Biopolymers are polymers being synthesized by living organisms with the help of enzymes that connects the building blocks like sugars, hydroxyl fatty acids, and amino acids to produce molecules with high molecular weight.
- cell factories
- bacterial biopolymers
- pathogenesis
- biomaterials
- synthetic biology
1. Introduction
Biopolymers are polymers being synthesized by living organisms with the help of enzymes that connects the building blocks like sugars, hydroxyl fatty acids, and amino acids to produce molecules with high molecular weight. Bacteria produce a diverse variety of biopolymers like polyamides (amino acids linked by peptide bonds), polysaccharides (sugars or sugar acids linked by glycosidic bonds), polyphosphates (inorganic phosphates connected by anhydride bonds), and polyesters (hydroxyl fatty acids connected by ester bonds). Over a long period of time, scientists have tried to understand the pathways involved in the biosynthesis of biopolymers, since they are involved in bacterial pathogenesis and persistence. These polymers can act as shielding capsules to protect the cells and storage components and form the matrix component involved in biofilm formation that causes almost 60–80% of total human infections [1]. The physicochemical properties of these biopolymers have various medicinal as well as industrial applications [2,3,4,5][2][3][4][5]. Over the recent few years, bioengineering and synthetic biological methods helped to produce new biopolymers having potential usage in medicines (like hyaluronate that acts as a biomaterial), ingredients in various food materials (Chitosan, dextran, and xanthan), and production of polyester that is used in packaging [6,7,8,9][6][7][8][9]. The designing of microbial cell factories producing biopolymers is today a significant matter of research and commercial interest.
Even though biopolymer production consumes both nutrients and chemical energy, it is supported by bacteria, since biopolymers help them grow and persist in the presence of a wide range of unfavorable conditions, such as overcoming the immune responses inside the host cells. They are involved in carrying out various other biological roles, namely protection, energy storage, and adhesion. The production of biopolymers is mainly dependent upon environmental stimuli [10]. The physicochemical properties of biopolymers are essential to maintain cellular behaviors like attachment over the abiotic or biotic surfaces, translocation, and shielding and endurance. Bacteria are known to produce extracellular polymeric substances (EPS) that entangle within themselves to provide a mesh like network encasing the bacterial cells. This EPS production is mainly essential in the formation of biofilms, which are referred to as potentially structured communities of bacterial cells [11], and these communities are considered to be one of the most persistent forms of life on Earth. EPS are the biopolymers that are synthesized by several strains of microorganisms, providing structural stability and nutrients during adhesion of the sessile microcolonies and the development of the biofilm. EPS produced by the microbial communities vary greatly in their biochemical composition, thus exhibiting varying chemical and physical properties. Some of these can be polyanionic, whereas others can be polycationic in nature [12]. The complex arrangement of structural units inside the EPS has been studied for decades. The macromolecules that are predominantly available within the EPS comprise of polysaccharides, proteins, peptidoglycans, lipids, enzymes, and extracellular DNA (eDNA). Nuclease that is present within the EPS acts as an important regulator for the formation of the biofilm [13]. The polysaccharides are a widely studied component of EPS. The analysis of various polysaccharides obtained from varied microbial species show that they vary largely in their composition and are made up of one or more different structural components with varied arrangement in EPS [14]. The commonly found polysaccharides within the EPS are highly soluble in salt solution and water. The polysaccharides forming the capsule help in adherence to the cell surface via covalent bonds with the other polymers that are present upon the surface. The extracellular polysaccharides are insoluble and cannot be separated from the cells, thereby making the determination of physical and chemical properties difficult. As the formation of biofilms serves as the main platform for the development of chronic infections [15], there are many pieces of research to understand the function of bacterial biopolymers in the formation of biofilms leading to pathogenesis. These biopolymers produced by bacterial cellular pathways and their biological roles have been considered as potential targets for the development of new antibacterial drugs.
Besides these, researches are still going on to harness the novel materialistic properties of these bacterial biopolymers like dextran [16], cellulose [17], polyesters [18], and xanthan [19] to be used in industries to produce medicines and other materials. Over the last few years, advanced molecular methodologies and genome sequencing have provided a large amount of information regarding the role of bacterial biopolymers to cause pathogenesis and also to devise bacteria to be used as cell factories producing tailor-made biomaterials. These biodegradable and renewable materials have the potential to replace the oil-based type of materials and can also be exploited to develop novel high-end biomaterials in order to provide treatments for unfulfilled medical requirements, because these materials are frequently biocompatible. Thus, this review focuses on the bacterial pathway for polymer biosynthesis and its potential role in causing pathogenesis, as well as how it can have diverse industrial, technical, and medical applications.
2. Types of Bacterial Biopolymers
2.1. Polysaccharides
Polysaccharides are mainly divided into two categories, namely heteropolymers and homopolymers. These polysaccharides can be uncharged or charged, repeating or non-repeating, and un-branched or branched. A plethora of bacteria can produce polysaccharides and reserve them within their cells, such as glycogen, or they may also secrete polysaccharides, which are attached to the cell surfaces or free exopolysaccharides that help in the matrix formation in biofilms (cellulose and alginate mainly) [7]. On being motile, pathogenic organisms produce various virulence factors and other toxic substances such as exotoxins. On the other hand, while in sessile state, pathogens produce different exopolysaccharides such as cellulose, alginate, and hyaluronate, acting as components of biofilm matrix. This shifting to sessile biofilm state defines the onset of chronic infectious diseases, since the encapsulated or embedded cells are shielded from host immune responses and anti-microbial drugs [11]. For example, alginate present inside the matrix of biofilm in Pseudomonas aeruginosa contributes to enhancing the surviving chances of the bacterial cells by shielding them from phagocytosis [1]. Alginates react with divalent cations forming dense hydrogels with increased water-retention capacity [20]. Again, cellulose production accounts for identical advantages to enterococcal pathogens [21]. Escherichia coli is known to produce phosphoethanolamine cellulose that can form a mortar-like structure for stabilization of proteinaceous curli fibers. These fibers strongly interconnect the biofilm forming cells, providing resistivity against conditions of high stress [22]. Other pathogens like Bacillus cereus G9241 and Streptococcus pyogenes consist of a capsule made of hyaluronate, which is a linear heteropolysaccharide with negative charge and a structural homolog of hyaluronate that is found in the connective tissues of humans. Thus, these pathogens can easily escape from being exposed to phagocytosis and opsonization. Serogroup B Neisseria meningitidis leads to invasive diseases of meningitis because of the presence of the capsular polysaccharide consisting of sialic acid homopolymers (N-acetylneuraminic acid) having α-2, 8 sialic acid bonds. This polymeric structure of the moieties of polysialic acid resembles the structure of the antigens present in human tissues, and this molecular analogy accounts for reduced immunogenic properties of the capsular polysaccharides, thereby rendering the pathogen non-immunogenic to the host [23]. Streptococcus pneumoniae is one of the leading pathogens in causing severe lung diseases and consists of over 100 serotypes producing diverse capsular polysaccharides for evasion of adaptive host immune response [24]. Capsular and secreted polysaccharides are widely used as antigens for producing conjugate vaccines. Designing of serotype-independent vaccines is increasing because of the varying serotypes of N. meningitidis and S. pneumoniae, which decreases the effectiveness of these vaccines [1]. Glycogen is a water-soluble polymer consisting of α-1, 6 and α-1, 4 glycosidic linkages, and it is the most well-known form of carbon and energy reserve for survival during starvation. In case of intracellular infections, glycogen helps the pathogens like Salmonella enterica subsp. Mycobacterium tuberculosis and Chlamydia trachomatis to survive [25] (Figure 1).
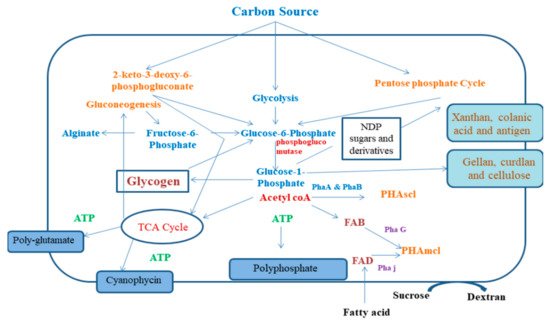
Figure 1. Mechanism of bacterial polymer synthesis pathway. FAB: fatty acid de novo biosynthesis; FAD: fatty acid β-oxidation; KDPG: 2-keto-3-deoxy-6-phosphogluconate pathway; NDP: nucleoside 5ʹ-diphosphate; P: phosphate; PGI: phosphoglucoisomerase; PGM: phosphoglucomutase; Pha: polyhydroxyalkanoate synthesis enzyme; TCA cycle: tricarboxylic acid cycle.
Glycogen is secreted from the capsular polysaccharides that are associated with the surface of the cell and result in the development of the matrix of the biofilm [7]. Alginates that are produced by P. aeruginosa are responsible for the protection of the cells from phagocytosis [1]. Phosphoethanolamine cellulose produced by E. coli helps in the stabilization of fibers, which in turn form a complex biofilm by interlinkage of these fibres and develop resistance against high shear conditions. E. coli produces phosphoethanolamine cellulose, which forms mortar-like structures to stabilize proteinaceous curli fibers. These fibers mediate strong connections between cells in complex biofilms and provide resistance in high-shear conditions. Hyaluronate produced by Streptococcus pyrogens and Bacillus cereus helps in mimicking hyaluronate being present within the connective tissues, thus protecting them from phagocytosis [26]. It has also been observed that capsular polysaccharide comprising of sialic acid produced by Neisseria meningitides resembles the polysialic moieties of human antigen, thereby making it invisible within the host exerting its pathogenicity [23].
Apart from acting as virulence factors, bacterial polysaccharides exhibit distinctive materialistic properties. Synthesis of polysaccharides chemically is tedious, expensive, and restricted to low molecular weight molecules and could be done only for a few types of polysaccharides. Thus, microbial cell factories are essential for manufacturing these polymers. As hydrophilic groups (carboxyl and hydroxyl groups) are present on the polysaccharides, these polysaccharides actually have high water-retention capacity allowing crosslinking (polymer–polymer, drug–polymer, host tissue–polymer, and cellular interactions) and intermolecular interactions. Polysaccharides are able to form porous hydrogels to be used in drug delivery and controlled delivery of anti-cancer drugs [27], enzyme immobilization [28], and therapeutic entrapment of cells, thereby protecting transplanted cells from the host immunity [29] and tissue engineering [30]. Hydrogels that are made from bacterial cellulose can serve as efficient matrices, fiber composites, or hydrogel nanofibrillar scaffold network, having biomedical applications such as wound dressing that can deliver human dermal fibroblasts and epidermal keratinocytes [24]. Cellulose from Komagataeibacter xylinus finds application in large scale production of rayon-based fibers to be used as wearable textiles. Hyaluronate produced by non-pyogenic Streptococcus zooepidemicus also has wide biomedical applications [31]. Commercialized formulations of hyaluronate forms a gel-like fluid that can be used in injections to treat arthritis pain in knee joints.
Some enzymes naturally alter the materialistic properties of polysaccharides to modify their biological functions. For example, the existence of acetyl groups on chains of polysaccharides remarkably modify structural conformities, thereby affecting chain–chain interactions, water-retention capacity, solubility, molecular weight, and viscoelasticity [32]. Using genetically engineered polysaccharide-modifying enzymes in microbial cell factories or using these enzymes to modify polysaccharides in vitro allows the generation of tailor-made polysaccharides. The fabrication of these materials after modification changes on being blended with other non-polymeric or polymeric components (esterification of stearic acid, crosslinking of citric acid, and plasticizers). Blending accounts for modification of properties like degree of gelation, viscoelasticity, material strength, and porosity. These materials are being used as feedstock components and as bioinks in 3D bioprinting with varied engineering and biomedical applications like drug testing and drug delivery and tissue engineering. 3D cell-loaded scaffolds made from hyaluronate or alginate can be extensively used as artificial extracellular matrix (ECM), providing for a temporary environment for supporting adhesion, invasion, proliferation, and differentiation of different types of cells such as fibroblasts, mesenchymal stem cells, embryonic stem cells, chondrocytes, and osteoblasts [33]. Thus, bacteria can be considered as an important natural source for the secretion of a wide variety of polysaccharides having medicinal uses and potential industrial applications (Table 1).
| Type of Polymer | Localization of the Polymer | Primary Structure | Major Component | Precursors | Enzyme for Polemerization and Operon | Producer | Industrial Application | Reference |
|---|---|---|---|---|---|---|---|---|
| Polysaccharides | ||||||||
| Hyaluronic acid | Produced extracellularly | β-(1,4) linkage | N-acetyl glucosamine and Glucuronate | UDP–N-acetyl glucosamine and UDP–d-glucuronate | Hyaluron synthase (HasA) has operon | Pasteurella Multocida and Streptococcus sp. |
Drug delivery, cosmetic, Viscosupplementation, and repair of tissue | [34] |
| Cellulose | Produced extracellularly | β-(1,4) linkage | D-glucose | UDP-D-glucose | Cellulose synthetase (BcsA) bcs operon | Betaproteobacteria, Alphaproteobacteria, Gammanproteobacteria, and Gram-positive bacteria | Wound dressing and in food industry | [34] |
| K30 antigen | Capsular | β-(1,2) linkage | Glucuronate Mannose,= and galactose | UDP–D-glucuronate UDP–D-galactose, and UDP–D-glucose, | Polysaccharide polymerase (Wzy) | Escherichia coli | NA | [34] |
| Colanic acid | Extracellular | β-(1,4) linkage | Glucuronate, glucose fucose, and galactose | UDP–D-glucose, UDP–D-galactose, GDP–L-fucose, and UDP–D-glucuronate | Colanic acid polymerase (WcaD) | Shigella sp., E. coli, Enterobacter sp., and Salmonella sp | NA | [34] |
| Gellan | Produced extracellularly | β-(1,3) linkage | Glucuronate, rhamnoseand Glucose | dTDP–rhamnose, UDP–glucuronate, and UDP–glucose | Gellan synthase (Gel G) | Sphingomonas sp. | Food additive, culture media additive for encapsulation | [34] |
| Cudlan | Produced extracellularly | β-(1,3) linkage | Glucose | UDP-glucose | Curdlan synthase (Crd S) | Rhizobium sp., Cellulomonas spp, and Agrobacterium sp. | Food additives | [34] |
| Glycogen | Produced intracellularly | α-(1,6)-branched and α-(1,4)-linked polymer | Glucose | ADP-glucose | Glycogen synthase (GlgA) glg operon | Archea and Bacteria | NA | [34] |
| Alginate | Produced extracellularly | β-(1,4) linkage | Guluronic acid and Mannuronic acid | GDP–mannuronic acid | Glycosyl transferase (Alg 8) alg operon | Azotobacter sp. and Pseudomonas sp. | Development of biomaterials | [34] |
| GBS polysaccharides | Capsular | Galactose, Glucose, and N-acetylneuraminic acid; N-acetylglucosamine or rhamnose | Streptococcus agalactiae | Currently finding its application in the investigation of vaccines | [34] | |||
| Pel | Acetylated -(1,4) linkage | -- | N-acetylgalactosamine and N-acetylglucosamine | P. aeruginosa | [34] | |||
| Psl | L-rhamnose, D-mannose, and D-glucose | P. aeruginosa | MEDI3902d (IgG1 mAb) targets Psl | [34] | ||||
2.2. Polyamides
Bacteria are capable of producing polyaminoacids or polyamides like secreted poly-L-lysine and poly-D-glutamic acid or the intracellularly produced cyanophycin (copolymer of L-arginine and L-asapartic acid), and they can serve as biofilm matrix components or capsular polymer and also storage materials [35]. Just like the polysaccharides of biofilm matrix, polyamide biofilm or capsule is less immunogenic, thereby protecting pathogens like Bacillus anthracis from being attacked by the host immune system [33]. Polyamide-based materials are also produced by various non-pathogenic bacteria such as Bacillus megaterium, Bacillus licheniformis, and many of the cyanobacteria [36]. Polyamides have high charges and may be polycationic or polyanionic. They are considered as non-toxic, biodegradable, and renewable. Metabolic engineering helps in the production of biotechnologically enhanced polyamides. Polyamides potentially substitute chemically produced polymers in industries. For example, poly-D-glutamic acid can be utilized as a flocculant for replacing synthetic flocculants (polyacrylamide or polyaluminium chloride) for treating wastewater [34]. Poly-L-lysine possesses antibacterial properties, since it can disrupt the integrity of membrane, thereby damaging the cross links, and is used in antimicrobial coatings [34].
2.3. Polyesters
Polyhydroxyalkanoates (PHA) are bacterially produced bioplastics. They have a linear symmetry and are arranged in hydrophobic spheric inclusion, functioning as a carbon and energy reserving material [10]. Even though many gram-negative and gram-positive bacteria can secrete PHAs, the functions of PHAs and the potential genes involved in PHA-mediated pathogenesis and persistence are still unknown. PHA
mutants of
reveal decreased attachment to surfaces of glass and diminished stress tolerance in presence of biofilms, therefore revealing a significant contribution of PHA in persistence in infections [37]. In one of the plant pathogens
(causes major rice production losses), the regulatory protein called PhaR represses the synthesis of PHA and also suppresses EPS production, thus affecting virulence and phenotypic changes modifying lifestyle of the bacteria [38]. PHAs are reported to be electron sinks, which in anaerobic conditions (terminal electron or oxygen absence) enhance survival [38]. Synthesis and mobilization of PHA are controlled under responses such as environmental stimuli and environmental and nutritional stresses, rendering an advantageous survival.
PHAs are known to be a distinctive bioplastic that can be chemically modified or bioengineered to be used as high-value medical biomaterials such as scaffolds in tissue engineering, sutures, particulate vaccines, and drug carriers or to be used as low-value bioplastic [34]. Synthesis of tailor-made PHAs through physical blending, bioengineering, or chemical modification produced enhanced materialistic properties that met the requirements of medical and industrial usage. Microbial cell factories can be biologically engineered to produce PHA inclusions that can be heavily coated with proteins of functional interest. These functionalized beads of PHA were stable even after being separated from the cell mass of bacteria, indicating high-value performance as immunodiagnostics, vaccines, enzyme carriers, bio separation resins, and tools required in the production of recombinant proteins. Additionally, the functional performance of these protein-coated, non-porous beads of PHA can be tuned further by regulated encapsulation inside porous microspheres of alginate allowing flow-through usage [39,40][39][40].
2.4. Polyphosphates
Polyphosphate (polyP) is a polymer consisting of condensed phosphates (several inorganic phosphates), which have a high negative charge along with high-energy anhydride bonds. PolyP serves as an energy storing polymer. PolyP biosynthesis results from an evolutionary activity of bacteria, and this helps in providing chemical energy for the various biosynthetic pathways inside bacterial cells and can act as buffers against bases. Additionally, polyPs can act as a metal chelating agent contributing to the formation of channel complexes in order to uptake DNA. PolyPs aid in cell signalling regulation, thus affecting the lifestyle of bacteria, their growth, persistence, viability, virulence, and stress tolerance [41].
Since polyPs can eminently act as an energy-storing material, they have been widely used in industries to provide energy in carrying out enzyme-catalyzed reactions. They are used as biologically active biomaterials in the production of regenerative medicines for repairing cartilages and in the regeneration of bones or can be also used as vehicles for drug-delivery. As polyPs can react with cationic polymers (like hyaluronate and alginate), inorganic cations (like Mg2+, Ca2+, Zn2+, Na+, Fe2+, and K+) or other organic alkaline components (like peptides, polyamines, and amino acids), they can be converted into hydrogels or nanoparticles to be used in biomineralization of bones [42] or for various biomedical uses. Essentially, physical properties like functionality and stability and mechanical durability of polyP-associated complex nanoparticles or hydrogels may change depending upon the class of blended polymeric substances or interacting counterions. This modification furnishes considerable space designing for the generation of a wide variety of materialistic properties for bioinks and smart biomaterials in regenerative medicines. Living organisms, particularly bacteria, are considered as the only source of polyp, since there are no known abiotic polyP minerals found on Earth. Bacteria of the genera Corynebacterium and Mycobacterium can produce granules of polyP with increased yield and therefore can be used as potential producers for polyP manufacture [43].
2.5. Other Bacterial Polymers
Bacteria can even produce various types of biopolymers that can act as proteinaceous components and extracellular DNA. These types of biopolymers are not only responsible for pathogenesis in bacteria, but can be also used for biomaterial production. Extracellular DNA forms when a cell is lysed, releasing the intracellular DNA. In the case of biofilms, a cellular subpopulation lyses to produce extracellular DNA, like for example in stalks of mushroomlike microcolonies formed by P. aeruginosa [44]. Because of the high negative charge, extracellular DNA has multiple roles in the adherence and stability of the matrix of biofilm by interaction with cationic polysaccharides (like Pel) and other cations and can also act as a source of nutrients at times of starvation conferring resistivity to antibiotics.
Secreted polypeptides are composed of alternating hydrophobic and hydrophilic residues of amino acids and proteins like pilin, fibrillin, and flagellin. These polypeptides can serve as molecular elementary units to form extracellular self-built structures like pili, fimbiriae, flagella, and functional amyloids such as curli fibers. These self-assembled structures are capable of forming nanotubes or nanofibers, mediating cell adhesion on abiotic and biotic surfaces, developing matrix of biofilm, or providing motility during pathogenesis. Various features like increased ratio of surface area to volume, the explicit arrangement of building blocks of proteins, and polymorphic transformations of these structures are a result of their responses against chemical and physical stimulations, making them a potential component in industrial and biomedical applications. These properties make them important biomaterials and bio templates for the designing of novel nanodevices, nanostructures, and multiple layered lattices to be used in nanomedicines (drug delivery) and bioengineering [45]. The genetic programmability and the simplicity of engineering polypeptides, proteins, and extracellular DNA make them attractive for manufacturing programmable biomaterial-based platforms, which can be hardly manufactured with other types of biopolymers like polyesters and polysaccharides. Moreover, the uncomplicated genetic programmability helped in the development of bioengineered living materials in which the live cells are bioengineered for autonomous self-assembly of all the materials, having tunable and novel properties for different purposes like microbial electrosynthesis, electronic devices for monitoring, biosensors, and bioremediation [46].
(References would be added automatically after the entry is online)
References
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39.
- Costa, D.; Briscoe, W.H.; Queiroz, J. Polyethylenimine coated plasmid DNA–surfactant complexes as potential gene delivery systems. Colloids Surfaces B Biointerfaces 2015, 133, 156–163.
- Pati, S.; Chatterji, A.; Dash, B.P. Chitosan from the carapace of Indian horseshoe crab (Tachypleus gigas, müller): Isolation and its characterization. Adv. Biores 2018, 9, 52–64.
- Pati, S.; Chatterji, A.; Dash, B.P.; Nelson, B.R.; Sarkar, T.; Shahimi, S.; Edinur, H.A.; Abd Manan, T.S.B.; Jena, P.; Mohanta, Y.K.; et al. Structural characterization and antioxidant potential of chitosan by γ-irradiation from the carapace of horseshoe crab. Polymers 2020, 12, 2361.
- Albuquerque, T.; Faria, R.; Sousa, Â.; Neves, A.R.; Queiroz, J.A.; Costa, D. Polymer-peptide ternary systems as a tool to improve the properties of plasmid DNA vectors in gene delivery. J. Mol. Liq. 2020, 309, 113157.
- Costa, D.; Miguel, M.G.; Lindman, B. Responsive Polymer Gels: Double-Stranded versus Single-Stranded DNA. J. Phys. Chem. B 2007, 111, 10886–10896.
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496.
- Sarkar, T.; Nayak, P.; Chakraborty, R. Storage study of mango leather in sustainable packaging condition. Mater. Today Proc. 2020, 22, 2001–2007.
- Pati, S.; Jena, P.; Shahimi, S.; Nelson, B.R.; Acharya, D.; Dash, B.P.; Chatterji, A. Characterization dataset for pre- and post-irradiated shrimp waste chitosan. Data Brief 2020, 32, 106081.
- Rehm, B.H.A. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592.
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575.
- Sutherland, I. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9.
- Kiedrowski, M.R.; Kavanaugh, J.S.; Malone, C.L.; Mootz, J.M.; Voyich, J.M.; Smeltzer, M.S.; Bayles, K.W.; Horswill, A.R. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e26714.
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636.
- Bartell, J.A.; Sommer, L.M.; Haagensen, J.A.J.; Loch, A.; Espinosa, R.; Molin, S.; Johansen, H.K. Evolutionary highways to persistent bacterial infection. Nat. Commun. 2019, 10, 629.
- Li, L.; Eyckmans, J.; Chen, C.S. Designer biomaterials for mechanobiology. Nat. Mater. 2017, 16, 1164–1168.
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; An, D.; Li, X.; Xian, M.; Zhang, H. A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat. Commun. 2019, 10, 437.
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362.
- Mondal, S.; Varenik, M.; Bloch, D.N.; Atsmon-Raz, Y.; Jacoby, G.; Adler-Abramovich, L.; Shimon, L.J.W.; Beck, R.; Miller, Y.; Regev, O.; et al. A minimal length rigid helical peptide motif allows rational design of modular surfactants. Nat. Commun. 2017, 8, 14018.
- Bruchet, M.; Melman, A. Fabrication of patterned calcium cross-linked alginate hydrogel films and coatings through reductive cation exchange. Carbohydr. Polym. 2015, 131, 57–64.
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8.
- Thongsomboon, W.; Serra, D.O.; Possling, A.; Hadjineophytou, C.; Hengge, R.; Cegelski, L. Phosphoethanolamine cellulose: A naturally produced chemically modified cellulose. Science 2018, 359, 334–338.
- Tzeng, Y.-L.; Thomas, J.; Stephens, D.S. Regulation of capsule in Neisseria meningitidis. Crit. Rev. Microbiol. 2016, 42, 759–772.
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899.
- Sambou, T.; Dinadayala, P.; Stadthagen, G.; Barilone, N.; Bordat, Y.; Constant, P.; Levillain, F.; Neyrolles, O.; Gicquel, B.; Lemassu, A.; et al. Capsular glucan and intracellular glycogen of Mycobacterium tuberculosis: Biosynthesis and impact on the persistence in mice. Mol. Microbiol. 2008, 70, 762–774.
- Wilkening, R.V.; Federle, M.J. Evolutionary Constraints Shaping Streptococcus pyogenes-Host Interactions. Trends Microbiol. 2017, 25, 562–572.
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071.
- Mohan, T.; Rathner, R.; Reishofer, D.; Koller, M.; Elschner, T.; Spirk, S.; Heinze, T.; Stana-Kleinschek, K.; Kargl, R. Designing Hydrophobically Modified Polysaccharide Derivatives for Highly Efficient Enzyme Immobilization. Biomacromolecules 2015, 16, 2403–2411.
- Kim, H.; Jeong, H.; Han, S.; Beack, S.; Hwang, B.W.; Shin, M.; Oh, S.S.; Hahn, S.K. Hyaluronate and its derivatives for customized biomedical applications. Biomaterials 2017, 123, 155–171.
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513.
- De Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.V.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb. Cell Fact. 2016, 15, 119.
- Pauly, M.; Ramírez, V. New Insights Into Wall Polysaccharide O-Acetylation. Front. Plant Sci. 2018, 9, 1210.
- Jang, J.; Cho, M.; Lee, H.-R.; Cha, K.; Chun, J.-H.; Hong, K.-J.; Park, J.; Rhie, G.-E. Monoclonal antibody against the poly-gamma-D-glutamic acid capsule of Bacillus anthracis protects mice from enhanced lethal toxin activity due to capsule and anthrax spore challenge. Biochim. Biophys. Acta 2013, 1830, 2804–2812.
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210.
- Watzer, B.; Forchhammer, K. Cyanophycin Synthesis Optimizes Nitrogen Utilization in the Unicellular Cyanobacterium Synechocystis sp. Strain PCC 6803. Appl. Environ. Microbiol. 2018, 84.
- Du, J.; Li, L.; Zhou, S. Microbial production of cyanophycin: From enzymes to biopolymers. Biotechnol. Adv. 2019, 37, 107400.
- Ushimaru, K.; Hamano, Y.; Katano, H. Antimicrobial Activity of ε-Poly-l-lysine after Forming a Water-Insoluble Complex with an Anionic Surfactant. Biomacromolecules 2017, 18, 1387–1392.
- Long, J.-Y.; Song, K.-L.; He, X.; Zhang, B.; Cui, X.-F.; Song, C.-F. Mutagenesis of PhaR, a Regulator Gene of Polyhydroxyalkanoate Biosynthesis of Xanthomonas oryzae pv. oryzae Caused Pleiotropic Phenotype Changes. Front. Microbiol. 2018, 9, 3046.
- Winnacker, M. Polyhydroxyalkanoates: Recent Advances in Their Synthesis and Applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101.
- Akinmulewo, A.B.; Nwinyi, O.C. Polyhydroxyalkanoate: A biodegradable polymer (a mini review). J. Phys. Conf. Ser. 2019, 1378, 42007.
- Wang, L.; Yan, J.; Wise, M.J.; Liu, Q.; Asenso, J.; Huang, Y.; Dai, S.; Liu, Z.; Du, Y.; Tang, D. Distribution Patterns of Polyphosphate Metabolism Pathway and Its Relationships with Bacterial Durability and Virulence. Front. Microbiol. 2018, 9, 782.
- Müller, W.E.G.; Tolba, E.; Ackermann, M.; Neufurth, M.; Wang, S.; Feng, Q.; Schröder, H.C.; Wang, X. Fabrication of amorphous strontium polyphosphate microparticles that induce mineralization of bone cells in vitro and in vivo. Acta Biomater. 2017, 50, 89–101.
- Lindner, S.N.; Knebel, S.; Pallerla, S.R.; Schoberth, S.M.; Wendisch, V.F. Cg2091 encodes a polyphosphate/ATP-dependent glucokinase of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2010, 87, 703–713.
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358.
- Bera, S.; Mondal, S.; Xue, B.; Shimon, L.J.W.; Cao, Y.; Gazit, E. Rigid helical-like assemblies from a self-aggregating tripeptide. Nat. Mater. 2019, 18, 503–509.
- Gilbert, C.; Ellis, T. Biological Engineered Living Materials: Growing Functional Materials with Genetically Programmable Properties. ACS Synth. Biol. 2019, 8, 1–15.
