.; Gueguen, M.; Vernoux, J.P. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J. Med. Microbiol. 2013, 62, 637–649.
- Singh, T.P., Kaur, G., Kapila, S., Malik, R.K. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 2017, 8, 486. doi: 10.3389/fmicb.2017.00486.Singh, T.P.; Kaur, G.; Kapila, S.; Malik, R.K. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 2017, 8, 486.
- Ouwehand, A.C., Salminen, S. (2003) In vitro adhesion assays for probiotics and their in vivo relevance: a review. Microb. Ecol. Health Dis., 2003, 15, 175-184, doi: 10.1080/08910600310019886Ouwehand, A.C.; Salminen, S. In vitro adhesion assays for probiotics and their in vivo relevance: A review. Microb. Ecol. Health Dis. 2003, 15, 175–184.
- Lye, H.S.; Rahmat-Ali, G.R.; Liong, M.T. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int. Dairy J. 2010, 20, 169–175. doi: 10.1016/j.idairyj.2009.10.003.Lye, H.S.; Rahmat-Ali, G.R.; Liong, M.T. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int. Dairy J. 2010, 20, 169–175.
- Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 2010, 11, 2499-2522. doi: 10.3390/ijms11062499.Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522.
- Lye, H.S.; Alias, K.A.; Rusul, G.; Liong, M.T. Ultrasound treatment enhances cholesterol removal ability of lactobacilli. Ultrason. Sonochem. 2012, 19, 632-641. doi: 10.1016/j.ultsonch.2011.08.004.Lye, H.S.; Alias, K.A.; Rusul, G.; Liong, M.T. Ultrasound treatment enhances cholesterol removal ability of lactobacilli. Ultrason. Sonochem. 2012, 19, 632–641.
- Wilkinson, M.G. Flow cytometry as a potential method of measuring bacterial viability in probiotic products: a review. Trends Food Sci. Technol. 2018, 78, 1-10. doi: 10.1016/j.tifs.2018.05.006.Wilkinson, M.G. Flow cytometry as a potential method of measuring bacterial viability in probiotic products: A review. Trends Food Sci. Technol. 2018, 78, 1–10.
- Mora, D.; Filardi, R.; Arioli, S.; Boeren, S.; Aalvink, S.; de Vos, W.M. Development of omics‐based protocols for the microbiological characterization of multi‐strain formulations marketed as probiotics: the case of VSL# 3. Microb. Biotechnol. 2019, 12, 1371-1386. doi: 10.1111/1751-7915.13476.Mora, D.; Filardi, R.; Arioli, S.; Boeren, S.; Aalvink, S.; de Vos, W.M. Development of omics-based protocols for the microbiological characterization of multi-strain formulations marketed as probiotics: The case of VSL# 3. Microb. Biotechnol. 2019, 12, 1371–1386.
- Pane, M.; Allesina, S.; Amoruso, A.; Nicola, S.; Deidda, F.; Mogna, L. (2018). Flow cytometry: evolution of microbiological methods for probiotics enumeration. J. Clin. Gastroenterol. 2018, 52, S41-S45. doi: 10.1097/MCG.0000000000001057.Pane, M.; Allesina, S.; Amoruso, A.; Nicola, S.; Deidda, F.; Mogna, L. Flow cytometry: Evolution of microbiological methods for probiotics enumeration. J. Clin. Gastroenterol. 2018, 52, S41–S45.
- Klein, G.; Schanstra, J.P.; Hoffmann, J.; Mischak, H.; Siwy, J.; Zimmermann, K. Proteomics as a quality control tool of pharmaceutical probiotic bacterial lysate products. PloS One 2013, 8, e66682. doi: 10.1371/journal.pone.0066682.Klein, G.; Schanstra, J.P.; Hoffmann, J.; Mischak, H.; Siwy, J.; Zimmermann, K. Proteomics as a quality control tool of pharmaceutical probiotic bacterial lysate products. PLoS ONE 2013, 8, e66682.
- Soejima, T.; Tanaka, M.; Yamauchi, K.; Abe, F. Exclusive use of digital PCR allows an absolute assay of heat-killed Lactobacilli in foods targeting multiple copies of 16S rDNA. Sci. Rep. 2020, 10, 12691. doi: 10.1038/s41598-020-69206-5.Soejima, T.; Tanaka, M.; Yamauchi, K.; Abe, F. Exclusive use of digital PCR allows an absolute assay of heat-killed Lactobacilli in foods targeting multiple copies of 16S rDNA. Sci. Rep. 2020, 10, 12691.
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. doi: 10.1016/j.cofs.2020.03.009.Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123.
- Reale, A.; Di Renzo, T.; Coppola, R. Factors affecting viability of selected probiotics during cheese-making of pasta filata dairy products obtained by direct-to-vat inoculation system. LWT-Food Sci. Technol. 2019, 116, 108476. doi: 10.1016/j.lwt.2019.108476.
- Reale, A.; Ianniello, R.G.; Ciocia, F.; Di Renzo, T.; Boscaino, F.; Ricciardi, A.; et al. Effect of respirative and catalase-positive Lactobacillus casei adjuncts on the production and quality of Cheddar-type cheese. Int. Dairy J. 2016, 63, 78-87. doi: 10.1016/j.idairyj.2016.08.005.
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic viability in yoghurt: a review of influential factors. Int. Dairy J. 2020, 109, 104793. doi: 10.1016/j.idairyj.2020.104793
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic delivery through fermentation: dairy vs. non-dairy beverages. Fermentation 2017, 3, 67. doi: 10.3390/fermentation3040067.
- Wang, Y.; Jiang, Y.; Deng, Y.; Yi, C.; Wang, Y.; Ding, M.; et al. Probiotic supplements: Hope or hype? Front. Microbiol. 2020, 11, 160. doi: 10.3389/fmicb.2020.00160.
- Nishida, K.; Sawada, D.; Kawai, T.; Kuwano, Y.; Fujiwara, S.; Rokutan, K. Para-psychobiotic Lactobacillus gasseri CP2305 ameliorates stress-related symptoms and sleep quality. J. Appl. Microbiol. 2017, 123, 1561-1570. doi: 10.1111/jam.13594.
- Sawada, D.; Sugawara, T.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I. Effect of continuous ingestion of a beverage prepared with Lactobacillus gasseri CP2305 inactivated by heat treatment on the regulation of intestinal function. Food Res. Int. 2016, 79, 33-39. doi: 10.1016/j.foodres.2015.11.032.
- Nobutani, K.; Sawada, D.; Fujiwara, S.; Kuwano, Y.; Nishida, K.; Nakayama, .J.; et al. The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. J. Appl. Microbiol. 2017, 122, 212-224. doi: 10.1111/jam.13329.
- Zeng, J.; Jiang, J.; Zhu, W.; Chu, Y. Heat-killed yogurt-containing lactic acid bacteria prevent cytokine-induced barrier disruption in human intestinal Caco-2 cells. Ann. Microbiol. 2016, 66, 171–178. doi: 10.1007/s13213-015-1093-2.
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Laiola, M.; Paparo, L.; Calignano, A.; et al. Specific signatures of the gut microbiota and increased levels of butyrate in children treated with fermented cow's milk containing heat-killed Lactobacillus paracasei CBA L74. Appl. Environ. Microbiol. 2017, 83, e01206-17. doi: 10.1128/AEM.01206-17.
- Liu, Z.M.; Xu, Z.Y.; Han, M.; Guo, B.H. Efficacy of pasteurised yoghurt in improving chronic constipation: A randomised, double-blind, placebo-controlled trial. Int. Dairy J. 2015, 40, 1-5. doi: 10.1016/j.idairyj.2014.08.009.
- Rodríguez-Figueroa, J.C.; González-Córdova, A.F.; Astiazaran-García, H.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. Antihypertensive and hypolipidemic effect of milk fermented by specific Lactococcus lactis strains. J. Dairy Sci. 2013, 96, 4094-4099. doi: 10.3168/jds.2012-6014.
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on non-dairy probiotics and their use in non-dairy based products. Fermentation 2020, 6, 30. doi: 10.3390/fermentation6010030.
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. doi: 10.1080/10408398.2018.1462760.
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s milk allergy: From allergens to new forms of diagnosis, therapy and prevention. Methods 2014, 66, 22-33. doi: 10.1016/j.ymeth.2013.08.005.
- Vijaya Kumar, B.; Vijayendra, V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products - a review. J. Food Sci. Technol. 2015, 52, 6112–6124. doi: 10.1007/s13197-015-1795-2.
- Liu, Y.; Poon, S.; Seeman, E.; Hare, D.L.; Bui, M.; Iuliano, S. Fat from dairy foods and ‘meat’ consumed within recommended levels is associated with favourable serum cholesterol levels in institutionalised older adults. J. Nutr. Sci. 2019, 8, e10. doi: 10.1017/jns.2019.5.
- Fehér, A.; Gazdecki, M.; Véha, M.; Szakály, M.; Szakály, Z.A. Comprehensive review of the benefits of and the barriers to the switch to a plant-based diet. Sustainability 2020, 12, 4136. doi: 10.3390/su12104136.
- Welsh, J.; Braun, H.; Brown, N.; Um, C.; Ehret, K.; Figueroa, J.; Boyd Barr, D. Production-related contaminants (pesticides, antibiotics and hormones) in organic and conventionally produced milk samples sold in the USA. Public Health Nutr. 2019, 22, 2972-2980. doi:10.1017/S136898001900106X.
- Pimentel, T.C.; Costa, W.K.A.D.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. doi: 10.1016/j.foodres.2020.110033.
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605-616. doi: 10.1038/s41575-019-0173-3.
- Mattila-Sandholm, T.; Myllärinen, P.; Crittenden, R.; Mogensen, G.; Fondèn, R.; Saarela, M. Technological challenges for future probiotic foods. Int. Dairy J. 2002, 12, 173-182. doi: 10.1016/S0958-6946(01)00099-1.
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. doi: 10.3390/ijms20102534.
- Murata, M.; Kondo, J.; Iwabuchi, N.; Takahashi, S.; Yamauchi, K.; Abe, F.; Miura, K. Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Benef Microbes 2018, 9, 855–864. doi: 10.3920/bm2017.0197.
- Othman, M.B.; Sakamoto, K. Effect of inactivated Bifidobacterium longum intake on obese diabetes model T mice (TSOD). Food Res. Int. 2020, 129, 108792. doi: 10.1016/j.foodres.2019.108792.
- Buckley, M.; Lacey, S.; Doolan, A.; Goodbody, E.; Seamans, K. The effect of Lactobacillus reuteri supplementation in Helicobacter pylori infection: A placebo-controlled, single-blind study. BMC Nutr. 2018, 4, 48. doi: 10.1186/s40795-018-0257-4.
- Mehling, H.; Busjahn, A. Non-viable Lactobacillus reuteri DSMZ 17648 (Pylopass™) as a new approach to Helicobacter pylori control in humans. Nutrients 2013, 5, 3062-3073. doi: 10.3390/nu5083062.
- Liévin-Le Moal, V.; Sarrazin-Davila, L.E.; Servin, A.L. An experimental study and a randomized, double-blind, placebo-controlled clinical trial to evaluate the antisecretory activity of Lactobacillus acidophilus strain LB against nonrotavirus diarrhea. Pediatrics 2007, 120, e795–e803. doi: 10.1542/peds.2006-2930.
- Xiao, S.D.; De Zhang, Z.; Lu, H.; Jiang, S.H.; Liu, H.Y.; Wang, G.S.; et al. Multicenter, randomized, controlled trial of heat-killed Lactobacillus acidophilus LB in patients with chronic diarrhea. Adv. Ther. 2003, 20, 253–260. doi: 10.1007/BF02849854.
- Sawada, D.; Kuwano, Y.; Tanaka, H.; Hara, S.; Uchiyama, Y.; Sugawara, T.; et al. Daily intake of Lactobacillus gasseri CP2305 relieves fatigue and stress-related symptoms in male university Ekiden runners: A double-blind, randomized, and placebo-controlled clinical trial. J. Funct. Food. 2019, 57, 465-476. doi: 10.1016/j.jff.2019.04.022.
- Sugawara, T.; Sawada, D.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; et al. Regulatory effect of paraprobiotic Lactobacillus gasseri CP2305 on gut environment and function. Microb. Ecol. Health Dis. 2016, 27, 30259. doi: 10.3402/mehd.v27.30259.
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Sugawara, T.; Aoki, Y.; et al. Daily administration of paraprobiotic Lactobacillus gasseri CP2305 ameliorates chronic stress-associated symptoms in Japanese medical students. J. Funct. Food. 2017, 36, 112–121. doi: 10.1016/j.jff.2017.06.031.
- Barros C.P.; Grom, L.; Guimaraes, J.T.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; et al. Paraprobiotic obtained by ohmic heating added in whey-grape juice drink is effective to control postprandial glycemia in healthy adults. Food Res. Int. 2020, 140, 109905. doi: 10.1016/j.foodres.2020.109905.
- Azad, M.A.K.; Manobendro Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018, 8063647. doi: 10.1155/2018/8063647.
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics importance and their immunomodulatory properties. J. Cell Physiol. 2019, 234, 8008-8018. doi: 10.1002/jcp.27559.
- Vincenzi, A.; Goettert, M.I.; Volken de Souza, C.F. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-alpha) signaling and gene expression. Cytokine Growth Factor Rev. 2021, 57, 27-38. doi: 10.1016/j.cytogfr.2020.10.004.
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160-174. doi: 10.1159/000342079.
- Westendorf, A.M.; Fleissner, D.; Hansen, W.; Buer, J. T cells, dendritic cells and epithelial cells in intestinal homeostasis. Int. J. Med. Microbiol. 2010, 300, 11-18. doi: 10.1016/j.ijmm.2009.08.009.
- Chapot-Chartier, M.P.; Vinogradov, E.; Sadovskaya, I.; Andre, G.; Mistou, M.Y.; Trieu-Cuot, P.; et al. Cell surface of Lactococcus lactis is covered by a protective polysaccharide pellicle. J. Biol. Chem. 2010, 285, 10464–1047. doi: 10.1074/jbc.M109.082958.
- Siciliano, R.A.; Lippolis, R.; Mazzeo, M.F. Proteomics for the investigation of surface-exposed proteins in probiotics. Front Nutr. 2019, 6, 52. doi: 10.3389/fnut.2019.00052.
- Besselink, M.G.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. doi: 10.1016/S0140-6736(08)60207-X.
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.; Hörmannsperger, G.; et al. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164-185. doi: 10.4161/gmic.1.3.12127.
- Zawistowska-Rojek, A.; Tyski, S. Are probiotic really safe for humans? Pol. J. Microbiol. 2018, 67, 251–258. doi: 10.21307/pjm-2018-044.
- Chuang, L.; Wu, K.G.; Pai, C.; Hsieh, P.S.; Tsai, J.J.; Yen, J.H.; Lin, M.Y. Heat-killed cells of lactobacilli skew the immune response toward T helper 1 polarization in mouse splenocytes and dendritic cell-treated T cells. J. Agric. Food Chem. 2007, 55, 11080-6. doi: 10.1021/jf071786o
- Jorjão, A.L.; de Oliveira, F.E.; Leão, M.V.P.; Carvalho, C.A.T.; Jorge, A.O.C.; de Oliveira, L.D. Live and heat-killed Lactobacillus rhamnosus ATCC 7469 may induce modulatory cytokines profiles on macrophages RAW 264.7. Sci. World J., 2015: 716749. doi: 10.1155/2015/716749.
- Song, M.W.; Jang, H.J.; Kim, K.T.; Paik, H.D. Probiotic and Antioxidant Properties of Novel Lactobacillus brevis KCCM 12203P Isolated from Kimchi and Evaluation of Immune-Stimulating Activities of Its Heat-Killed Cells in RAW 264.7 Cells. J. Microbiol. Biotechnol. 2019, 29:1894-1903. doi: 10.4014/jmb.1907.07081.
- Castro-Herrera, V.M.; Rasmussen, C.; Wellejus, A.; Miles, E.A.; Calder, P.C. In Vitro Effects of Live and Heat-Inactivated Bifidobacterium animalis Subsp. Lactis, BB-12 and Lactobacillus rhamnosus GG on Caco-2 Cells. Nutrients. 2020, 12, 1719. doi: 10.3390/nu12061719
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and paraprobiotics in viral infection: Clinical application and effects on the innate and acquired immune systems. Curr. Pharm. Des. 2018, 24, 710–717. doi: 10.2174/1381612824666180116163411.
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; et al. Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immun. 2019, 10, 2247. doi: 10.3389/fimmu.2019.02247.
- Keilich, S.R.; Bartley, J.M.; Haynes, L. Diminished immune responses with aging predispose older adults to common and uncommon influenza complications. Cell. Immunol. 2019, 345, 103992. doi: 10.1016/j.cellimm.2019.103992.
- Oh, S.J.; Lee, J.K.; Shin, O.S. Aging and the immune system: The impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 2019, 19, e37. doi: 10.4110/in.2019.19.e37.
- Kawase, M.; He, F.; Miyazawa, K.; Kubota, A.; Yoda, K.; Hiramatsu, M. Orally administered heat-killed Lactobacillus gasseri TMC0356 can upregulate cell-mediated immunity in senescence-accelerated mice. FEMS Microbiol. Lett. 2012, 326, 125-130. doi: 10.1111/j.1574-6968.2011.02440.x.
- Kimoto-Nira, H.; Mizumachi, K.; Okamoto, T.; Sasaki, K.; Kurisaki, J. Influence of long-term consumption of a Lactococcus lactis strain on the intestinal immunity and intestinal flora of the senescence-accelerated mouse. Br. J. Nutr. 2009, 102, 181-185. doi: 10.1017/S0007114508143574.
- Kotani, Y.; Shinkai, S.; Okamatsu, H.; Toba, M.; Ogawa, K.; Yoshida, H.; et al. Oral intake of Lactobacillus pentosus strain b240 accelerates salivary immunoglobulin A secretion in the elderly: A randomized, placebo-controlled, double-blind trial. Immun. Ageing 2010, 7, 11. doi: 10.1186/1742-4933-7-11.
- Shinkai, S.; Toba, M.; Saito, T.; Sato, I.; Tsubouchi, M.; Taira, K.; et al. Immunoprotective effects of oral intake of heat-killed Lactobacillus pentosus strain b240 in elderly adults: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2013, 109, 1856–1865. doi: 10.1017/ S0007114512003753.
- Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Yoshikai, Y.; Tsuru, T. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J. Nutr. 2006, 136, 3069-3073. doi: 10.1093/jn/136.12.3069.
- Suganya, K.; Koo, B.S. Gut-brain axis: Role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. Int. J. Mol. Sci. 2020, 21, 7551. doi: 10.3390/ijms21207551.
