Rheumatoid arthritis (RA) is the most prevalent systemic autoimmune inflammatory disease affecting approximately 1% of the adult population worldwide.
- rheumatoid arthritis
- risk factors
- epidemiology
- diet
1. Introduction
The etiopathogenesis of RA is only partially understood. The current knowledge is that in genetically susceptible individuals, environmental factors induce a pathological activation of the immune system that eventually leads to clinical onset of RA [1]. The European League Against Rheumatism (EULAR) has proposed a terminology for specific preclinical phases of RA development, which are not necessarily consecutive or mutually exclusive (Figure 1) [1][2]. Interactions between genetic factors, environmental factors and the presence of autoantibodies lead to increased risk of developing RA.
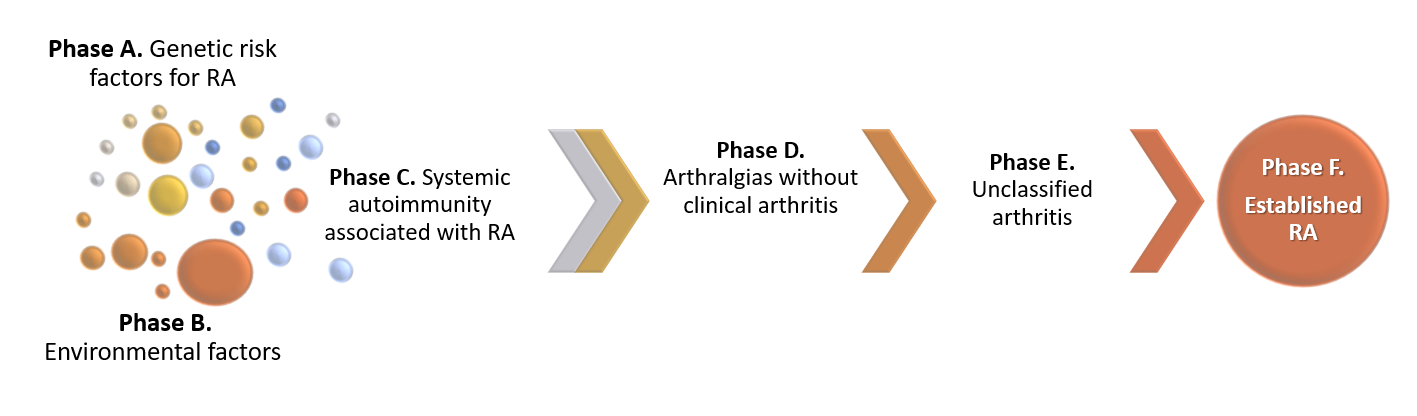
Figure 1. Proposed preclinical phases of RA development. Genetic, environmental factors and systemic autoimmunity interactions lead to RA development. The progression from one pre-clinical phase to another is not necessarily linear, and the phases may be overlapping.
Several studies suggest environmental factors play an important role in the etiology of the disease [3][4]. Smoking is the environmental factor more consistently associated with RA development [5]. However, patients are frequently also concerned about the effect of diet on the development of RA. Human diet has gone through extensive transformation globally, with increasing consumption of processed foods, salt and carbohydrate enriched products, contributing to the development of obesity and other chronic diseases, such as hypertension, type 2 diabetes mellitus and cardiovascular diseases. Some nutritional factors may contribute to the pathologic activation of immune system, eventually leading to RA, and some others may be protective. Studies have suggested that the initial steps of the pathological autoimmune response associated with RA take place at mucosal sites, such as intestinal or airway mucosa, rather than in the joints [6], and are associated with higher abundance of particular bacterial species [7]. Diet modifications affect the composition and function of the intestinal microbiota and it is possible that part of the observed effect of nutrition on RA is mediated by changes in the microbiota.
2. Nutrition and Development of Systemic Autoimmunity Associated with RA
‘Systemic autoimmunity associated with RA’ is a pre-clinical phase of RA, often considered the immune onset of the disease and characterized by the presence of autoantibodies, such as the rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPAs). Few studies have analyzed the impact of nutritional factors on the development of systemic autoimmunity associated with RA, in individuals at risk of RA.
2.1. Omega-3 and Omega-6 Fatty Acids
Omega-3 fatty acids have been suggested to be protective against the development of autoimmunity associated with RA. In a nested case-control study in the Studies of the Etiology of RA (SERA), healthy FDR-RA individuals who developed ACPAs had used less frequently omega-3 supplements (Odds ratio, OR 0.14, 95% Confidence Interval, CI 0.03–0.68) and had significantly lower concentrations of omega-3 fatty acids in red blood cell membranes than controls (30 cases vs 47 controls) [8]. The SERA research group further analyzed, in a larger number of FDR-RA individuals, whether omega-3 fatty acids were also associated with RF and whether these associations were modified by shared epitope (SE) positivity. Individuals with RF and SE positivity or with ACPA and SE positivity had lower concentrations of omega-3 fatty acids in red blood cell membranes (OR 0.27, 95% CI 0.10–0.79 and OR 0.42, 95% CI 0.20–0.98, respectively) [9]. These results suggest a potential protective effect of omega-3 fatty acids on RA-related autoimmunity, which may be more prominent in those with genetic susceptibility to RA.
3. Nutrition and Development of RA
Several studies have analyzed associations of dietary patterns, individual foods and beverages with established RA. We are going to review potentially protective and hazardous factors and discuss controversial factors.
3.1. Protective Factors
alcohol
In animal models, adding small doses of ethanol to mice’s drinking water delays the onset of collagen-induced arthritis, suggesting preventive properties of low dose and persistent alcohol consumption [10]. In humans, moderate alcohol consumption (defined as 5.0–9.9 g/day) has been described as a protective factor against RA [11][12]. A meta-analysis of nine observational studies found a protective effect of alcohol on the development of RA (OR 0.78, 95% CI 0.63–0.96), and even more pronounced in ACPA-positive RA (OR 0.52, 95% CI 0.36–0.76) [13].
3.2. Hazardous Factors
Salt Consumption
High salt consumption has been suggested a risk factor for the development of RA, in particular in smokers [14][15]. In a nested case-control study from Sweden, 386 patients with RA were compared to 1886 matched controls [16]. High sodium intake doubled the risk of RA among smokers (OR 2.26, 95% CI 1.06–4.81) but not in nonsmokers. A study by same authors compared ACPA positive RA vs ACPA negative RA, and after stratification by salt consumption, ever-smokers with medium to high sodium consumption had an increased risk of ACPA-positive RA (OR 1.7, 95% CI 1.2–2.4) [17]. In a Spanish cohort study of 18,555 individuals, 392 persons developed RA [18]. Persons with high daily sodium intake (>4.55 g) had a higher risk of developing RA adjusting by confounders, such as physical activity, hypertension, cardiovascular diseases, diabetes, cancer and smoking (OR 1.5, 95% CI 1.1–2.1). However, in this study nonsmokers had a higher association than ever smokers.
3.3. Sugar-Sweetened Beverages
In the Nurses’ Health Study (NHS), regular consumption of sugar-sweetened sodas, meaning >1 daily serving, significantly increased the risk of developing RA [19]. The association was independent of obesity and other socio-economic factors and tended to be stronger for late-onset RA (HR 2.64, 95% CI 1.56–4.46). No causal relation was found with diet soda or between sugar-sweetened soda and seronegative RA [19]. An interaction between sugar sweetened soda consumption and smoking was described.
3.4. Controversial Factors
Despite the large number of studies examining the role of individual foods, dietary factors, dietary supplements and beverages in the development of RA, many controversies remain.
Controversial Dietary Factors
-
Mediterranean diet is characterized by high consumption of vegetables, legumes, olive oil, alcohol, and fish. This dietary pattern has been associated with a number of chronic diseases, including RA. In the NHS, 913 incident cases of RA were documented during 3,511,050 cumulative person-years of follow-up. After adjustment for several lifestyle and dietary variables, adherence to Mediterranean dietary pattern was not associated with increased risk of RA in women [20]. A nested case-control study in the Swedish EIRA cohort, analyzed data of 1721 patients with incident RA and 3667 controls and found that a Mediterranean diet was inversely associated with the risk of RA, particularly among men (OR 0.49, 95% 0.33–0.73) and with RF and ACPA positivity (OR 0.69, 95% CI 0.54–0.88 and 0.72, 95% IC 0.57–0.92, respectively) [21]. Recently, a French cohort, the E3N study identified 480 incident cases among 62,629 women and found that a Mediterranean diet was associated with a decreased risk of RA among ever smokers (HR 0.86, 95% CI 0.84–0.99) [22].
-
Meat and dairy products consumption. During 12 years of follow-up in a Swedish cohort study (381,456 person-years), 368 individuals developed RA. No associations between the development of RA and the consumption of meat and meat products or total consumption of milk and dairy products were found (HR 1.08, 95% CI 0.77–1.53 and HR 1.09, 95% CI 0.76–1.55, respectively) [23]. Other analyses related to meat consumption are ongoing, such as a large prospective Danish cohort which aims to investigate the impact of fiber, red meat and processed meat on risk of late-onset chronic inflammatory diseases, including RA [24].
-
Vegan diet. Vegan diet has been associated with reduced inflammation markers. In a randomized control trial, markers of inflammation relevant for RA were compared in individuals who were on vegan diet against individuals on meat-rich diet during four weeks. Vegan diet reduced neutrophils, monocytes and platelets related to branched-chain amino acids. These findings suggested a mode of action via the mTOR signaling pathway [25]. Another study reported improved signs and symptoms of RA with a gluten-free vegan diet and the effects on arthritis correlated with a reduction in antibodies to food antigens [26]. However, no protective effect of vegan diet on RA development has been demonstrated.
-
Fasting has been reported as beneficial on RA disease activity [27][28][29]. A systematic review reported 31 studies examining the effects of fasting in patients with RA, but only four controlled studies analyzed follow-up data over at least three months after fasting, and showed statistically and clinically significant beneficial effects [30]. However, a protective effect of fasting on RA development has not yet been demonstrated.
-
Elemental diet. A small study compared elemental diet with oral prednisolone for 2 weeks in RA patients. Elemental diet appeared as effective as a course of oral prednisolone 15 mg daily in improving subjective clinical parameters of RA [31]. In a smaller but longer study, patients with active RA were randomized either to a liquid elemental peptide-diet for four weeks or usual diet. Elemental diet produced transient but statistically significant improvement in pain and disability measured with Health Assessment Questionnaire (HAQ)-score [31]. Similarly, to the vegan diet and fasting, the role of elemental diet on RA development has not been explored.
Omega-3, Omega-6 Fatty Acids and Fish Consumption
A study compared polyunsaturated fatty acids (PUFA), including omega-3 and omega-6, between pre-RA individuals (measurement prior to disease onset) and matched controls from the European Prospective Investigation into Cancer and Nutrition (EPIC). Omega-6 PUFA levels of the erythrocyte were inversely associated with risk of RA, but no association were observed for omega-3 [32]. However, in a large cohort study from Sweden, in which 205 RA cases among 32,232 women were recorded, a similar analysis supported the hypothesis that omega-3 PUFA may play a role in RA development. In this study, long-lasting intake of omega-3 fatty acids higher than 0.21 g/day decreased the development of subsequent RA by 52% (95% CI 29–67%) [33], as well as a regular consumption of fish at least once per week (risk ratio (RR), 0.71, 95% CI 0.48–1.04). A meta-analysis examining the association between fish consumption and subsequent development of RA suggested a trend towards a protective effect with one to three portions of fish per week (RR 0.76, 95% CI 0.57–1.02) [34]. In a large prospective cohort study with 1080 incident RA cases in 3,863,909 person years of follow-up, no clear protective effect of omega-3 fatty acids intake on RA risk was found. However, authors reported a significant interaction between tobacco smoking and fish consumption. Frequent fish consumption among ever smokers women attenuated the strong association of smoking and RA, particularly in young-onset RA (diagnosed at 55 years of age or younger) [35].
Vitamin D
Vitamin D has immunomodulatory properties [36]. Low vitamin D levels may contribute to increased immune activation and may lead to RA development [37]. Several studies have reported vitamin D deficiency in RA patients, in up to 76% of patients and inverse association between vitamin D levels and disease activity [37][38][39]. However, the evidence is controversial as reverse causation may explain some of these findings and a beneficial effect of vitamin D supplementation on RA disease onset has not been demonstrated.
Coffee and Tea
In the large prospective NHS, the authors did not find a significant association between coffee, decaffeinated coffee, or tea consumption and the risk of RA in women [40]. Another prospective cohort study reported decaffeinated coffee consumption (≥4 cups by day) was associated with increased RA onset (RR 2.58, 95% CI 1.63–4.06), while tea consumption was inversely associated with RA (RR 0.39, 95% CI 0.16–0.97) [41].
Obesity
The role of obesity as a risk factor for RA is controversial, as it has been described as a risk factor in women, but as a protective factor in men [11][42][43]. Obese women (BMI ≥ 30.0 kg/m2) in the NHS tended to have an increased risk of RA, particularly those diagnosed at younger ages (HR 1.65, 95% CI 1.34–2.05) and in those obese during adolescence (HR 1.35, 95% CI 1.10–1.66) [11]. Similar results were found in Europe, with obesity increasing the risk for seronegative RA in women (HR 1.6, 95% CI 1.2–2.2) [42]. In men, the effect of obesity was less obvious and some studies have even described a reduced risk of RA in men [43]. From two large population-based health surveys (30,447 and 33,346 participants), excess weight or obesity in men was associated with a reduced risk of RA development (OR 0.33, 95%CI 0.14–0.76 and OR 0.60, 95%CI 0.39–0.91, respectively) [43].
4. Conclusion
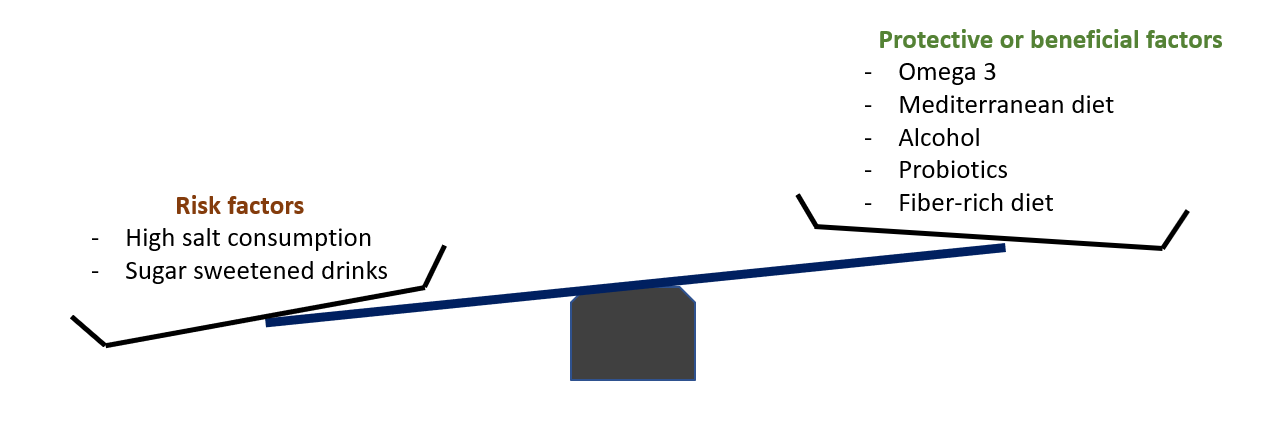
Though available literature is limited, the interest about the role of diet in the development of RA is growing. Several studies have recently suggested that the use of omega-3 and moderate alcohol consumption may have a protective effect on RA development, particularly among smokers or individuals at high risk. On the contrary, high-salt diet and sugar sweetened soft-drinks are considered risk factors. However, it is still unclear how these dietary factors are mechanistically related to the onset of RA. One could hypothesizethe the gut microbiota and the intestinal barrier homeostasis to be a missing link, but such research has so far only been conducted in mouse models.
References
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Harris, H.E.; Ulfgren, A.K.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46.
- Mankia, K.; Emery, P. Preclinical Rheumatoid Arthritis: Progress Toward Prevention. Arthritis Rheumatol. 2016, 68, 779–788.
- Svendsen, A.J.; Holm, N.V.; Kyvik, K.; Petersen, P.H.; Junker, P. Relative importance of genetic effects in rheumatoid arthritis: Historical cohort study of Danish nationwide twin population. BMJ 2002, 324, 264–266.
- Hensvold, A.H.; Magnusson, P.K.E.; Joshua, V.; Hansson, M.; Israelsson, L.; Ferreira, R.; Jakobsson, P.J.; Holmdahl, R.; Hammarström, L.; Malmström, V.; et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: An epidemiological investigation in twins. Ann. Rheum. Dis. 2015, 74, 375–380.
- Sugiyama, D.; Nishimura, K.; Tamaki, K.; Tsuji, G.; Nakazawa, T.; Morinobu, A.; Kumagai, S. Impact of smoking as a risk factor for developing rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2010, 69, 70–81.
- Holers, V.M.; Demoruelle, M.K.; Kuhn, K.A.; Buckner, J.H.; Robinson, W.H.; Okamoto, Y.; Norris, J.M.; Deane, K.D. Rheumatoid arthritis and the mucosal origins hypothesis: Protection turns to destruction. Nat. Rev. Rheumatol. 2018, 14, 542–557.
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78.
- Gan, R.W.; Young, K.A.; Zerbe, G.O.; Demoruelle, M.K.; Weisman, M.H.; Buckner, J.H.; Gregersen, P.K.; Mikuls, T.R.; O’Dell, J.R.; Keating, R.M.; et al. Lower omega-3 fatty acids are associated with the presence of anti-cyclic citrullinated peptide autoantibodies in a population at risk for future rheumatoid arthritis: A nested case-control study. Rheumatology (Oxford) 2016, 55, 367–376.
- Gan, R.W.; Demoruelle, M.K.; Deane, K.D.; Weisman, M.H.; Buckner, J.H.; Gregersen, P.K.; Mikuls, T.R.; O’Dell, J.R.; Keating, R.M.; Fingerlin, T.E.; et al. Omega-3 fatty acids are associated with a lower prevalence of autoantibodies in shared epitope-positive subjects at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 147–152.
- Jonsson, I.-M.; Verdrengh, M.; Brisslert, M.; Lindblad, S.; Bokarewa, M.; Islander, U.; Carlsten, H.; Ohlsson, C.; Nandakumar, K.S.; Holmdahl, R.; et al. Ethanol prevents development of destructive arthritis. Proc. Natl. Acad. Sci. USA 2007, 104, 258–263.
- Lu, B.; Hiraki, L.T.; Sparks, J.A.; Malspeis, S.; Chen, C.-Y.; Awosogba, J.A.; Arkema, E.V.; Costenbader, K.H.; Karlson, E.W. Being overweight or obese and risk of developing rheumatoid arthritis among women: A prospective cohort study. Ann. Rheum. Dis. 2014, 73, 1914–1922.
- Källberg, H.; Jacobsen, S.; Bengtsson, C.; Pedersen, M.; Padyukov, L.; Garred, P.; Frisch, M.; Karlson, E.W.; Klareskog, L.; Alfredsson, L. Alcohol consumption is associated with decreased risk of rheumatoid arthritis: Results from two Scandinavian case-control studies. Ann. Rheum. Dis. 2009, 68, 222–227.
- Scott, I.C.; Tan, R.; Stahl, D.; Steer, S.; Lewis, C.M.; Cope, A.P. The protective effect of alcohol on developing rheumatoid arthritis: A systematic review and meta-analysis. Rheumatology (Oxford) 2013, 52, 856–867.
- Sigaux, J.; Semerano, L.; Favre, G.; Bessis, N.; Boissier, M.-C. Salt, inflammatory joint disease, and autoimmunity. Joint Bone Spine 2018, 85, 411–416.
- Sharif, K.; Amital, H.; Shoenfeld, Y. The role of dietary sodium in autoimmune diseases: The salty truth. Autoimmun. Rev. 2018, 17, 1069–1073.
- Sundström, B.; Johansson, I.; Rantapää-Dahlqvist, S. Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: Results from a nested case-control study. Rheumatology (Oxford) 2015, 54, 487–493.
- Jiang, X.; Sundström, B.; Alfredsson, L.; Klareskog, L.; Rantapää-Dahlqvist, S.; Bengtsson, C. High sodium chloride consumption enhances the effects of smoking but does not interact with SGK1 polymorphisms in the development of ACPA-positive status in patients with RA. Ann. Rheum. Dis. 2016, 75, 943–946.
- Salgado, E.; Bes-Rastrollo, M.; de Irala, J.; Carmona, L.; Gómez-Reino, J.J. High Sodium Intake Is Associated With Self-Reported Rheumatoid Arthritis: A Cross Sectional and Case Control Analysis Within the SUN Cohort. Medicine 2015, 94, e924.
- Hu, Y.; Costenbader, K.H.; Gao, X.; Al-Daabil, M.; Sparks, J.A.; Solomon, D.H.; Hu, F.B.; Karlson, E.W.; Lu, B. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am. J. Clin. Nutr. 2014, 100, 959–967.
- Hu, Y.; Costenbader, K.H.; Gao, X.; Hu, F.B.; Karlson, E.W.; Lu, B. Mediterranean diet and incidence of rheumatoid arthritis in women. Arthritis Care Res. 2015, 67, 597–606.
- Johansson, K.; Askling, J.; Alfredsson, L.; Di Giuseppe, D.; EIRA study group. Mediterranean diet and risk of rheumatoid arthritis: A population-based case-control study. Arthritis Res. Ther. 2018, 20, 175.
- Nguyen, Y.; Salliot, C.; Gelot, A.; Gambaretti, J.; Mariette, X.; Boutron-Ruault, M.-C.; Seror, R. Mediterranean diet and risk of rheumatoid arthritis: Findings from the French E3N-EPIC cohort study. Arthritis Rheumatol. 2020.
- Sundström, B.; Ljung, L.; Di Giuseppe, D. Consumption of Meat and Dairy Products Is Not Associated with the Risk for Rheumatoid Arthritis among Women: A Population-Based Cohort Study. Nutrients 2019, 11, 2825.
- Rasmussen, N.F.; Rubin, K.H.; Stougaard, M.; Tjønneland, A.; Stenager, E.; Lund Hetland, M.; Glintborg, B.; Bygum, A.; Andersen, V. Impact of red meat, processed meat and fibre intake on risk of late-onset chronic inflammatory diseases: Prospective cohort study on lifestyle factors using the Danish ’Diet, Cancer and Health’ cohort (PROCID-DCH): Protocol. BMJ Open 2019, 9, e024555.
- Lederer, A.-K.; Maul-Pavicic, A.; Hannibal, L.; Hettich, M.; Steinborn, C.; Gründemann, C.; Zimmermann-Klemd, A.M.; Müller, A.; Sehnert, B.; Salzer, U.; et al. Vegan diet reduces neutrophils, monocytes and platelets related to branched-chain amino acids—A randomized, controlled trial. Clin. Nutr. 2020, 39, 3241–3250.
- Hafström, I.; Ringertz, B.; Spångberg, A.; von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology (Oxford) 2001, 40, 1175–1179.
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Laerum, E.; Eek, M.; Mowinkel, P.; Hovi, K.; Førre, O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902.
- Sundqvist, T.; Lindström, F.; Magnusson, K.-E.; Sköldstam, L.; Stjernström, I.; Tagesson, C. Influence of Fasting on Intestinal Permeability and Disease Activity in Patients with Rheumatoid Arthritis. Scand. J. Rheumatol. 1982, 11, 33–38.
- Sköldstam, L.; Larsson, L.; Lindström, F.D. Effects of Fasting and Lactovegetarian Diet on Rheumatoid Arthritis. Scand. J. Rheumatol. 1979, 8, 249–255.
- Müller, H.; de Toledo, F.W.; Resch, K.L. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review. Scand. J. Rheumatol. 2001, 30, 1–10.
- Podas, T.; Nightingale, J.M.D.; Oldham, R.; Roy, S.; Sheehan, N.J.; Mayberry, J.F. Is rheumatoid arthritis a disease that starts in the intestine? A pilot study comparing an elemental diet with oral prednisolone. Postgrad. Med. J. 2007, 83, 128–131.
- De Pablo, P.; Romaguera, D.; Fisk, H.L.; Calder, P.C.; Quirke, A.-M.; Cartwright, A.J.; Panico, S.; Mattiello, A.; Gavrila, D.; Navarro, C.; et al. High erythrocyte levels of the n-6 polyunsaturated fatty acid linoleic acid are associated with lower risk of subsequent rheumatoid arthritis in a southern European nested case-control study. Ann. Rheum. Dis. 2018, 77, 981–987.
- Di Giuseppe, D.; Wallin, A.; Bottai, M.; Askling, J.; Wolk, A. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: A prospective cohort study of women. Ann. Rheum. Dis. 2014, 73, 1949–1953.
- Di Giuseppe, D.; Crippa, A.; Orsini, N.; Wolk, A. Fish consumption and risk of rheumatoid arthritis: A dose-response meta-analysis. Arthritis Res. Ther. 2014, 16, 446.
- Sparks, J.A.; O’Reilly, É.J.; Barbhaiya, M.; Tedeschi, S.K.; Malspeis, S.; Lu, B.; Willett, W.C.; Costenbader, K.H.; Karlson, E.W. Association of fish intake and smoking with risk of rheumatoid arthritis and age of onset: A prospective cohort study. BMC Musculoskelet. Disord. 2019, 20, 2.
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647.
- Bragazzi, N.L.; Watad, A.; Neumann, S.G.; Simon, M.; Brown, S.B.; Abu Much, A.; Harari, A.; Tiosano, S.; Amital, H.; Shoenfeld, Y. Vitamin D and rheumatoid arthritis: An ongoing mystery. Curr. Opin. Rheumatol. 2017, 29, 378–388.
- Raczkiewicz, A.; Kisiel, B.; Kulig, M.; Tłustochowicz, W. Vitamin D status and its association with quality of life, physical activity, and disease activity in rheumatoid arthritis patients. J. Clin. Rheumatol. 2015, 21, 126–130.
- Buondonno, I.; Rovera, G.; Sassi, F.; Rigoni, M.M.; Lomater, C.; Parisi, S.; Pellerito, R.; Isaia, G.C.; D’Amelio, P. Vitamin D and immunomodulation in early rheumatoid arthritis: A randomized double-blind placebo-controlled study. PLoS ONE 2017, 12, e0178463.
- Karlson, E.W.; Mandl, L.A.; Aweh, G.N.; Grodstein, F. Coffee consumption and risk of rheumatoid arthritis. Arthritis Rheum. 2003, 48, 3055–3060.
- Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Merlino, L.; Mudano, A.S.; Burma, M.; Folsom, A.R.; Saag, K.G. Coffee, tea, and caffeine consumption and risk of rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2002, 46, 83–91.
- Wesley, A.; Bengtsson, C.; Elkan, A.C.; Klareskog, L.; Alfredsson, L.; Wedrén, S.; Epidemiological Investigation of Rheumatoid Arthritis Study Group. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: Results from a population-based case-control study. Arthritis Care Res. 2013, 65, 107–112.
- Turesson, C.; Bergström, U.; Pikwer, M.; Nilsson, J.-Å.; Jacobsson, L.T.H. A high body mass index is associated with reduced risk of rheumatoid arthritis in men, but not in women. Rheumatology (Oxford) 2016, 55, 307–314.
