A working definition of heart failure (HF) in children is “a progressive clinical and pathophysiological syndrome caused by cardiovascular and noncardiovascular abnormalities that results in characteristic signs and symptoms including edema, respiratory distress, growth failure, and exercise intolerance and accompanied by circulatory, neurohormonal, and molecular derangements”.
- acute heart failure syndrome
- pediatric heart failure
- pharmacotherapy for heart failure
1. Introduction
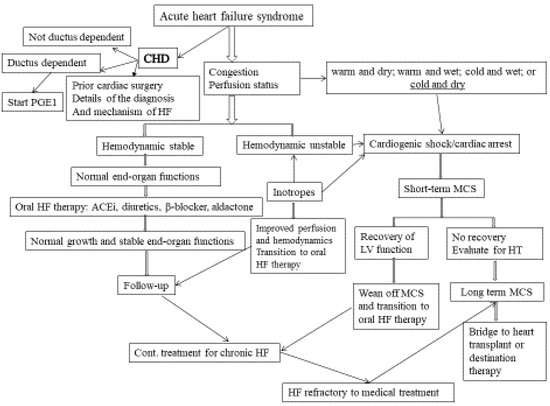
Figure 1. Approaches to acute HF in infants and children. (MCS: mechanical circulatory support; HF: heart failure; CHD: congenital heart disease; H/O: history of; PGE1: prostaglandin 1; ACEi: angiotensin-converting enzyme inhibitor).
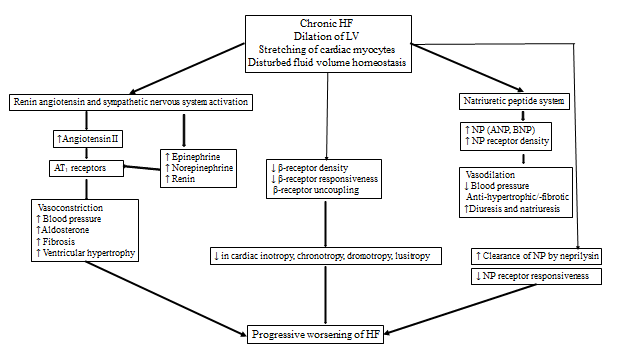 Figure 2. Pathophysiology of progression of Chronic Heart Failure (LV: left ventricle; ANP: atrial natriuretic peptide, BNP: brain type natriuretic peptide; AT: angiotensin )
Figure 2. Pathophysiology of progression of Chronic Heart Failure (LV: left ventricle; ANP: atrial natriuretic peptide, BNP: brain type natriuretic peptide; AT: angiotensin )
 Figure-2: Pathophysiology of progression of Chronic Heart Failure (LV: left ventricle; ANP: atrial natriuretic peptide, BNP: brain type natriuretic peptide; AT: angiotensin )
Figure-2: Pathophysiology of progression of Chronic Heart Failure (LV: left ventricle; ANP: atrial natriuretic peptide, BNP: brain type natriuretic peptide; AT: angiotensin )
2. Acute Heart Failure Syndrome
This review discusses the current and future pharmacological therapies in children with acute and chronic HF, the mechanism of action of drugs, and the need for future clinical trials in children for the safety and efficacy of newer drugs that are used in adults. Acute HF syndrome is described as a structural or functional alteration in the heart that occurs rapidly, followed by congestion, malperfusion, hypotension, and end-organ dysfunction resulting in a need for hospitalization and urgent therapy.
The goals of acute HF management in children are to improve hemodynamics and prevent progression (Figure 1). Current management for chronic HF includes stabilization with intravenous inotropes/vasopressors, diuretics, mechanical ventilation, treatment of arrhythmia, progression to mechanical circulatory support, and heart transplantation if needed. After presenting with AHFS, when the hemodynamics stabilize and end-organ functions recover, the child is often left with the lingering diagnosis of chronic HF syndrome, with or without a genetically based or syndrome/systemic disease-based diagnosis. Several regulatory neurohumoral and counter-regulatory pathways are involved in the pathogenesis of CHFS (Figure 2). The goal of the clinical management of CHFS in children is to permit recovery, maintain stability, prevent progression, and provide a reasonable milieu to allow somatic growth and optimal development into adult life. Typically, a multi-drug approach is required, with either sequential combination therapy or upfront combination of angiotensin-converting enzyme inhibitors, β-blockers, diuretics, and aldosterone antagonists. Herein we review the current and future landscape of drug therapies for chronic HF in adults with evidence for randomized clinical trials to highlight the knowledge gap in pediatric HF.
This entry discusses the current and future pharmacological therapies in children with acute and chronic HF, the mechanism of action of drugs, and the need for future clinical trials in children for the safety and efficacy of newer drugs that are used in adults. Acute HF syndrome is described as a structural or functional alteration in the heart that occurs rapidly, followed by congestion, malperfusion, hypotension, and end-organ dysfunction resulting in a need for hospitalization and urgent therapy.
The goals of acute HF management in children are to improve hemodynamics and prevent progression (Figure 1). Current management for chronic HF includes stabilization with intravenous inotropes/vasopressors, diuretics, mechanical ventilation, treatment of arrhythmia, progression to mechanical circulatory support, and heart transplantation if needed. After presenting with AHFS, when the hemodynamics stabilize and end-organ functions recover, the child is often left with the lingering diagnosis of chronic HF syndrome, with or without a genetically based or syndrome/systemic disease-based diagnosis. Several regulatory neurohumoral and counter-regulatory pathways are involved in the pathogenesis of CHFS (Figure 2). The goal of the clinical management of CHFS in children is to permit recovery, maintain stability, prevent progression, and provide a reasonable milieu to allow somatic growth and optimal development into adult life. Typically, a multi-drug approach is required, with either sequential combination therapy or upfront combination of angiotensin-converting enzyme inhibitors, β-blockers, diuretics, and aldosterone antagonists. Herein we review the current and future landscape of drug therapies for chronic HF in adults with evidence for randomized clinical trials to highlight the knowledge gap in pediatric HF.
Despite recent positive trials in children and adults with drug therapies for heart failure (HF) with reduced ejection fraction, the risk for death or transplantation in these patients remains high. Mechanical circulatory support with left ventricular assist devices is approved for therapy in patients with end-stage HF. Currently approved devices are used as bridge-to-transplantation or destination therapy. Device-based therapies are increasingly being investigated for the treatment of HF. This is important as side effects from drugs can limit their use and specific structural abnormalities may require targeted solutions. Implanted technologies have an advantage as they operate independent of patient compliance. Adult HF clinical trials may serve as guidelines to design compelling drug trials in children but are not substitutes. In the meantime, it is necessary to specifically think through the mechanism of HF and the mechanism of pharmacotherapy for selecting appropriate drugs in children with HF. In children, due to small case numbers of HF, there are no incentives for the industry to develop children-specific HF therapies. The federal government should support such clinical trials to expedite the testing of drug and device therapies in pediatric HF. Novel clinical trial designs may be considered that allow for early market access by accelerating the development, assessment, and review processes, and linking reimbursement from the Centers for Medicare and Medicaid Services to FDA marketing approval.
Despite recent positive trials in children and adults with drug therapies for heart failure (HF) with reduced ejection fraction, the risk for death or transplantation in these patients remains high. Mechanical circulatory support with left ventricular assist devices is approved for therapy in patients with end-stage HF. Currently approved devices are used as bridge-to-transplantation or destination therapy. Device-based therapies are increasingly being investigated for the treatment of HF. This is important as side effects from drugs can limit their use and specific structural abnormalities may require targeted solutions. Implanted technologies have an advantage as they operate independent of patient compliance. Adult HF clinical trials may serve as guidelines to design compelling drug trials in children but are not substitutes. In the meantime, it is necessary to specifically think through the mechanism of HF and the mechanism of pharmacotherapy for selecting appropriate drugs in children with HF. In children, due to small case numbers of HF, there are no incentives for the industry
to develop children-specific HF therapies. The federal government should support such clinical trials to expedite the testing of drug and device therapies in pediatric HF. Novel clinical trial designs may be considered that allow for early market access by accelerating the development, assessment, and review processes, and linking reimbursement from the Centers for Medicare and Medicaid Services to FDA marketing approval.
