The fibrosis-4 (FIB-4) index is the first triaging tool for excluding advanced fibrosis because of its accuracy, simplicity, and cheapness, especially for general physicians or endocrinologists, although the FIB-4 index has several drawbacks. Accumulating evidence has suggested that vibration-controlled transient elastography (VCTE) and the enhanced liver fibrosis (ELF) test may become useful as the second step after triaging by the FIB-4 index. The leading cause of mortality in MAFLD is cardiovascular disease (CVD), extrahepatic malignancy, and liver-related diseases. MAFLD often complicates chronic kidney disease (CKD), resulting in increased simultaneous liver kidney transplantation. The FIB-4 index could be a predictor of not only liver-related mortality and incident hepatocellular carcinoma, but also prevalent and incident CKD, CVD, and extrahepatic malignancy.
- hepatic fibrosis
- hepatocellular carcinoma
- cardiovascular disease
- FIB-4
1. Background
2. Which Fibrosis Stage Should We Pick up in MAFLD?
| Index | Formula | Strengths | Weaknesses |
|---|---|---|---|
| FIB-4 index [15][16][15,16] |
(age [years] × AST [U/L]/(platelet count [109/L] × √ALT [U/L]) https://www.eapharma.co.jp/medicalexpert/product/livact/fib-4/calculator.html (accessed on 25 January 2021) |
|
|
| NAFLD fibrosis score [26] |
−1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio–0.013 × platelet count (×109/L) − 0.66 × albumin (g/dL) http://nafldscore.com/ (accessed on 25 January 2021) |
|
| Cutoff Values | No. of Studies (No. of Patients) | Summary Sensitivity, %, Mean (Range) | Summary Specificity, %, Mean (Range) | Summary PPV, %, Mean (Range) | Summary NPV, %, Mean (Range) |
|---|
| APRI | |||||||||
| 0.452–0.50 | 5 (729) | 72.9 (50.0–87.4) |
|
||||||
| 67.7 | (43.1–91.0) | 44.8 | (22.9–71.0) |
89.4 (84.9–95.0) |
APRI [27] |
AST to platelet ratio index |
|
|
|
| = 1 point | |||||||||
| 0.54–0.98 | 7 (1,351) | 68.6 (61.0–76.2) |
72.7 (59.4–86.0) |
61.4 (46.9–76.2) |
77.6 (59.4–94.0) |
BARD [28] |
BMI > 28 kg/m2AST/ALT ratio > 0.8 = 2 points Diabetes = 1 point |
| |
| 1.00 | 4 (1101) |
|
|||||||
| 43.2 | (27.0–67.0) | 86.1 (81.0–89.0) |
33.5 (26.0–40.0) |
89.8 (84.0–95.0) |
CA-fibrosis index [29] |
||||
| 1.50 | 1.5 × type IV collagen 7S (ng/mL) + 0.0264 × AST (IU/l) | 4 (682) |
|
|
|||||
| ELF test [22] |
−7.412 + (In [HA] × 0.681) + (In [P3NP] × 0.775) + (In [TIMP1] × 0.494) |
|
|
||||||
3. The Usefulness of FIB-4 Index to Evaluating Severe Fibrosis in MAFLD
| APRI× | ||||||||||
| BARD× | ||||||||||
| NAFLD | ||||||||||
| [ | ||||||||||
| 58 | ||||||||||
| ] | ||||||||||
| 646 | ||||||||||
| Japan | ||||||||||
| Biopsy | FIB-4 ○ | FIB-4 ○ | FIB-4 × | |||||||
| NAFLD [64] | 4073 | Japan | US | NFS ○ | NFS ○ | |||||
| NAFLD with diabetes [63] |
284 | Australia | US | 51.4 (range 6.1–146). months |
NFS × | |||||
| FIB-4 × | ||||||||||
| APRI × | ||||||||||
| NAFLD [60] | 11,154 | US | US | 14.5 years | FIB-4 ○ | FIB-4 ○ | ||||
| NFS ○ | NFS ○ | |||||||||
| APRI ○ | APRI ○ | |||||||||
| NASH [69] |
148 | Canada | biopsy | Median: 5 years (IQR: 3–8) | FIB-4 ○ | |||||
| NFS ○ | ||||||||||
| APRI ○ | ||||||||||
| NAFLD [59] |
153 | US | biopsy | Median 104.8 (range, 3–317) months | NFS ◎ | |||||
| FIB-4 ○ | ||||||||||
| APRI ○ |
7. FIB-4 Index and Risk of Cardiovascular Disease
8. FIB-4 Index and Risk of Chronic Kidney Disease
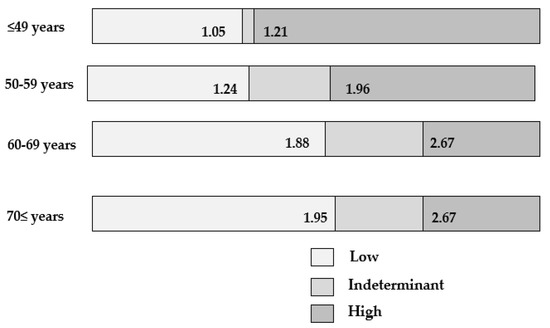
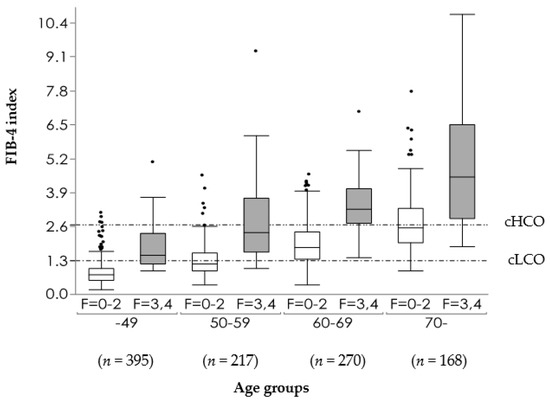
9. Distribution of FIB-4 Index in MAFLD Population
10. Drawbacks of FIB-4 Index
| Over-Referral | Under-Referral | ||||
|---|---|---|---|---|---|
| FIB-4 index low COI | 1.3 | 1.45 | |||
| GP | Work ↓ | Work ↑ | |||
| Hepatologists | Work ↑ | Work ↓ | |||
| Unnecessary liver biopsy | |||||
| 32.9 | (6.3–70.0) | 90.5 (74.5–97.0) |
55.5 (40.0–72.1) |
79.1 (73.2–87.2) |
|
| FIB-4 index | |||||
| 1.24–1.45 | 10 (2759) | 77.8 (63.0–90.0) |
71.2 (55.5–88.0) |
40.3 (24.0–50.6) |
92.7 (88.0–98.0) |
| 1.51–2.24 | 8 (1533) | 77.0 (70.6–89.5) |
79.2 (67.1–93.6) |
66.4 (37.4–85.7) |
83.9 (58.6–97.2) |
| 2.67 | 6 (1910) | 31.9 (12.0–63.2) |
95.7 (88.3–98.7) |
66.0 (51.1–80.0) |
85.0 (79.4–92.6) |
| 3.25 | 6 (1890) | 37.3 (5.0–56.0) |
95.8 (89.0–100) |
72.5 (37.0–100) |
87.3 (78.5–94.0) |
| 5.31–10.62 | 4 (543) | 67.5 (50.0–100) |
80.8 (54.0–100) |
90.0 (80.0–100) |
85.1 (80.0–90.2) |
| BARD | |||||
| 1.5 | 1 (242) | 83.0 | 59.0 | 34.0 | 93.0 |
| 2 | 14 (3057) | 75.2 (41.7–100) |
61.6 (32.5–88.9) |
38.3 (15.0–79.8) |
88.7 (49.6–100) |
| 3–4 | 5 (736) | 59.4 (33.3–85.2) |
75.1 (59.9–91.8) |
55.2 (24.0–69.2) |
81.0 (71.4–90.1) |
| NFS | |||||
| (−26.93)–(−2.16) | 2 (106) | 80.5 (78.0–83.0) |
69.5 (69.0–70.0) |
None | None |
| −1.455 | 10 (3057) | 72.9 (22.7–96.0) |
73.8 (42.9–100) |
50.4 (24.0–100) |
91.8 (81.3–98.1) |
| (−1.31)–(0.156) | 5 (963) | 78.2 (69.0–86.4) |
71.7 (60.0–83.0) |
58.4 (34.0–80.8) |
82.1 (54.1–95.0) |
| 0.67–0.676 | 14 (3896) | 43.1 (8.3–100) |
88.4 (25.0–100) |
66.9 (26.0–100) |
88.5 (78.6–100) |
| 0.735 | 1 (235) | 68.4 | 88.3 | 53.0 | 93.5 |
4. The Compassion between FIB-4 Index and VCTE
5. FIB-4 Index and Carcinogenesis
6. FIB-4 Index and Mortality
| Subjects | N | Nation | Dx | Observation Period | Over-all Mortality /Morbidity |
Liver-Related Mortality/Morbidity | Liver Event | HCC | CVD Mortality | Extrahepatic Cancer |
|---|
| NAFLD [62] | 646 | Sweden | Biopsy | 19.9 ±8.7 years |
FIB-4 ○ | FIB-4 ○ | ||||||
| NFS ○ | NFS ○ | |||||||||||
| Viral hepatitis-negative adults [61] |
14,841 | USA | General population | Median 19.3 years (IRQ, 17.5–21.1) years | APRI ○ | APRI ○ | FIB-4 ○ | APRI ○ | ||||
| FIB-4 ○ | FIB-4 ○ | |||||||||||
| May increase | May reduce | NFS ○ | NFS ○ | |||||||||
| HCC early detection | Possible? | May delay diagnosis? | Forns score ○ | |||||||||
| Heath economic costs | Forns score○ | |||||||||||
| High? | Low? | NAFLD [57] | 153 | Israel | Biopsy | 100 months (mean) |
FIB-4 ○ | FIB-4 ○ | FIB-4 ○ | |||
| NFS ○ | NFS ○ | NFS ○ | ||||||||||
| APRI × | APRI ○ | APRI ○ | ||||||||||
| NAFLD [68] | 180 | China | US | 6.6 (range 0.5–14.8) years |
NFS ◎ | |||||||
| FIB-4 ○ | ||||||||||||
FAST score =e−1.65+1.07×In(LSM)+2.66×10−8×CAP863.3×AST−11+e−1.65+1.07×In(LSM)+2.66×10−8×CAP3−63.3×AST−1
11. Two-Step Diagnostic Algorithm Using FIB-4 Index as the First Step
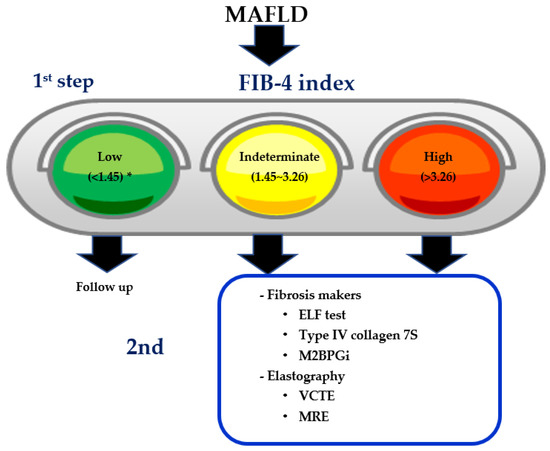
12. FIB-4 Index as Milestones of Treatment in MAFLD
| Author | Subjects | Outcomes | Parameter Correlated with Pathological Improvement |
|---|---|---|---|
| Hamaguchi [121] |
MAFLD (n = 39) | Hepatic fibrosis | ⊿HbA1c reduction |
| Seko [122] |
Steatohepatitis (n = 52) | NAS Hepatic fibrosis |
⊿ALT reduction ≥ 30% from baseline |
| Hoofnagle [123] |
Steatohepatitis (n = 139) without DM PIVENS trial |
NAS Hepatic fibrosis |
⊿ALT reduction ≥ 30% from baseline or post-treatment ALT ≤ 40 IU/L |
| Vilar-Gomez [124] |
Steatohepatitis (n = 261) | NASH resolution w/o worsening fibrosis | ⊿BW reduction, absence of T2D ALT normalization, younger age, NAS < 5 |
| Vuppalanchi | Adult steatohepatitis (n = 231) Pediatric MAFLD (n = 152) |
Histological improvement | ⊿CK18 reduction (inferior to ⊿ALT reduction) |
| Siddiqui [119] |
MAFLD (n = 292) | Hepatic fibrosis | ⊿FIB-4 index, ⊿NFS, ⊿APRI |
| Jayakumar [111] |
Steatohepatitis, stage 2–3 (n = 54) Selonsertib (Phase 2) |
Hepatic fibrosis | ⊿MRE |
| Hepatic steatosis | MRI-PDFF > 25% reduction | ||
| Chalasani [120] |
Steatohepatitis (n = 200) FLINT trial (Phase 2) Placebo vs. OCA 72wk |
Hepatic fibrosis | ⊿FIB-4 index ⊿APRI (⊿NFS: no correlation) |
| Loomba | NAS ≥ 2 points reduction without worsening fibrosis | OCA(+), pretreatment NAS > 5, TG ≤ 154 mg/dL, INR < 1, AST < 49 IU/L, ⊿ALT at 24wk (>17 IU/L) |