Postbiotics are health-promoting microbial metabolites delivered as a functional food or a food supplement. They either directly influence signaling pathways of the body or indirectly manipulate metabolism and the composition of intestinal microflora. Cancer is the second leading cause of death worldwide and even though the prognosis of patients is improving, it is still poor in the substantial part of the cases. The preventable nature of cancer and the importance of a complex multi-level approach in anticancer therapy motivate the search for novel avenues of establishing the anticancer environment in the human body. This article summarizes the principal findings demonstrating the usefulness of both natural and synthetic sources of postbotics in the prevention and therapy of cancer. Specifically, the effects of crude cell-free supernatants, the short-chain fatty acid butyrate, lactic acid, hydrogen sulfide, and β-glucans are described. Contradictory roles of postbiotics in healthy and tumor tissues are highlighted. In conclusion, the application of postbiotics is an efficient complementary strategy to combat cancer.
- microbiome
- colorectal cancer
- intestinal metabolome
- GPR81
- SCFA
- functional food
Introduction
-
Prebiotics are non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of a limited number of bacteria in the colon, thus improving the host’s health.
-
Probiotics are living microorganisms that, when administered in adequate amounts, confer a health benefit on the host.
-
Synbiotics are combinations of prebiotics and probiotics that have beneficial effects on the gut microbiome.
-
Postbiotics are substances released by or produced through the metabolic activity of the microorganisms which directly exert beneficial effects on the host.
While the research on probiotics has begun more than 100 years ago, postbiotics are a newcomer in the field of science, even though the beneficial effects of fermented food have been known for millennia. Until recently, the studies have been focused on the effects of probiotics, mainly lactic acid-producing bacteria such as Lactobacillus or Bifidobacterium, and on prebiotics such as dietary fiber, human milk oligosaccharides, lactulose, and inulin derivatives. However, the increasing body of evidence suggests that the overall metabolism of the intestinal microflora is more important than the presence or absence of any particular microbial species. The postbiotics research was initiated by studies employing the cell-free supernatants (CFSs) from bacterial fermentation followed by primary microbial metabolites such as lactic acid and short-chain fatty acids (SCFA). However, there are numerous other interesting molecules including the odorous gas hydrogen sulfide (H2S).
Metabolic signaling in cancer
Here, we hypothesize that an important component of the anti-cancer effects of postbiotics is the induction of cell response to metabolic and oxidative stress. As microbial metabolic waste products, postbiotics disturb the metabolic homeostasis of host cells, causing metabolic and oxidative stress. Lactate has been shown to stimulate mitochondrial production of reactive oxygen species (ROS) by increasing the NADH/NAD+ ratio and serving as a substrate of lactate oxidase. Butyrate has also been shown to stimulate ROS levels, although the exact mechanism is unknown. H2S covalently modifies sulfhydryl moieties of proteins and inhibits mitochondrial cytochrome c oxidase enhancing the ROS release.
The adaptive response of the cells is driven by increased activity of stress signaling pathways, including, but not limited to, the energy sensor AMP-activated protein kinase (AMPK), the family of sirtuin deacetylases (SIRT1-7), the metabolic transcription coactivator PGC1α, the antioxidant transcription factor NFE2L2 (Nrf2), and the stimulation of specific survival processes including mitochondrial biogenesis and autophagy. The enhanced stress signaling protects normal tissues against carcinogenesis by suppressing cell proliferation, mutagenesis, and tissue inflammation. Specifically, AMPK activity mimics the cancer-preventing effects of caloric restriction by enhancing autophagy and suppressing mTOR signaling. It is also an activator of sirtuins and PGC1α that inhibit the cell cycle and nuclear factor kappa B (NFκB) signaling. In addition, Nrf2-driven transcription stimulates the antioxidant, detoxification, and DNA repair capacity of the cells. It is important to note that butyrate induces Nrf2 directly through its effect on histone acetylation.
On the other hand, the increased production of mitochondrial ROS accompanied by the enhanced stress signaling frequently occurs in cancer cells as a byproduct of metabolic transformation. Moreover, it is indispensable for the progression of the established malignant tumors due to its pro-survival effect. However, such deregulated signaling may be overwhelmed by external metabolic stress, including postbiotics. Therefore, postbiotics also suppress the progression of established tumors by exaggerating the metabolic and oxidative stress, causing programmed cell death (Figure 1). [1], [2],
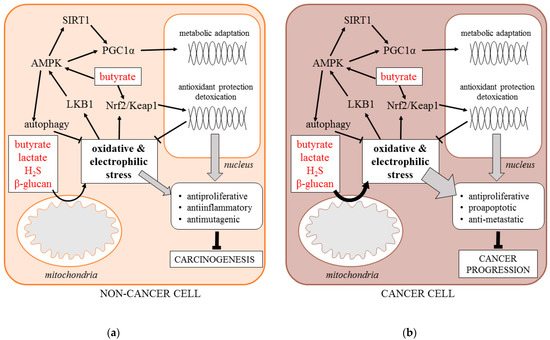
Figure 1. Signaling response of non-cancer (a) and cancer cell (b) to postbiotics (AMPK—5’ AMP-activated protein kinase, H2S—hydrogen sulfide, Keap1—Kelch Like ECH Associated Protein 1, LBK1—liver kinase B1, Nrf-2—Nuclear factor erythroid 2-related factor 2, PGC1α—Peroxisome proliferator-activated receptor gamma coactivator 1-alpha, SIRT1—Sirtuin 1).
Tributyrin
Tributyrin – a triacylglycerol containing three molecules of butyrate was tested as a postbiotic agent avoiding the disgusting odor and a short half-life of free butyrate. Several in vitro studies on different cancer cell lines showed, that tributyrin induces the apoptosis at millimolar levels. More importantly, the treatment with tributyrin also induced apoptosis in some in vivo studies. Giermasz et al. showed that the systematic administration of tributyrin significantly retarded growth of B16F10 melanoma in mice. This effect was also observed in another in vivo study on mouse with transplanted prostate cancer cell lines. These results motivated phase I clinical trials for tributyrin tolerability in cancer patients, which proved good tolerance and promising effects on the disease progression. In addition, the preventive effects of tributyrin also deserve the attention. The treatment with tributyrin reduced the frequency of preneoplastic lesions in a rat model of chemical hepatocarcinogenesis. Tributyrin feeding also protected against intestinal injury and inflammation in various animal models, both phenomena being the well-known contributors to the intestinal carcinogenesis.
Cancer prevention with fermented dairy products
There is about 0.9 % of lactic acid in yogurts and 2 % in kefirs. In 2008, a large prospective study of the effect of fermented milk on 82 002 Swedish women and men was published. This study was focused on the bladder cancer, because most metabolites are excreted through the urinary bladder providing a visible effect of orally administered dairy products. The results suggest that high intake of cultured milk may reduce the risk of developing bladder cancer. Other studies have shown that a regular consumption of yogurt but not milk decreases the incidence of colorectal cancer in Europe and US. A higher adolescent dairy intake was associated with lower rectal and advanced adenoma risk later in life in a large cohort of nurses. A regular yogurt intake versus rare intake was associated with decreased odds of hyperplastic polyps and adenomatous polyps in colon.
Conclusions
Postbiotics represent an emerging concept appreciating the importance of microbial metabolites in the maintenance of health. Butyrate, the prototypical SCFA, derived fror more info see:m the intestinal metabolism of fiber or supplemented as tributyrin inhibits carcinogenesis and selectively induces apoptosis in tumor cells. Lactate produced by LAB in fermented food or in the gut serves as a signaling molecule in the host. It may inhibit an early carcinogenesis by its anti-inflammatory and mitohormetic effects. However, the endogenously produced lactate is the important contributor to cancer progression. Therefore, LAB-fermented food is the important part of a cancer-preventing diet but the application of lactate in the anti-cancer therapy is unlikely. Hydrogen sulfide is also a double-edge sword in the fight against cancer. While its endogenous moderate production is indispensable for the cancer cell survival, the consumption of high-dose H2S [1]donors, [2]especially those of plant origin, is an emerging anticancer strategy. Taken together, the molecules inspired by microbiota-derived functional metabolites represent a novel and promising class of remedies applicable in cancer prevention and treatment.
For more info see: [3]
References
- Jaroslav Zelenka; Martina Koncošová; Tomáš Ruml; Targeting of stress response pathways in the prevention and treatment of cancer. Biotechnology Advances 2018, 36, 583-602, 10.1016/j.biotechadv.2018.01.007.
- Jaroslav Zelenka; Ales Dvorak; Lukáš Alán; L-Lactate Protects Skin Fibroblasts against Aging-Associated Mitochondrial DysfunctionviaMitohormesis. Oxidative Medicine and Cellular Longevity 2015, 2015, 1-14, 10.1155/2015/351698.
- Nikola Vrzáčková; Tomáš Ruml; Jaroslav Zelenka; Postbiotics, Metabolic Signaling, and Cancer. Molecules 2021, 26, 1528, 10.3390/molecules26061528.
