Sperm cryopreservation is one of the most efficient ways to preserve rabbit strains because it is easy to collect ejaculate repeatedly from a single male and inseminate artificially into multiple females. During the cooling, freezing and thawing process of sperms, the plasma membrane, cytoplasm and genome structures could be damaged by osmotic stress, cold shock, intracellular ice crystal formation, and excessive production of reactive oxygen species.
- rabbit
- sperm quality
- cryopreservation
- animal model
- assisted reproductive technology
1. Introduction
Rabbits have been indispensable for human life because they are not only valuable for agriculture but also for biomedical research. Rabbits are widely used as a source of meat, hair and fur, and it is estimated that each year, around 300 million rabbits (and hares) are used in the world [1]. Because of their tame characters, rabbits are also raised as a pet. In addition, rabbits are the most-used animals for antibody production for biomedical research. Furthermore, rabbits are similar to humans in terms of cardiovascular physiology and lipid metabolism, and they play an important role in studying human diseases such as atherosclerosis and hypercholesterolemia [2][3]. Along with the development of genetic engineering, many gene-modified rabbits have been created as experimental models of human diseases. In addition to transgenic rabbits produced with the conventional pronuclear microinjection technique, knockout rabbits have been established using CRISPR/Cas9 genome editing technology [4]. These genetically modified rabbits are valuable and thus it is vitally important to breed and maintain rabbit strains for different purposes and preserve them as bio-resources [5].
2. Major Ways to Preserve Rabbit Strains
There are two major ways to preserve rabbit strains. The common way to maintain a rabbit colony is carried out simply by repeat breeding. However, several difficulties with this method exist including space and cost. In particular, rabbit shows severe inbreeding depression [6][7][8][9], thus a considerable number of rabbits are required to keep a colony. For laboratory rabbits, they are usually housed in strictly controlled conditions in terms of temperature, humidity, illumination and microbiological examinations. Furthermore, living animals have a risk of annihilation or escape in the case of a disaster or accident.
The second method of maintaining the rabbit colony is the cryopreservation of gametes. Cryopreservation of gametes requires less space and cost than animal breeding. It is generally believed that properly cryopreserved zygotes and gametes can be preserved semi-eternally in a liquid nitrogen tank to keep their fertile and developmental ability. In the case of employing ovum or embryo preservation, ova or embryos are generally obtained with oviduct–uterus dissection from sacrificed females, and a skillful surgical operation is required for the embryo transfer. In contrast, ejected semen can be collected repeatedly without sacrificing males (Figure 1) and artificially inseminated into females can be conducted without specific skills, and thus, sperm cryopreservation would be the first choice for preservation of rabbit strains even though sperms preserved can bring paternal hereditary information only, and immediate offspring is always heterozygosity. However, when concerned with one specific transgene, homozygotes can be obtained in the second generation. Even in the case of livestock animals concerning pedigree related with polygenetic factors, the inbreeding can be avoided by mating live females and cryopreserved sperm with a generation gap.
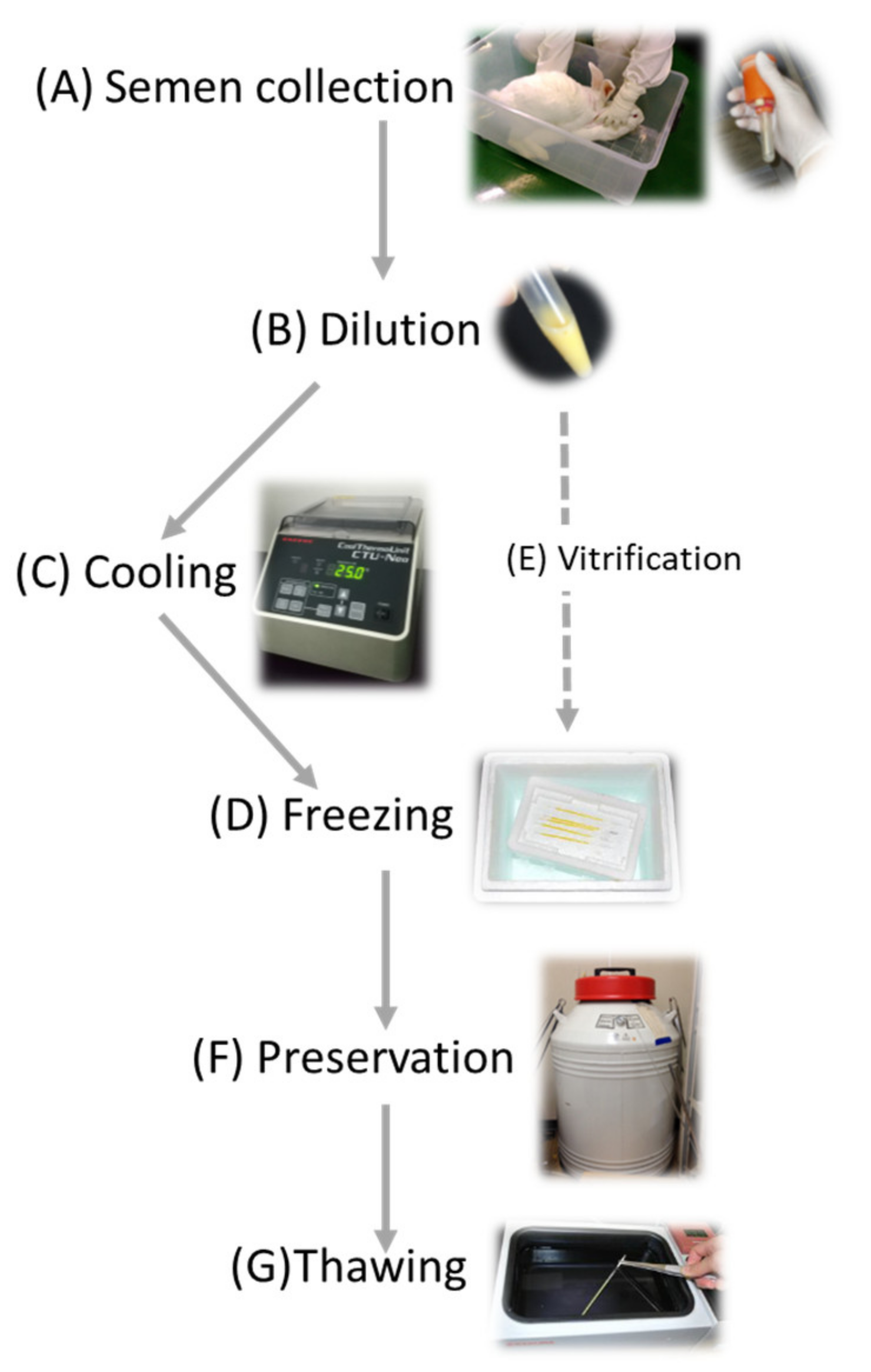
Figure 1.
A
B
C
D
E
F
G
As mentioned above, the successful preservation of rabbits depends on the efficiency and reliability of procedures in sperm cryopreservation. It is known that the process of sperm cryopreservation, including cooling, freezing and thawing, leads to cellular damage on membrane, cytoplasm and genome structures [10][11] caused by osmotic stress, cold shock, intracellular ice crystal formation, and excessive production of reactive oxygen species (ROS) [12] (Figure 2). During the cooling process, the sperm membrane is injured by cold shock which can be diminished by cooling rate [13] or materials stabilizing the membrane including egg yolk or skim milk [14]. The addition of cryoprotectants causes osmotic and toxic stress, which increases due to prolonged exposure during slow cooling [15]. In the following freezing process, major problem is ice crystal formation which grows larger by recrystallization and injures cell [16]. The freezing rate and cryoprotectants application should be considered to diminish the problem. Recrystallization occurs during the thawing process because of entry through the recrystallization temperature zone. Since sperms suffer from oxidative stress throughout the cryopreservation process [12], supplementation of antioxidants is considerable for the improvement of sperm quality.
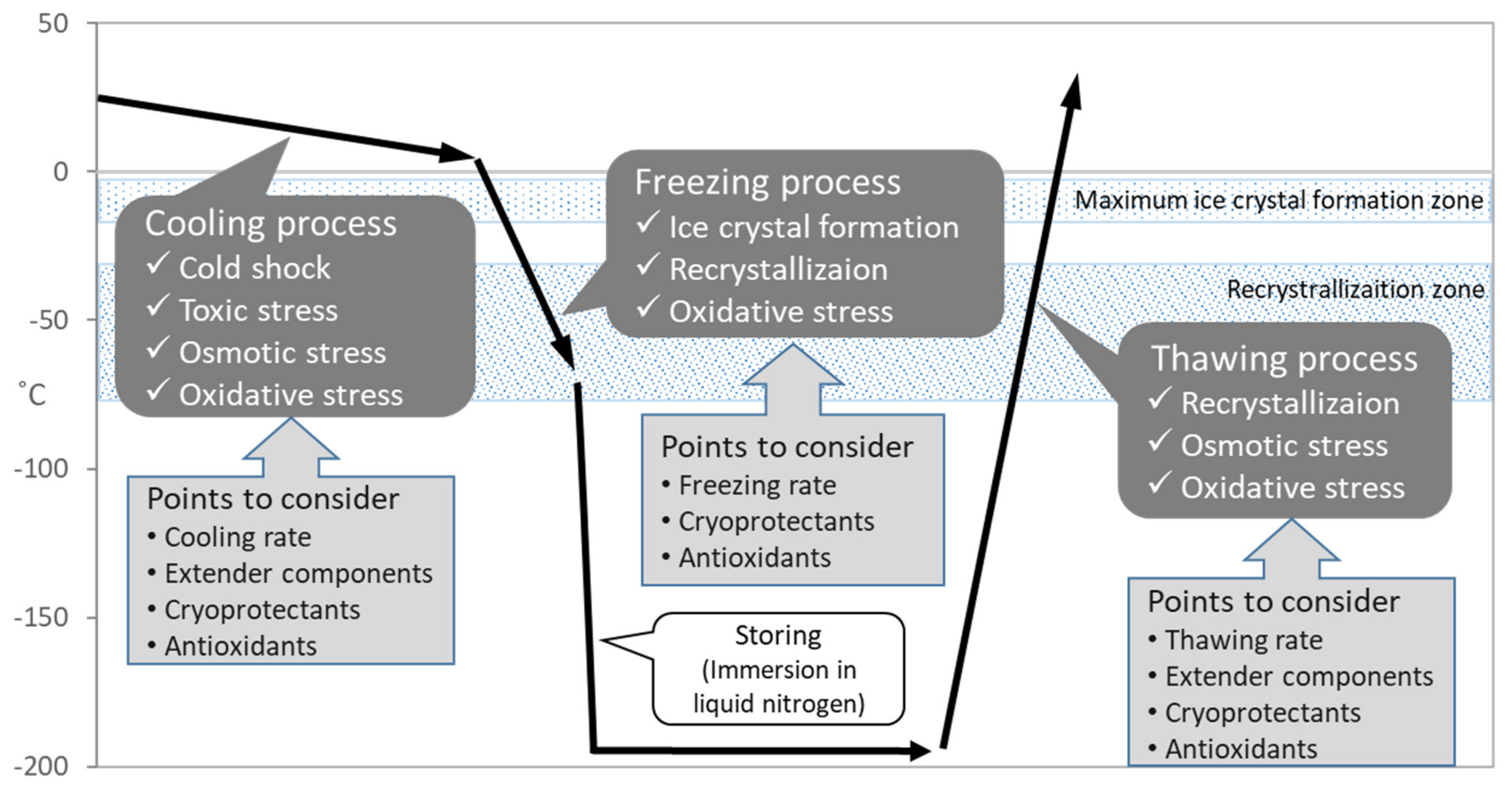
Figure 2.
3. Conclusions and Perspectives
Numerous studies on improving rabbit sperm cryopreservation have been conducted from various aspects including freezing procedure, type, concentration and combination of cryoprotectants. In spite of this, a standard procedure for rabbit sperm cryopreservation has not been well established due to various and irreproducible results from each study.
One of the reasons for the irreproducibility in rabbit sperm cryopreservation may be derived from the differences in sperm conditions. It is known that freezabilities of rabbit sperms differ among individuals [17] or breeds [18]. However, rabbit breeds used in some reproductive studies have not strictly and well defined rather than other livestock animals such as cattle, horses or pigs, and some authors even did not provide enough information about the rabbit breed examined. There are several reports which indicate associations between the sperm freezability and abundance of particular components in seminal plasma including proteins and fatty acids in some species [19][20][21][22][23][24]. The individual and breed difference in sperm freezability can be explained by such seminal plasma traits. It would be possible to improve the sperm freezability and resolve the individual or breed differences by complemental supplementations according to the seminal plasma trait. In the rabbit, it was revealed that genotype, i.e., breed, affects the abundance of some seminal plasma proteins [25], which are associated with sperm quality [26]. Furthermore, there is no information about the association between sperm freezability and seminal plasma traits in rabbits, and further studies are needed. Again, lack of information about the rabbit breed can disturb the improvement of rabbit sperm cryopreservation efficiency.
Another possible reason for the irreproducibility is disunified evaluation criteria of the quality of the sperms among the reports. Some studies report both the quality of the frozen–thawed sperms and their fertility and prolificacy, and others do only one of them. Examination of fertility and prolificacy involves artificial insemination procedures which affect the results of the study. On the other hand, fertility and prolificacy cannot be estimated by the sperm quality alone, even though obtaining more motile sperms is generally advantageous for efficient reproduction [27][28][29]. Additionally, the rate of rapid and progressive motile sperm would be important for successful artificial insemination, since the inseminated sperm need to reach the ova via a long reproductive tract [30][31][32].
In any case, to achieve a consensus on the efficient method for rabbit sperm cryopreservation, extensive investigations are required under unified evaluation criteria and conditions except for factors like extenders, cryoprotectants and procedures to be examined. On the other hand, it seemed that the supplements like antioxidants generally just add their effects without interfering with other components in the extender. Therefore, most of the supplements exert expected effects on the quality of frozen–thawed rabbit sperm even in various conditions including the extender (Table 1). The dose and combination of the supplements can be a key subject for highly efficient rabbit sperm cryopreservation in future studies.
As Dr. Robert G. Edwards was awarded The Nobel Prize in Physiology or Medicine for the development of in vitro fertilization (IVF) in 2010, assisted reproductive technology (ART) including IVF is an indispensable medical procedure in this modern age. The rabbit is also known as a prime reproductive model for human health, because of (1) exact staging of early embryonic developmental and maternal pregnancy stages, (2) large-sized blastocysts amenable to micromanipulation, (3) cell-lineage-specific analyses, (4) gastrulation stages representative of mammalian development, and (5) placental morphology and function similar to the human [33]. Therefore, the development of reproductive technology in rabbits, which leads to the improvement of medical procedures in humans, is very important and desirable.
References
- Food and Agriculture Organization of the United Nations. FAO Database. 2019. Available online: (accessed on 14 March 2021).
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015, 146, 104–119.
- Shiomi, M. The History of the WHHL Rabbit, an Animal Model of Familial Hypercholesterolemia (I)—Contribution to the Elucidation of the Pathophysiology of Human Hypercholesterolemia and Coronary Heart Disease. J. Atheroscler. Thromb. 2020, 27, 105–118.
- Matsuhisa, F.; Kitajima, S.; Nishijima, K.; Akiyoshi, T.; Morimoto, M.; Fan, J. Transgenic Rabbit Models: Now and the Future. Appl. Sci. 2020, 10, 7416.
- Mazur, P.; Leibo, S.P.; Seidel, G.E., Jr. Cryopreservation of the germplasm of animals used in biological and medical research: Importance, impact, status, and future directions. Biol. Reprod. 2007, 78, 2–12.
- Casellas, J.; Vidal-Roqueta, D.; Flores, E.; Casellas-Vidal, D.; Llach-Vila, M.; Salgas-Fina, R.; Casellas-Molas, P. Epistasis for founder specific inbreeding depression in rabbits. J. Hered. 2011, 102, 157–164.
- Chai, C.K. Effects of inbreeding in rabbits. Inbred lines, discrete characters, breeding performance, and mortality. J. Hered. 1969, 60, 64–70.
- Chai, C.K.; Degenhardt, K.H. Developmental anomaties in inbred rabbits. J. Hered. 1962, 53, 174–182.
- Ragab, M.; Sánchez, J.P.; Baselga, M. Effective population size and inbreeding depression on litter size in rabbits. A case study. J. Anim. Breed. Genet. 2015, 132, 68–73.
- Isachenko, E.; Isachenko, V.; Katkov, I.I.; Dessole, S.; Nawroth, F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: From past practical difficulties to present success. Reprod. Biomed. Online 2003, 6, 191–200.
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492.
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank 2016, 17, 745–756.
- Parrish, J.J.; Foote, R. Fertility of cooled and frozen rabbit sperm measured by competitive fertilization. Biol. Reprod. 1986, 35, 253–257.
- Mocé, E.; Vicente, J.S. Rabbit sperm cryopreservation: A review. Anim. Reprod. Sci. 2009, 110, 1–24.
- Mazur, P. The role of intracellular freezing in the death of cellscooled at supraoptimal rates. Cryobiology 1977, 14, 251–272.
- Koshimoto, C.; Mazur, P. Effects of warming rate, temperature, and antifreeze proteins on the survival of mouse spermatozoa frozen at an optimal rate. Cryobiology 2002, 45, 49–59.
- Mocé, E.; Lavara, R.; Vicente, J.S. Influence of the donor male on the fertility of frozen-thawed rabbit sperm after artificial insemination of females of different genotypes. Reprod. Domest. Anim. 2005, 40, 516–521.
- Kulíková, B.; Oravcová, M.; Baláži, A.; Supuka, P.; Chrenek, P. Factors affecting storage of Slovak native rabbit semen in the gene bank. Zygote 2017, 25, 592–600.
- Martínez-Soto, J.C.; Landeras, J.; Gadea, J. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology 2013, 1, 365–375.
- Rickard, J.P.; Leahy, T.; Soleilhavoup, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Lynch, G.W.; Druart, X.; de Graaf, S.P. The identification of proteomic markers of sperm freezing resilience in ram seminal plasma. J. Proteom. 2015, 126, 303–311.
- Vilagran, I.; Castillo, J.; Bonet, S.; Sancho, S.; Yeste, M.; Estanyol, J.M.; Oliva, R. Acrosin-binding protein (ACRBP) and triosephosphate isomerase (TPI) are good markers to predict boar sperm freezing capacity . Theriogenology 2013, 80, 443–450.
- Vilagran, I.; Yeste, M.; Sancho, S.; Castillo, J.; Oliva, R.; Bonet, S. Comparative analysis of boar seminal plasma proteome from different freezability ejaculates and identification of Fibronectin 1 as sperm freezability marker. Andrology 2015, 3, 345–356.
- Wang, P.; Wang, Y.F.; Wang, H.; Wang, C.W.; Zan, L.S.; Hu, J.H.; Li, Q.W.; Jia, Y.H.; Ma, G.J. HSP90 expression correlation with the freezing resistance of bull sperm. Zygote 2014, 22, 239–245.
- Yeste, M.; Estrada, E.; Casas, I.; Bonet, S.; Rodríguez-Gil, J.E. Good and bad freezability boar ejaculates differ in the integrity of nucleoprotein structure after freeze-thawing but not in ROS levels. Theriogenology 2013, 79, 929–939.
- Casares-Crespo, L.; Fernández-Serrano, P.; Vicente, J.S.; Marco-Jiménez, F.; Viudes-de-Castro, M.P. Rabbit seminal plasma proteome: The importance of the genetic origin. Anim. Reprod. Sci. 2018, 189, 30–42.
- Bezerra, M.J.B.; Arruda-Alencar, J.M.; Martins, J.A.M.; Viana, A.G.A.; Viana Neto, A.M.; Rêgo, J.P.A.; Oliveira, R.V.; Lobo, M.; Moreira, A.C.O.; Moreira, R.A.; et al. Major seminal plasma proteome of rabbits and associations with sperm quality. Theriogenology 2019, 128, 156–166.
- Baker, R.D.; Dziuk, P.J.; Norton, H.W. Effect of volume of semen, number of sperm and drugs on transport of sperm in artificially inseminated gilts. J. Anim. Sci. 1968, 27, 88–93.
- Flowers, W.L. Increasing fertilization rate of boars: Influence of number and quality of spermatozoa inseminated. J. Anim. Sci. 2002, 80, E47–E53.
- Langford, G.A.; Marcus, G.J. Influence of sperm number and seminal plasma on fertility of progestagen-treated sheep in confinement. J. Reprod. Fertil. 1982, 65, 325–329.
- Berker, B.; Şükür, Y.E.; Kahraman, K.; Atabekoğlu, C.S.; Sönmezer, M.; Özmen, B.; Ateş, C. Absence of rapid and linear progressive motile spermatozoa “grade A” in semen specimens: Does it change intrauterine insemination outcomes? Urology 2012, 80, 1262–1266.
- Bollendorf, A.; Check, J.H.; Lurie, D. Evaluation of the effect of the absence of sperm with rapid and linear progressive motility on subsequent pregnancy rates following intrauterine insemination or in vitro fertilization. J. Androl. 1996, 17, 550–557.
- Nagata, M.P.B.; Endo, K.; Ogata, K.; Yamanaka, K.; Egashira, J.; Katafuchi, N.; Yamanouchi, T.; Matsuda, H.; Goto, Y.; Sakatani, M.; et al. Live births from artificial insemination of microfluidic-sorted bovine spermatozoa characterized by trajectories correlated with fertility. Proc. Natl. Acad. Sci. USA 2018, 115, E3087–E3096.
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Navarrete Santos, A.; Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 2012, 144, 1–10.
