The European Water Framework Directive 2000/60/EC (WFD) has been implemented over the past 20 years, using physicochemical, biological and hydromorphological elements to assess the ecological status of surface waters. Benthic diatoms (i.e., phytobenthos) are one of the most common biological quality elements (BQEs) used in surface water monitoring and are particularly successful in detecting eutrophication, organic pollution and acidification. Herein, we reviewed their implementation in river biomonitoring for the purposes of the WFD, highlighting their advantages and disadvantages over other BQEs, and we discuss recent advances that could be applied in future biomonitoring. Until now, phytobenthos have been intercalibrated by the vast majority (26 out of 28) of EU Member States (MS) in 54% of the total water bodies assessed and was the most commonly used BQE after benthic invertebrates (85% of water bodies), followed by fish (53%), macrophytes (27%) and phytoplankton (4%). To meet the WFD demands, numerous taxonomy-based quality indices have been developed among MS, presenting, however, uncertainties possibly related to species biogeography. Recent development of different types of quality indices (trait-based, DNA sequencing and predictive modeling) could provide more accurate results in biomonitoring, but should be validated and intercalibrated among MS before their wide application in water quality assessments.
The European Water Framework Directive 2000/60/EC (WFD) has been implemented
over the past 20 years, using physicochemical, biological and hydromorphological elements to assess
the ecological status of surface waters. Benthic diatoms (i.e., phytobenthos) are one of the most
common biological quality elements (BQEs) used in surface water monitoring and are particularly
successful in detecting eutrophication, organic pollution and acidification. Herein, we reviewed their
implementation in river biomonitoring for the purposes of the WFD, highlighting their advantages
and disadvantages over other BQEs, and we discuss recent advances that could be applied in future
biomonitoring. Until now, phytobenthos have been intercalibrated by the vast majority (26 out of 28)
of EU Member States (MS) in 54% of the total water bodies assessed and was the most commonly used
BQE after benthic invertebrates (85% of water bodies), followed by fish (53%), macrophytes (27%)
and phytoplankton (4%). To meet the WFD demands, numerous taxonomy-based quality indices
have been developed among MS, presenting, however, uncertainties possibly related to species
biogeography. Recent development of different types of quality indices (trait-based, DNA sequencing
and predictive modeling) could provide more accurate results in biomonitoring, but should be
validated and intercalibrated among MS before their wide application in water quality assessments.
- phytobenthos
- biological quality indices
- ecological status
- surface waters
- water quality
1. Background
[1
[1]
[2]
[2]
[9]
for Europe (WISE) database (https://water.europa.eu, last accessed on 29 January 2021) to retrieve data for the ecological quality of MS after the second river basin management plan.
2. Benthic Diatoms in Biomonitoring
2.1. Importance of Benthic Diatoms as Biological Indicators
[10]
[16]
[27]
[28]
[11]
[33]
[25]
[35]
[34]
2.2. Advantages of Benthic Diatoms over Other Biological Quality Elements (BQEs)
[18]
[39]
[25]
[45]
[46]
[46]
[37]
[47]
[25]
[46]
[48]
[49]
[50]
[20]
[53]
[53]
2.3. Benthic Diatoms in the Water Framework Directive
[56]
[57]
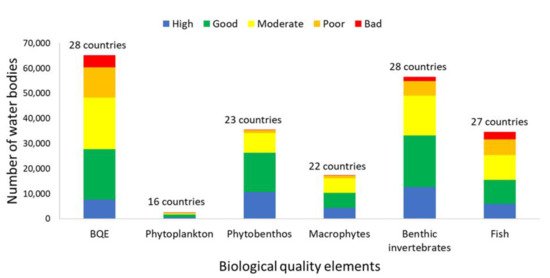
Figure 1.
[62]
[7]
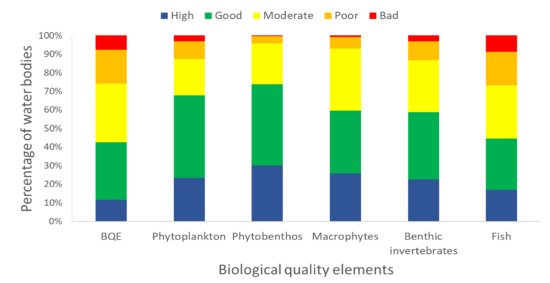
Figure 2.
2.4. Diatom-Based Indices Used So Far in the Water Framework Directive
[30]
[74]
[75]
[76]
[79]
[79]
[79]
[80]
[81]
[18]
[82]
[81]
[83]
[80]
To overcome the restrictions of the taxonomy-based indices, nontaxonomic measures emerged recently, taking into consideration functional traits (e.g., cell size, ecological guilds, life forms) and DNA sequences (e.g., operational taxonomic units, exact sequence variant, individual sequence units). Furthermore, assemblage structure methods, such as predictive models and statistical techniques (e.g., machine learning) have been developed to assess water quality using benthic diatom assemblages against different environmental parameters.
To overcome the restrictions of the taxonomy-based indices, nontaxonomic measures emerged recently, taking into consideration functional traits (e.g., cell size, ecological guilds, life forms) and DNA sequences (e.g., operational taxonomic units, exact sequence variant, individual sequence units). Furthermore, assemblage structure methods, such as predictive models and statistical techniques (e.g., machine learning) have been developed to assess water quality using benthic diatom assemblages against different environmental parameters.
During the last two decades, the WFD has been the main European legislation used for biological quality assessment of surface waters. Benthic diatoms, as the dominant part of phytobenthos, were used and successfully intercalibrated by 93% of EU MS in 54% of the total water bodies during the second river basin management plan. Their sensitivity to natural or anthropogenic disturbance, their ubiquitous nature (present in all types of natural and artificial substrates), their easy sampling and their fast response to environmental changes render the benthic diatoms as valuable bioindicators of biological assessment of aquatic systems.
During the last two decades, the WFD has been the main European legislation used for biological quality assessment of surface waters. Benthic diatoms, as the dominant part of phytobenthos, were used and successfully intercalibrated by 93% of EU MS in 54% of the total water bodies during the second river basin management plan. Their sensitivity to natural or anthropogenic disturbance, their ubiquitous nature (present in all types of natural and artificial substrates), their easy sampling and their fast response to environmental changes render the benthic diatoms as valuable bioindicators of biological assessment of aquatic systems.
Diatom quality indices are being implemented for the purpose of WFD, and many advances have been made in their development over the past 20 years. However, it seems that most of these advances were made toward the same direction, by adapting locally the same taxonomy-based indices. This resulted in more than half of the MS using the same index (IPS) irrespective of their ecoregion, raising doubts on the accuracy of the results. Development of HTS techniques have given a new boost in classic taxonomy-based indices, increasing the number of sequences that could be important for water quality status and probably introducing a more accurate classification. Agreement in quality classes has been proven high in cases tested, highlighting their future merits despite their long way before they can be generalized and used as a standalone method rather than a complementary tool in biomonitoring.
It was not until recently that research turned to other aspects of diatom assemblages, such as quality elements (functional traits, ecosystem processes and -diversity approaches). These new approaches could lead biomonitoring into a new era, by linking water quality assessment to ecosystem structure and function, thus towards the true objective of the WFD, i.e., a holistic ecosystem integrity approach. All of these new approaches should be validated and intercalibrated among MS, however, before their application in future water quality assessments.
References
- European Commission. Report from the Commission to the European Parliament and the Council on the Implementation of the WaterFramework Directive (2000/60/EC) River Basin Management Plans; Official Journal of the European Union: Luxemberg, 2012.
- European Commission. Carrying forward the Common Implementation Strategy for the Water Framework Directive-Progress and WorkProgramme for 2003/2004; Official Journal of the European Union: Luxemberg, 2003
- Sabater, S.; Guasch, H.; Ricart, M.; Romaní, A.; Vidal, G.; Klünder, C.; Schmitt-Jansen, M.; Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal. Bioanal. Chem 2007, 387, 1425-1434.
- Johnson, R.K.; Hering, D.; Response of taxonomic groups in streams to gradients in resource and habitat characteristics. J. Appl. Ecol 2009, 46, 175-186.
- Johnson, S.; Ringler, N.; The response of fish and macroinvertebrate assemblages to multiple stressors: A comparative analysis of aquatic communities in a perturbed watershed (Onondaga Lake, NY). Ecol. Indic 2014, 41, 198-208.
- Karaouzas, I.; Smeti, E.; Kalogianni, E.; Skoulikidis, N.T.; Ecological status monitoring and assessment in Greek rivers: Do macroinvertebrate and diatom indices indicate same responses to anthropogenic pressures?. Ecol. Indic 2019, 101, 126-132.
- Poikane, S.; Kelly, M.; Cantonati, M.; Benthic algal assessment of ecological status in European lakes and rivers: Challenges and opportunities. Sci. Total Environ 2016, 568, 603-613.
- Cetin, T.; Demir, N.; The use of phytobenthos for the ecological status assessment in Upper Sakarya Basin, Turkey. Ecol. Environ. Res. 2019, 17, 10155-10172.
- C´ iric´, M.; Nikolic´, N.; Krizmanic´, J.; Gavrilovic´, B.; Pantelic´, A.; Petrovic´, V.M.; Diatom diversity and ecological status of the Lasovaˇcka and Lenovaˇcka streams near Zajeˇcar: Consideration of WFD implementation in Serbia. Arch. Biol. Sci. 2018, 70, 691-698.
- Round, F.E.. The Diatoms; Cambridge University Press: Cambridge, UK, 1990; pp. 747.
- Mann, D.G.; The species concept in diatoms. Phycologia 1999, 38, 437-495.
- Falciatore, A.; Bowler, C; Revealing the Molecular Secrets of Marine Diatoms. Annu. Rev. Plant Biol 2002, 53, 109-130.
- Allen, A.E.; Vardi, A.; Bowler, C; An ecological and evolutionary context for integrated nitrogen metabolism and related signaling pathways in marine diatoms. Curr. Opin. Plant Biol 2006, 9, 264-273.
- Pardo, I.; Delgado, C.; Abraín, R.; Gómez-Rodríguez, C.; García-Roselló, E.; García, L.; Reynoldson, T.B.; A predictive diatom-based model to assess the ecological status of streams and rivers of Northern Spain.. Ecol. Indic 2018, 90, 519-528.
- Kelly, M.G.; Chiriac, G.; Soare-Minea, A.; Hamchevici, C.; Birk, S.; Defining ecological status of phytobenthos in very large rivers: A case study in practical implementation of the Water Framework Directive in Romania. Hydrobiologia 2018, 828, 353-367.
- Kitner, M.; Poulíˇcková, A.; Littoral diatoms as indicators for the eutrophication of shallow lakes. Hydrobiologia 2003, 506, 519-524.
- Licursi, M.; Gómez, N; Benthic diatoms and some environmental conditions in three lowland streams. Ann. Limnol. Int. J. Limnol. 2002, 38, 109-118.
- Hering, D.; Johnson, R.K.; Kramm, S.; Schmutz, S.; Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw. Biol 2006, 51, 1757-1785.
- Delgado, C.; Pardo, I.; García, L.; Diatom communities as indicators of ecological status in Mediterranean temporary streams (Balearic Islands, Spain). Ecol. Indic. 2012, 15, 131-139.
- Vilmi, A.; Karjalainen, S.M.; Freshwater diatoms as environmental indicators: Evaluating the effects of eutrophication using species morphology and biological indices. Environ. Monit. Assess. 2015, 187, 243.
- Giorgio, A.; De Bonis, S.; Guida, M.; Macroinvertebrate and diatom communities as indicators for the biological assessment of river Picentino (Campania, Italy). Ecol. Indic 2016, 64, 85-91.
- Cabecinha, E.; Cortes, R.; Cabral, J.A.; Ferreira, T.; Lourenço, M.; Pardal, M. Ângelo; Multi-scale approach using phytoplankton as a first step towards the definition of the ecological status of reservoirs.. Ecol. Indic 2009, 9, 240-255.
- Wu, N.; Faber, C.; Sun, X.; Qu, Y.; Wang, C.; Ivetic, S.; Riis, T.; Ulrich, U.; Fohrer, N; Importance of sampling frequency when collecting diatoms. Sci. Rep. 2016, 6, 36950.
- Karaouzas, I.; Smeti, E.; Vourka, A.; Vardakas, L.; Mentzafou, A.; Tornés, E.; Sabater, S.; Muñoz, I.; Skoulikidis, N.T.; Kalogianni, E.; et al. Assessing the ecological effects of water stress and pollution in a temporary river—Implications for water man-agement.. Total Environ. 2018, 618, 1591-1604,.
- Solak, C.N.; Peszek, Ł.; Yilmaz, E.; Ergül, H.A.; Kayal, M.; Ekmekçi, F.; Várbíró, G.; Yüce, A.M.; Canli, O.; Binici, M.S.; et al. Diatoms in Monitoring the Sakarya River Basin, Turkey. Water 2020, 12, 703.
- Cimarelli, L.; Singh, K.S.; Mai, N.T.N.; Dhar, B.C.; Brandi, A.; Brandi, L.; Spurio, R.Int. J. Environ. Res. Public Health; Molecular tools for the selective detection of nine diatom species biomarkers of various water quality levels.. Int. J. Environ. Res. Public Health 2015, 12, 5485-5504.
- Smeti, E.; Schiller, D.; Von Karaouzas, I.; Vardakas, L.; Sabater, S.; Tornés, E.; Monllor-alcaraz, L.S.; Guillem-argiles, N.; Martinez, E.; Barceló, D.; et al. Multiple stressor effects on biodi-versity and ecosystem functioning in a Mediterranean temporary river. Total Environ 2019, 647, 1179-1187.
- Laine, M.; Morin, S.; Tison-Rosebery, J; A multicompartment approach—Diatoms, macrophytes, benthic macroinverte-brates and fish—To assess the impact of toxic industrial releases on a small French river.. PLoS ONE 2014, 9, e102358.
- Fernández, M.R.; Martín, G.; Corzo, J.; de la Linde, A.; García, E.; López, M.; Sousa, M.; Design and Testing of a New Diatom-Based Index for Heavy Metal Pollution. Arch. Environ. Contam. Toxicol 2017, 74, 170-192.
- Falasco, E.; Bona, F.; Badino, G.; Hoffmann, L.; Ector, L; Diatom teratological forms and environmental alterations: A review.. Hydrobiologia 2009, 623, 1-35.
- Tornes, E.; Mor, J.R.; Mandaric, L.; Sabater, S.; Diatom responses to sewage inputs and hydrological alteration in Mediterranean streams.. Environ. Pollut. 2018, 238, 369–378..
- Montesanto, B.; Zille, S.; Coste, M.; Diatomees epilithiques et qualite biologique de ruisseaux du mont Stratonikon, Chalkidiki (Grece). . Cryptogam. Algol. 1999, 3, 235–251..
- Martínez-Carreras, N.; Wetzel, C.E.; Frentress, J.; Ector, L.; McDonnell, J.J.; Hoffmann, L.; Pfister, L.; Hydrological connectivity inferred from diatom transport through the riparian-stream system.. Hydrol. Earth Syst. Sci. 2015, 19, 3133-3151.
- Vidal, T.; Pereira, J.L.; Abrantes, N.; Soares, A.M.V.M.; Gonçalves, F; Ecotoxicological Assessment of Contaminated River Sites as a Proxy for theWater Framework Directive: An Acid Mine Drainage Case Study. Water Air Soil Pollut 2012, 223, 6009-6023.
- Morin, S.; Licursi, M.; Tison-Rosebery, J.. Benthic Diatom Monitoring and Assessment of Freshwater Environments: Standard Methods and Future Challenges. In Aquatic Biofilms: Ecology, Water Quality and Wastewater Treatment;; Romaní, A.M., Guasch, H., Balaguer, M.D., Eds.; Caister Academic Press: Poole: UK, 2016; pp. 111-124.
- Pandey, L.K.; Bergey, E.A.; Lyu, J.; Park, J.; Choi, S.; Lee, H.; Depuydt, S.; Oh, Y.T.; Lee, S.M.; Han, T.; et al. The use of diatoms in ecotoxicology and bioassessment: Insights, advances and challenges. Water Res. 2017, 118, 39-58.
- Dahm, V.; Hering, D.; Nemitz, D.; Graf,W.; Schmidt-Kloiber, A.; Leitner, P.; Melcher, A.; Feld, C.K.; Effects of physicochemistry, land use and hydromorphology on three riverine organism groups: A comparative analysis with monitoring data from Germany and Austria. Hydrobiologia 2013, 704, 389-415.
- Mangadze, T.; Bere, T.; Mwedzi, T.; Epilithic diatom flora in contrasting land-use settingsin tropical streams, Manyame Catchment, Zimbabwe.. Hydrobiologia 2015, 753, 163-173.
- Borojevi´c, K.K.; Udoviˇc, M.G.; Žutini´c, P.; Várbíró, G.; Plenkovi´c-Moraj, A.; Do benthic diatom assemblages reflect abiotic typology: A case study of Croatian streams and rivers. Acta Bot. Croat. 2017, 76, 80-90.
- Janauer, G.; Dokulil, M.. Macrophytes and Algae in Running Waters, Biological Monitoring of Rivers: Applications and Perspectives;; Ziglio, G., Siligardi, M., Flaim, G., Eds.; Wiley Online Library: Hudson County, NJ, USA, 2006; pp. ..
- Feipeng, L.; Haiping, Z.; Yiping, Z.; Yihua, X.; Ling, C.; Effect of flow velocity on phytoplankton biomass and composition in a freshwater lake. Sci. Total Environ 2013, 447, 64-71.
- Wehr, J.D.; Minireview Use of phytoplankton in large river management. J. Phycol 1998, 34, 741-749.
- Salmaso, N.; Braioni, M.G.; Factors controlling the seasonal development and distribution of the phytoplankton community in the lowland course of a large river in Northern Factors controlling the seasonal development and distribution of the phytoplankton community in the lowland course of a large river in Northern Italy (River Adige). Aquat. Ecol 2008, 42, 533-545.
- P. A. Chambers; E. E. Prepas; H. R. Hamilton; M. L. Bothwell; Current Velocity and Its Effect on Aquatic Macrophytes in Flowing Waters. Ecological Applications 1991, 1, 249-257, 10.2307/1941754.
- Susane, C.S.; Lawniczak, A.E.; Picinska-Faltynowicz, J.; Szoszkiewicz, K.; Do macrophytes, diatoms and non-diatom benthic algae give redundant information? Results from a case study in Poland.. Limnologica 2012, 42, 204-211.
- Marzin, A.; Archaimbault, V.; Belliard, J.; Chauvin, C.; Pont, D.; Ecological assessment of running waters: Do macrophytes, macroinvertebrates, diatoms and fish show similar responses to human pressures? Ecological assessment of running waters: Do macrophytes, macroinvertebrates, diatoms and fish show similar responses to human pressures. Ecol. Indic 2012, 23, 56-65.
- Qu, X.; Peng, W.; Liu, Y. Identifying the Impacts of Water Quality on Macroinvertebrate Degration in the Taizi River with aReconsideration of Water Quality Grades in China. In Proceedings of the 38th IAHR World Congress, Panama City, Panama, 1–6September 2019.
- Evangelia Smeti; Eleni Kalogianni; Ioannis Karaouzas; Sofia Laschou; Elisabet Tornés; Núria De Castro-Català; Evangelia Anastasopoulou; Maria Koutsodimou; Argyro Andriopoulou; Leonidas Vardakas; et al.Isabel MuñozSergi SabaterNikolaos Th. Skoulikidis Effects of olive mill wastewater discharge on benthic biota in Mediterranean streams. Environmental Pollution 2019, 254, 113057, 10.1016/j.envpol.2019.113057.
- S. Blanco; E. Bécares; Are biotic indices sensitive to river toxicants? A comparison of metrics based on diatoms and macro-invertebrates. Chemosphere 2010, 79, 18-25, 10.1016/j.chemosphere.2010.01.059.
- Giorgio Pace; Valentina Della Bella; Mariachiara Barile; Paolo Andreani; Laura Mancini; Carlo Belfiore; A comparison of macroinvertebrate and diatom responses to anthropogenic stress in small sized volcanic siliceous streams of Central Italy (Mediterranean Ecoregion). Ecological Indicators 2012, 23, 544-554, 10.1016/j.ecolind.2012.05.010.
- Snyder, D. Electrofishing and Its Harmful Effects on Fish; Information and Technology Report, USGS/BRD/ITR; US GovernmentPrinting Office: Denver, CO, USA, 2003; p. 13.
- Dodds,W.; Whiles, M.. Fish Ecology Fisheries and Aquaculture in Freshwater Ecology, 3rd ed.; Academic Press: London, UK, 2020; pp. Volume:23.
- Barbour, M.T.; Faulkner, C.; Gerritsen, J.. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates, and Fish, 2nd ed; George Gibson: Washington, DC, USA, 1999; pp. ..
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M.. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; English Edition with Updated Taxonomy and Added Species in Freshwater Benthic Diatoms of Central Europe; Cantonati, M., Kelly, M.G., Lange-Bertalot, H., Eds.; Koeltz Botanical Books: Frankfurt, Germany, 2017; pp. ..
- Blažena Brabcová; Petr Marvan; Libuše Opatřilová; Karel Brabec; Markéta Fránková; Jiří Heteša; Diatoms in water quality assessment: to count or not to count them?. Hydrobiologia 2017, 795, 113-127, 10.1007/s10750-017-3123-5.
- Salomé F.P. Almeida; Carmen Elias; João Ferreira; Elisabet Tornés; Camilla Puccinelli; François Delmas; Gerald Dörflinger; Gorazd Urbanič; Stefania Marcheggiani; Juliette Tison-Rosebery; et al.Laura ManciniSergi Sabater Water quality assessment of rivers using diatom metrics across Mediterranean Europe: A methods intercalibration exercise. Science of The Total Environment 2014, 476-477, 768-776, 10.1016/j.scitotenv.2013.11.144.
- European Environment Agency. European Waters Assessment of Status and Pressures; Report No 7/2018; EEA: Copenhagen,Denmark, 2018.
- Naicheng Wu; Hans Thodsen; Hans Estrup Andersen; Henrik Tornbjerg; Annette Baattrup-Pedersen; Tenna Riis; Flow regimes filter species traits of benthic diatom communities and modify the functional features of lowland streams - a nationwide scale study. Science of The Total Environment 2019, 651, 357-366, 10.1016/j.scitotenv.2018.09.210.
- Gunta Springer; Leonard Sandin; Agrita Briede; Agnija Skuja; Biological quality metrics: their variability and appropriate scale for assessing streams. Hydrobiologia 2006, 566, 153-172, 10.1007/s10750-006-0099-y.
- Eleni Kalogianni; Aikaterini Vourka; Ioannis Karaouzas; Leonidas Vardakas; Sofia Laschou; Nikolaos Th. Skoulikidis; Combined effects of water stress and pollution on macroinvertebrate and fish assemblages in a Mediterranean intermittent river. Science of The Total Environment 2017, 603-604, 639-650, 10.1016/j.scitotenv.2017.06.078.
- L. Vardakas; E. Kalogianni; S. Zogaris; Nicholas Koutsikos; T. Vavalidis; D. Koutsoubas; N. Th. Skoulikidis; Distribution patterns of fish assemblages in an Eastern Mediterranean intermittent river. Knowledge & Management of Aquatic Ecosystems 2015, 416, 30, 10.1051/kmae/2015026.
- Sandra Poikane; Fuensanta Salas Herrero; Martyn G. Kelly; Angel Borja; Sebastian Birk; Wouter van de Bund; European aquatic ecological assessment methods: A critical review of their sensitivity to key pressures. Science of The Total Environment 2020, 740, 140075, 10.1016/j.scitotenv.2020.140075.
- F Larson; K Sundbäck; Recovery of microphytobenthos and benthic functions after sediment deposition. Marine Ecology Progress Series 2012, 446, 31-44, 10.3354/meps09488.
- Calapez, A.R.; Elias, C.L.; Almeida, S.F.P.; Feio, M.J.; Extreme drought effects and recovery patterns in the benthic communities of temperate streams.. Limnetica 2014, 33, 281-296.
- Cemagref. Etude des Methodes Biologiques Quantitatives d’Appreciation de la Qualite des Eaux; Agence de l’eau Rhône MéditerranéeCorse: Lyon, France, 1982; p. 28.
- Coste, M.; Ayphasshorho, H. Etude de la Qualite des Eaux du Bassin Artois-Picardie a l’Aide des Communautes de Diatomees Benthiques(Application des Indices Diatomiques); Hal Inrae: Lyon, France, 1991; p. 227.
- M. G. Kelly; B. A. Whitton; The Trophic Diatom Index: a new index for monitoring eutrophication in rivers. Environmental Biology of Fishes 1995, 7, 433-444, 10.1007/bf00003802.
- Irene Álvarez-Blanco; Saúl Blanco; Cristina Cejudo-Figueiras; Eloy Bécares; The Duero Diatom Index (DDI) for river water quality assessment in NW Spain: design and validation. Environmental Monitoring and Assessment 2012, 185, 969-981, 10.1007/s10661-012-2607-z.
- Dell’uomo, A. Assessment of Water Quality of an Apennine River as a Piìot Study for Diatom-based Monitoring of ItalianWa-tercourses. In Use of Algae for Monitoring Rivers; Institut Fur Botanik, Universitàt: Vienna, Austria, 1996; pp. 65–72.
- Prygiel, J.; Management of the diatom monitoring networks in France. Environ. Biol. Fishes 2002, 14, 19-26.
- Martyn Kelly; Cathy Bennett; Michel Coste; Cristina Delgado; François Delmas; Luc Denys; Luc Ector; Claude Fauville; Martial Ferréol; Malgorzata Golub; et al.Amelie JarlmanMaria KahlertJohn LuceyBernadette Ní ChatháinIsabel PardoPeter PfisterJoanna Picinska-FaltynowiczJuliette Tison-RoseberyChristine SchranzJochen SchaumburgHerman Van DamSirje Vilbaste A comparison of national approaches to setting ecological status boundaries in phytobenthos assessment for the European Water Framework Directive: results of an intercalibration exercise. Hydrobiologia 2008, 621, 169-182, 10.1007/s10750-008-9641-4.
- M.G. Kelly; Role of benthic diatoms in the implementation of the Urban Wastewater Treatment Directive in the River Wear, North-East England. Journal of Applied Phycology 2002, 14, 9-18, 10.1023/a:1015236404305.
- Pertti Eloranta; Janne Soininen; Ecological status of some Finnish rivers evaluated using benthic diatom communities. Environmental Biology of Fishes 2002, 14, 1-7, 10.1023/a:1015275723489.
- C. Lecointe; M. Coste; J. Prygiel; ?Omnidia?: software for taxonomy, calculation of diatom indices and inventories management. Hydrobiologia 1993, 269-270, 509-513, 10.1007/bf00028048.
- Szczepocka, E.; Z˙ elazna-Wieczorek, J.; Diatom biomonitoring—Scientific foundations, commonly discussed issues and fre-quently made errors. Oceanol. Hydrobiol. Stud 2018, 47, 313-325.
- Martyn Kelly; Data rich, information poor? Phytobenthos assessment and the Water Framework Directive. European Journal of Phycology 2013, 48, 437-450, 10.1080/09670262.2013.852694.
- Philibert, A.; Prairie, Y.T. Diatom Inferred Paleolimnological Reconstructions: Do They Work in Nutrient Rich Lakes. In Proceedingsof the Sustainable Forest Management Network Conference, Edmonton, AB, Canada, 14–17 February 1999; pp. 155–160.
- Pipp, E.; A regional diatom-based trophic state indication system for running water sites in Upper Austria and its over regional applicability. Verh. Int. Ver. Limnol 2001, 27, 3376-3380.
- Kálmán Tapolczai; Agnès Bouchez; Csilla Stenger-Kovács; Judit Padisák; Frédéric Rimet; Trait-based ecological classifications for benthic algae: review and perspectives. Hydrobiologia 2016, 776, 1-17, 10.1007/s10750-016-2736-4.
- Otto Moog; Stefan Schmutz; Ilse Schwarzinger; Biomonitoring and Bioassessment. Riverine Ecosystem Management 2018, 8, 371-390, 10.1007/978-3-319-73250-3_19.
- Assane Anabi Toudjani; Abuzer Çelekli; E. Yonca Gümüş; Seda Kayhan; H. Ömer Lekesiz; Tolga Çetin; A new diatom index to assess ecological quality of running waters: a case study of water bodies in western Anatolia. Annales de Limnologie - International Journal of Limnology 2017, 53, 333-343, 10.1051/limn/2017012.
- McElligott, P. Developing Biocriteria as a Water Quality Assessment Tool in Canada: Scoping Assessment; Report; Canadian Council ofMinisters of the Environment: North Vancouver, BC, Canada, 2006.
- Tatenda Dalu; P. William Froneman; Diatom-based water quality monitoring in southern Africa: challenges and future prospects. Water SA 2016, 42, 551, 10.4314/wsa.v42i4.05.
- Tatenda Dalu; P. William Froneman; Diatom-based water quality monitoring in southern Africa: challenges and future prospects. Water SA 2016, 42, 551, 10.4314/wsa.v42i4.05.
- Otto Moog; Stefan Schmutz; Ilse Schwarzinger; Biomonitoring and Bioassessment. Riverine Ecosystem Management 2018, 8, 371-390, 10.1007/978-3-319-73250-3_19.
