Beetroot is a remarkable vegetable, as its rich nitrate and bioactive compound contents ameliorate cardiovascular and metabolic functions by boosting nitric oxide synthesis and regulating gene expressions or modulating proteins and enzyme activities involved in these cellular processes. Dietary nitrate provides a physiological substrate for nitric oxide production, which promotes vasodilatation, increases blood flow and lowers blood pressure.
- green leaves
- beetroot
- nitrate-rich diet therapy
- nitric oxide
- advanced hemodynamic parameters
- clinical trials
1. Dietary NO3− and Endothelial Dysfunction Therapy
Until a decade ago, NO
was considered an unfavorable dietary-derived toxic compound, as it was wrongly associated with the development of some malignancies, such as gastric cancer. Strict standards regarding the levels of this anion were regulated in food [1]. The World Health Organization (WHO) defined an acceptable daily intake (ADI) of 3.7 mg of NO
·kg
body weight, the same content adopted by the European Food Safety Authority. For a normal adult weighing 70 kg, this content is equivalent to ~260 mg of NO
·day
. However, vegetarian diets commonly contain >300 mg of NO
·day
for 70 kg adults, higher than the ADI [2].
Recently, researchers have become interested in the biological NO
role. Findings regarding the improvement of cardiovascular function have raised a biologically plausible and widely recognized hypothesis that the NO
present in vegetables may serve as a physiological substrate for NO generation which, in turn, promotes vasodilation and, consequently, improves cardiovascular function [3][4].
NO
is a nitric acid salt, while NO
is a nitrous acid salt compound, formed by a single nitrogen bonded to three or two oxygen atoms, respectively. Both compounds can be obtained from endogenous and/or exogenous sources. The endogenous formation of NO
and NO
occurs through the NO metabolism via the L-arginine/NO pathway, as mentioned previously. On the other hand, the main potential exogenous source for the acquisition of NO
and NO
is through the dietary route. Through this pathway, NO is then generated by a non-enzymatic pathway from NO
. Dietary NO
is reduced to NO
in the oral cavity by bacteria that produce the NO
-reductase enzyme [5]. The metabolic activities of commensal bacteria species, such as
,
,
,
,
,
, and
genera that inhabit the oral cavity have a significant influence on NO
to NO pathway. Previous studies have shown that individuals with a higher abundance of NO
-reducing bacteria are able to generate more salivary NO
and, consequently, NO, at a faster rate following dietary NO
ingestion [6]. However, enzymatic activity in the mouth and, consequently, the conversion of NO
to NO
may be disrupted by antibiotic use or mouthwash rinsing, since both substances inactivate bacteria cells [7]. Subsequently, NO
reaches the stomach and, in this acidic environment, is protonated, forming nitrous acid (HNO
), which decomposes non-enzymatically to NO and other bioactive nitrogen oxides such as nitrogen dioxide (NO
), dinitrogen trioxide (N
O
) and the nitrosonium ion (NO
and NO
in the jejunum are rapidly absorbed into the bloodstream or tissues, where their accumulation occurs in tandem with molecules endogenously synthesized by the L-arginine/NO pathway. Most NO
is excreted in urine, whereas a small portion is extracted by the salivary glands, concentrating this compound in the saliva, continuing the entero-salivary cycle [3][5]. A small part of plasma NO
and NO
concentrations may suffer the action of xanthine oxidoreductase (XOR), which displays similar activity to NO
-reductase. NO
can also be reduced to bioactive NO by deoxyhemoglobin (deoxyHb) and deoxymyoglobin (deoxyMb), especially when O
levels are low. Other enzymes and compounds exhibiting redox potential, such as aldehyde oxidase (AO), aldehyde dehydrogenase (ALDH), carbonic anhydrase (CA), vitamin C (Vit C.) and polyphenols, display the ability to synthesize NO from NO
reduction [3].
Several studies report beneficial effects of dietary NO
sources as a new physiological, therapeutic and nutritional approach to attain the intended cardioprotective effects by NO production stimulation [3][8][9]. However, dosage, supplementation regimen and individual health status must be considered to obtain the maximum cardioprotective effect following NO
intake. Furthermore, environmental factors such as temperature, exposure to sunlight, atmospheric humidity, water content and irradiation, as well as agricultural factors like plant genotype, fertilization, herbicide use, amount of available nitrogen, type of crop, soil conditions, nutrient availability and transport and, finally, storage conditions also influence NO
contents in plants, and, consequently dietary NO
supplementation [10].
2. Dietary NO3− Vegetable Sources
Vegetables are the main source of dietary NO
, corresponding to 85% of the daily intake, although NO
content can vary widely within members of distinct botanical families [11]. The NO
contents in plant organs can be classified from highest to lowest, as petiole ˃ leaf ˃ stem ˃ root ˃ tuber ˃ bulb ˃ fruit ˃ seed [12].
presents a list of vegetables commonly included in Western diets considered NO
sources, classified according to NO
contents, from the highest to the lowest.
Dietary NO
sources classified from the highest to the lowest according to mean [and range] NO
content.
| Vegetable | NO | 3− | Content/mg·kg | −1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| High NO | 3− | content (>1000 mg·kg | −1 | ) | Rocket or arugula ( | Eruca vesicaria | subsp. | sativa | ) | 2848 [2597–3100] |
| Green spinach ( | Spinacia oleracea | ) | 2500 [2013–2797] | |||||||
| Coriander ( | Coriandrum sativum | ) | 2445 | |||||||
| Basil ( | Ocimum basilicum | ) | 2292 [507–4695] | |||||||
| Celery ( | Apium graveolens | ) | 2200 [900–3500] | |||||||
| Parsley ( | Petroselinum crispum | ) | 2134 [1700–2101] | |||||||
| Radish ( | Raphanus raphanistrum | subsp. | sativus | ) | 2064 [1878–2250] | |||||
| Butter leaf lettuce ( | Lactuca sativa | variety | capitata | ) | 2000 | |||||
| Bok choy ( | Brassica rapa | subsp. | chinensis | ) | 1933 | |||||
| Lettuce ( | Lactuca sativa | ) | 1893 [970–2782] | |||||||
| Beet greens ( | Beta vulgaris | subsp. | vulgaris | ) | 1852 [1060–2600] | |||||
| Kohlrabi ( | Brassica oleracea | ) | 1769 | |||||||
| Swiss chard ( | Beta vulgaris | subsp. | maritima | ) | 1512 [1024–2000] | |||||
| Leaf chicory ( | Cichorium intybus | ) | 1452 | |||||||
| Beetroot ( | Beta vulgaris | subsp. | vulgaris | ) | 1300 [644–1950] | |||||
| Black radish ( | Raphanus raphanistrum | subsp. | sativus | ) | 1271 [667–1878] | |||||
| Mustard greens ( | Brassica juncea | ) | 1160 | |||||||
| Medium NO | 3− | content (100 to 1000 mg·kg | −1 | ) | Curly kale ( | Brassica oleracea | Acephala Group) | 987 [792–1181] | ||
| Broccoli raab ( | Brassica rapa | ) | 905 | |||||||
| Pumpkin ( | Cucurbita pepo | ) | 692 [445–939] | |||||||
| Turnip ( | Brassica rapa | subsp. | rapa | ) | 684 [307–1062] | |||||
| Endive ( | Cichorium endivia | ) | 663 | |||||||
| Cabbage ( | Brassica oleracea | var. | capitata | ) | 503 [85–920] | |||||
| Green beans ( | Phaseolus vulgaris | ) | 496 [449–585] | |||||||
| Green onion ( | Allium fistulosum | ) | 485 [99–870] | |||||||
| Courgette ( | Cucurbita pepo | ) | 416 | |||||||
| Fennel ( | Foeniculum vulgare | ) | 363 | |||||||
| Asparagus ( | Asparagus officinalis | ) | 355 [145–479] | |||||||
| Cauliflower ( | Brassica oleracea | var. | botrytis | ) | 331 [104–559] | |||||
| Savoy cabbage ( | Brassica oleracea | Savoy Cabbage Group) | 324 | |||||||
| Aubergine ( | Solanum melongena | ) | 314 | |||||||
| Broccoli ( | Brassica oleracea | var. | italica | ) | 300 [145–477] | |||||
| Carrot ( | Daucus carota | subsp. | sativus | ) | 300 [121–480] | |||||
| Cucumber ( | Cucumis sativus | ) | 240 [124–384] | |||||||
| Potato ( | Solanum tuberosum | ) | 220 [81–713] | |||||||
| Garlic ( | Allium sativum | ) | 183 [34–455] | |||||||
| Artichokes ( | Cynara scolymus | ) | 174 | |||||||
| Sweet pepper ( | Capsicum annuum | ) | 117 [93–140] | |||||||
| Green pepper ( | Capsicum annuum | ) | 111 [76–159] | |||||||
| Low NO | 3− | content (<100 mg·kg | −1 | ) | Onion ( | Allium cepa | ) | 87 [23–235] | ||
| Tomato ( | Solanum lycopersicum | ) | 69 [27–170] |
The NO
-rich vegetables within the
family comprise beetroot (1300 mg·kg
), beet greens (1852 mg·kg
), Swiss chard (1690 mg·kg
), and green spinach (≈2500 mg·kg
), while a
family representative consists of basil (2292 mg·kg
). Concerning the
family, the most representative members are bok choy (1933 mg·kg
), black radish (1271 mg·kg
), turnip (1018 mg·kg
), mustard greens (1160 mg·kg
), rocket or arugula (4677 mg·kg
), kohlrabi (1769 mg·kg
), and radish (≈2000 mg·kg
).
family members include coriander (2445 mg·kg
), celery (1100 mg·kg
) and parsley (2134 mg·kg
), whereas
family members include lettuce (≈1800 mg·kg
), leaf chicory (1452 mg·kg
), and butter leaf lettuce (2000 mg·kg
). All these vegetables are included in the high NO
-containing vegetable category of > 1000 mg·kg
. Vegetables such as cabbage (513 mg·kg
), curly kale (987 mg·kg
), broccoli (≈300 mg·kg
), broccoli raab (905 mg·kg
), cauliflower (202 mg·kg
) and Savoy cabbage (324 mg·kg
), which belong to the
family; carrot (≈300 mg·kg
) and fennel (363 mg·kg
), both members of the
family; artichokes (174 mg·kg
), asparagus chicory (355 mg·kg
), and endive (663 mg·kg
), belonging to the
family, garlic (183 mg·kg
) and green onion (≈450 mg·kg
) from the
family; aubergine (314 mg·kg
), capsicum (108 mg·kg
) and potato (220 mg·kg
), belonging
family; courgette (416 mg·kg
) and cucumber (240 mg·kg
), pumpkin (894 mg·kg
), from the
family member all contain intermediate NO
concentrations ranging from 100 to 1000 mg·kg
Among the vegetables considered the richest dietary NO
sources, as listed in
, beetroot, rocket and spinach have been the most tested concerning dietary interventions, and all resulted in effective improvements in cardiovascular performance estimated through blood pressure reduction and vascular function amelioration (
).
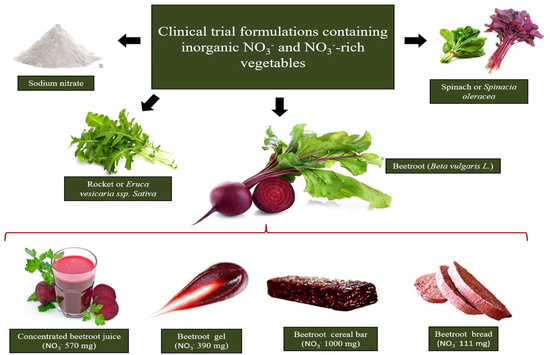
The richest sources of dietary NO
tested in clinical interventions are beetroot, rocket and spinach. Beetroot formulation choice to supplement dietary NO
relies on the design of beetroot-derived formulations containing pharmacological NO
doses in a small serving portion.
NO
vascular-effects depend on digestibility and bioavailability (bioacessibility), and better performances are obtained when NO
intake originates from food matrices compared to NaNO
salt administration [16]. The beneficial effects of different NO
-rich vegetables and NO
doses in NO stimulation production and biochemical, hemodynamic, and vascular parameters in healthy or cardiovascular-compromised patients are summarized in
. It is important to note that, to the best of our knowledge, NO
supplementation from green leaves has only been performed in healthy individuals, and it is unknown whether their effects can be extended to individuals presenting cardiovascular risk factors. In addition, although the cardiovascular protective effects of NO
-enriched vegetables have been clearly demonstrated in clinical trials with healthy individuals, the large volume of juice vegetables used to achieve effective dietary NO
concentrations can be a limiting factor in ensuring adherence to long-term nutritional interventions. However, this NO
limitation does not impact supplementation by beetroot juice. Beet juice and other beetroot formulations can be ingested in comfortable serving portions to achieve threshold NO
concentrations in order to promote beneficial cardiovascular function effects.
Selected clinical trials from 2012 to 2020 compared considering administered NO
content, intervention duration, level of systemic increase in NO evaluated by plasma NO
and NO
levels and improvements in primary and advanced hemodynamic parameters in healthy individuals and in patients presenting impaired vascular function.
| NO | 3− | Vegetable Intervention | NO | 3− | Content/Serving Portion Administered | Subjects | Duration of Administration | Trial Features | Effects | Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White beetroot bread ( | Beta vulgaris | L) Red beetroot bread ( | Beta vulgaris | L) | 99 mg·200 g | −1 | 112 mg·200 g | −1 | 14 healthy individuals | single intake | Randomized Placebo-controlled Single-blind Crossover |
↑ NO synthesis after 1 h of ingestion (through urinary NO | x | ) ↓ 24 h ambulatory SBP and DBP |
Hobbs et al. [38] | Hobbs et al. [17] | ||
| Beetroot bread ( | Beta vulgaris | L) | 68 mg·200 g | −1 | 23 healthy individuals | single intake | Randomized Placebo-controlled Open-label Crossover |
↑ NO synthesis after 1 h of ingestion (through plasma and urinary NO | 3− | and NO | 2− | ) ↓ iAUC (0–6 h after beet bread ingestion) for DBP ↑ iAUC (0–6 h after beet bread ingestion) for endothelium-independent microvascular vasodilation |
Hobbs et al. [39] | Hobbs et al. [18] | ||||
| Beetroot juice ( | Beta vulgaris | L) | 403 mg·70 mL | −1 | 24 overweight older individuals | 3 weeks | Randomized Placebo-controlled |
↓ daily resting DBP at home | Jajja et al. [40] | Jajja et al. [19] | ||||||||
| 400 mg·250 mL | −1 | 68 hypertensive individuals | 4 weeks | Randomized Placebo-controlled Double-blind Crossover |
↑ NO synthesis (by plasma and salivary NO | 3− | , NO | 2− | and plasma cGMP) ↓ home, clinic and 24 h ambulatorial SBP and DBP, and arterial stiffness (through reduction of PWV and AIx) ↑ endothelial function (through increased brachial artery diameter and time to peak dilatation after FMD) |
Kapil et al. [41] | Kapil et al. [20] | |||||||
| 100 mg·100 mL | −1 | 40 healthy individuals | single intake | Randomized Placebo-controlled Double-blind Crossover |
↑ NO synthesis (by urinary NO | 3− | and NO | 2− | ) No significant relationships between urinary NO | 3− | and NO | 2− | concentration and body mass after intervention were observed | Baião et al. [42] | Baião et al. [21] | |||
| 800 mg·200 mL | −1 | 14 non-hypertensive obese individuals |
single intake | Randomized Placebo-controlled Crossover |
↑ NO synthesis (through plasma NO | x | ) ↓ ambulatory SBP following 1–6 h of moderate-intensity aerobic exercise |
Bezerra et al. [43] | Bezerra et al. [22] | |||||||||
| Beetroot gel ( | Beta vulgaris | L) | 390 mg·100 g | −1 | 5 healthy individuals | single intake | - | ↑ NO synthesis (through plasma NO | 2− | ) ↓ ambulatory SBP, DBP and HR |
Silva et al. [44] | Silva et al. [23] | ||||||
| Beetroot cereal bar ( | Beta vulgaris | L) | 589 mg·60 g | −1 | women with 2 risk factors for CVD | 3 weeks | Randomized Placebo-controlled Double-blind Crossover |
↑ NO synthesis (through plasma NO | 3− | and NO | 2− | ) ↓ clinical DBP and SBP ↓ arterial stiffness (through reductions in AP, AIx, | ao | SP, | ao | PP, arterial age and PWV) ↑ endothelial function (through increased CVC peaks and AUC) |
Baião et al. [45] | Baião et al. [24] |
| Spinach ( | Spinacia oleracea | ) | 220 mg·250 g | −1 | 26 healthy individuals | single intake | Randomized Placebo-controlled Crossover |
↑ NO synthesis (through salivary NO | 3− | and NO | 2− | ) ↑ large artery elasticity index ↓ pulse pressure, SBP, estimated cardiac ejection time, estimated cardiac output, estimated stroke volume and total vascular impedance |
Liu et al. [46] | Liu et al. [25] | ||||
| 182 mg·200 g | −1 | 30 healthy individuals | single intake | Randomized Placebo-controlled Crossover |
↑ NO synthesis (through plasma RXNO, NO | 2− | and NO | x | ) ↑ ↑ endothelial function (through increases brachial artery diameter dilatation after FMD) ↓ ambulatory SBP and pulse pressure |
Bondonno et al. [47] | Bondonno et al. [26] | |||||||
| 800 mg·365 g | −1 | 18 healthy individuals | single intake | Semi randomized Crossover |
↑ NO synthesis (through plasma NO | 3− | and NO | 2− | ) ↓ ambulatory DBP and SBP |
Jonvik et al. [48] | Jonvik et al. [27] | |||||||
| Red spinach ( | Amaranthus dubius | ) | 1000 mg·90 mg | −1 | 15 healthy individuals | single intake | Placebo-controlled Double-blind Crossover |
↑ NO synthesis (through plasma NO | 2− | and NO | x | ) ↑ endothelial function (through increased reactive hyperemia and calf blood flow) |
Haun et al. [49] | Haun et al. [28] | ||||
| Rocket ( | Euruca vesicaria ssp. Sativa | ) | 800 mg·196 g | −1 | 18 healthy individuals | single intake | Semi randomized Crossover |
↑ NO synthesis (through plasma NO | 3− | and NO | 2− | ) ↓ DBP and SBP |
Jonvik et al. [48] | Jonvik et al. [27] |
A large volume of spinach comprising a serving portion of 250 g leaves containing 220 mg of NO
were administrated to twenty-six healthy individuals, resulting in an increase in NO synthesis evidenced by an eight-fold increase in salivary NO
and a seven-fold increase in salivary NO
at 120 min post-meal. Large artery elasticity indices were increased alongside lower pulse pressure and reduced systolic blood pressure (SBP) [25].
An amount of 800 mg NO
intake was supplied through four different vegetable drinks, namely beetroot juice (116 g), rocket salad (196 g), spinach (365 g) or NaNO
(1.1 g) prepared in water, which triggered an increase in NO
and NO
plasma concentrations. SBP declined after 150 min of beetroot juice ingestion (from 118 ± 2 to 113 ± 2 mm Hg) and a rocket salad beverage (from 122 ± 3 to 116 ± 2 mm Hg), which was sustained for at least 300 min after ingestion of the spinach beverage (from 118 ± 2 to 111 ± 3 mm Hg). Diastolic blood pressure (DBP) also declined after 150 min ingestion of all beverages and was sustained at lower levels for 300 min after rocket salad or spinach ingestion [27].
All NO
rich-vegetable drinks were more efficient than NaNO
in reducing both SBP and DBP, and beetroot was the most effective considering the food weight/NO
content ratio [27]. However, to the best of our knowledge, the lowest effective volume of beetroot able to promote beneficial vascular effects was 70 mL of beetroot juice containing 6.45 mmol NO
(403 mg), which was administered to 24 older and overweight volunteers for three weeks. This supplementation regimen and the offered dose promoted 2.3-fold and 7.3-fold increases in urinary and salivary NO
, respectively, and resulted in a 7.3 mm Hg decrease in SBP [29].
Beetroot consumption is noteworthy as a convenient and attractive alternative to obtain cardioprotective NO
effects in both healthy individuals and those presenting risk factors for CVD diseases, due to the distinct but smart formulations (traditional or novel) that can be prepared to fulfill effective pharmacological dietary NO
concentrations. An attractive and compact NO
-enriched-beetroot gel has been formulated in an attempt to provide an enriched NO
food product able to promote the claimed cardioprotective effects while still being easy to administer and facilitate adherence to nutritional therapy [23]. Acute supplementation with 100 g of beetroot gel containing 390 mg of NO
promoted a decrease in SBP (−6.2 mm Hg), DBP (−5.2 mm Hg), and heart rate (−7 bpm) in a pilot study conducted with healthy individuals. However, NO
supplementation had to be adjusted to treat hypertensive individuals, since similar doses in compromised vascular individuals do not alter hemodynamic parameters. The non-susceptibility of 27 treated hypertensive patients was clearly demonstrated by the intake of 7.0 mmol (434 mg) of NO
in 140 mL of beetroot juice for 7 days, resulting in increased NO synthesis, assessed by plasmatic, urinary and salivary NO
and NO
, but with no differences in home and 24 h ambulatory, SBP and DBP [26]. These results indicate that, in order to ameliorate primary hemodynamic parameters, high doses of dietary NO
combined with a long-term intervention can be applied to treat individuals presenting impaired endothelial function. Furthermore, in an unprecedented clinical trial, patients displaying at least three risk factors for the development of CVD, including hypertension, were chronically supplemented for three weeks with an enriched NO
beetroot-cereal bar providing 589 mg of NO
in 60 g of the intervention product, resulting in 14.0 mm Hg and 6.5 mm Hg decreases in SBP and DBP, respectively, in response to ~15-fold or ~7-fold increased plasma NO
and NO
concentrations, respectively. Endothelial function in the treated volunteers was improved and arterial stiffness was reduced by 14% [24][30].
3. Plasma NO3−/NO2− Increments on Cardiovascular Health and Impaired Cardiovascular Functions
It is known that plasma NO
and NO
concentrations are dependent on ingested NO
[31], but the minimum increase in NO
/NO
plasma levels necessary to promote hemodynamic responses may differ between healthy individuals and those with compromised cardiovascular function. In a clinical trial where healthy men received dietary supplementation, 3.5-fold and 1.6-fold increases of plasma NO
and NO
, respectively, resulted in significant DBP reductions and increases in endothelium-independent vasodilatation. This small but effective plasma increase was generated after the acute intake of beetroot bread (NO
1.1 mmol) [18]. On the other hand, Haun et al. [28] reported plasma NO
(~3-fold) and NO
(less than 1.5-fold) increases, albeit without any changes in hemodynamic parameters such as heart rate, DBP, SBP, FMD, radial artery pulse waves (PWA), central mean arterial pressure (CMAP) and central pulse pressure (CPP), after the acute intake of red spinach extract (NO
1.45 mmol) by 15 healthy subjects. Although the dose used by Haun et al. [28] was slightly higher than by Hobbs et al. [18] trial, plasma NO
(>3.5-fold) and NO
(>1.6-fold) increases should be a determinant factor in choosing the dose required to benefit healthy populations.
In individuals with impaired cardiovascular function, the administered NO
dose should be able to meet two requirements: (i) promote an increase in systemic NO
and NO
higher than observed in healthy individuals; (ii) be administered in a chronic and uninterrupted manner.
Hypertensive pregnant women exhibited ~10- and ~1.5-fold increases in plasma NO
and NO
, respectively, after 7 days of daily supplementation with NO
(6.45 mmol in beetroot juice). No significant differences were observed in plasma NO
and NO
levels measured 24 h after the initial dose, and even in the following 7-days of daily supplementation [32]. Similarly, a 1-week intake of beetroot juice (NO
~6.45 mmol) in 27 treated hypertensive individuals resulted in a three-fold increase in plasma NO
and NO
, with no differences in home and 24-h ambulatory blood pressures [33]. Finally, 24 overweight older subjects supplemented for 3 weeks with concentrated beet juice (~4.8–6.45 mmol) exhibited urinary NO
values ~3-fold higher greater than the baseline and beneficial SBP effects after juice intake. However, both urinary NO
and SBP returned to baseline levels 24 h after ingestion and in the first week following the end of supplementation [19]. These findings demonstrate that acute treatments able to promote systemic increases in NO
and NO
at levels similar to those observed in healthy individuals do not benefit individuals presenting cardiovascular risks.
On the other hand, clinical trials lasting more than 3 weeks or comprising higher NO
doses than usually applied (6–7 mmol) resulted in better hemodynamic outcomes. Hypertensive subjects treated for 4 weeks with beetroot juice (NO
6.4 mmol) exhibited substantial increases in NO
and NO
plasma levels (~5.5 and ~2.7, respectively). In addition, this intervention provided sustained BP lowering of 7.7/5.2 mm Hg 24 h after the treatment, with clinical BP reduced by 7.7/2.4 mm Hg and home BP, by 8.1/3.8 mm Hg [20]. In this trial, SBP and DBP reduction peaks occurred only in the last week, highlighting the importance of a prolonged intervention.
In another trial, supplementation for 3 weeks with a high dose of dietary NO
concentrate in a 60 g beetroot cereal bar (9.5 mmol) resulted in ~15- and ~7-fold increases in plasma NO
and NO
, respectively. This was accompanied by a considerable reduction in BP (−14.0/−6.5 mm Hg) and improvement in central hemodynamic and endothelial function parameters such as arterial stiffness, augmentation and index pressures, aortic systolic and pulse pressures and cutaneous microvascular conductance [24].
Based on these reports, individuals presenting physiopathological conditions that affect the cardiovascular system require a dietary therapy that associates high NO
doses capable of promoting systemic increases in NO
and NO
to levels higher than found in healthy individuals and in addition, is administered continuously (
).
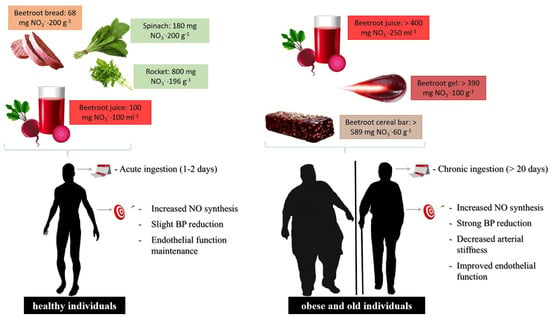
Food formulations and supplementation regimen of dietary NO
in healthy or cardiovascular-compromised patients. For individuals presenting risk factors for the development of cardiovascular disease, the dietary NO
dose should be higher to promote the systemic elevation of plasma NO
and NO
levels compared to healthy individuals, increasing NO generation by the NO
/NO
pathway, where increased levels must be administered through chronic and uninterrupted supplementation.
In short, the aforementioned studies discussed herein suggest that frequent daily dietary NO
doses for long periods of time would be necessary to promote beneficial effects on blood pressure and endothelial function in populations presenting compromised vascular responsiveness. A systematic review and meta-analysis study of randomized controlled trials demonstrated that supplementation of inorganic NO
from beetroot juice over 14 days provoked decreases in SBP (−3.55 mm Hg; 95% CI: −4.55, −2.54 mm Hg) and DBP (−1.32 mm Hg; 95% CI: −1.97, −0.68 mm Hg). Furthermore, beneficial dietary NO
effects on endothelial function were associated with dose, age, and body mass index (BMI), where chronic beetroot juice supplementation improved flow-mediated dilation (FMD) and endothelium functional effects according to the administered NO
contents (β = 0.04, SE = 0.01,
< 0.001), age (β = −0.01, SE = 0.004,
= 0.02) and BMI (β = −0.04, SE = 0.02,
= 0.05) [34]. A critical review of experimental data shows that chronic dietary NO
ingestion is a positive vascular endothelium effector promoting vasodilatation and reducing blood pressure in compromised vascular responsiveness individuals.
However, only beetroot supplementation has been tested in acute and chronic assays in individuals with impaired cardiovascular function. Although the NO
content of green leaves is able to fulfill the effective NO
concentration in such patients, beetroot formulations may be the best non-drug strategy, since beetroot-derived formulations can concentrate the pharmacological NO
dosage in a small serving portion of an attractive food product, favoring continuous intake and better adherence to this nutritional intervention. This may explain the well-documented and consistent cardioprotective effects of beet products in both healthy individuals and those presenting risk factors for the development of CVD when compared with other rich-NO
vegetables, such as green leaves, assayed in clinical trials.
Furthermore, it is important to note that, in addition to NO
, vegetables are also a source of numerous phytochemicals able to increase eNOS activity in endothelial cells and contribute to NO synthesis [35][36]. Due to the great variety of polyphenols and other bioactive compounds in vegetables, it is difficult to point out individual or synergistic effects on NO generation. However, only NO
has been directly associated to the cardioprotective effect, since it provides the physiological substrate for NO generation via the NO
-NO
/NO enterosalivary pathway [3]. The administration of the same food matrix, depleted in NO
, used as a placebo in the clinical trials had no effect on NO synthesis and hemodynamic parameters, proving that NO
is probably the active principle. The remaining phytochemicals after NO
removal, including polyphenols, which are preserved in the placebo, may promote a discrete increase in NO production but it seems they are not effective in promoting hemodynamic improvements, similar to the effect observed when NO
at concentrations under the pharmacological threshold is administered.
4. Conclusions
Vegetables are important health-promoting foods in a balanced diet, due to the presence of bioactive compounds, including dietary NO
. Vegetables that belong to the green leaf group, such as rocket, green spinach, basil, radish, Swiss chard and bok choy, in addition to red beetroot, are considered the richest sources of dietary NO
. Increasing dietary NO
ingestion results in beneficial effects in many physiological and clinical settings. Several clinical interventions with different NO
-rich vegetables have been reported as affecting metabolic and cardiovascular functions by increasing NO concentrations and improving endothelial function by reducing BP and arterial stiffness. However, minimal or no hemodynamic and vascular beneficial effects in healthy individuals have been observed following acute NO
ingestion. To obtain the maximum cardioprotective effects of NO
intake, patient health status, as well as NO
dosage and supplementation regimen, must be considered.
The aforementioned studies suggest that frequent daily doses up to 6.0 mmol of dietary NO
for long periods of time (≥3 weeks) are required to promote beneficial blood pressure and endothelial function effects, mainly in populations with compromised vascular responsiveness such as hypertensive, metabolic syndrome, obese and older individuals.
Only beetroot supplementation has been tested in acute and chronic assays in individuals with impaired cardiovascular function. Although the NO
content of green leaves or other vegetables could fulfill the effective NO
concentration in healthy individuals, patients with impaired vascular function require a higher dose able to provide systemic increases in NO
and NO
to levels higher than those achieved in healthy individuals. Beet formulations are easier, attractive, accessible and were the only vegetable shown to be effective in promoting increased systemic NO production at the magnitude necessary to achieve the expected pharmacological effects in individuals presenting cardiovascular disease risk factors.
References
- Mirvish, S.S. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995, 93, 17–48.
- Katan, M.B. Nitrate in foods: Harmful or healthy? Am. J. Clin. Nutr. 2009, 90, 11–12.
- Lidder, S.; Webb, A. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696.
- Lundberg, J.O.; Gladwin, M.T.; Ahluwalia, A.; Benjamin, N.; Bryan, N.S.; Butler, A.; Cabrales, P.; Fago, A.; Feelisch, M.; Ford, P.C.; et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009, 5, 865–869.
- Baião, D.S.; da Silva, D.V.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, bioactive and physicochemical characteristics of different beetroot formulations. Food Addit. 2017, 20–44.
- Blekkenhorst, L.C.; Bondonno, N.P.; Liu, A.H.; Ward, N.C.; Prince, R.L.; Lewis, J.R.; Devine, A.; Croft, K.D.; Hodgson, J.M.; Bondonno, C.P. Nitrate, the oral microbiome, and cardiovascular health: A systematic literature review of human and animal studies. Am. J. Clin. Nutr. 2018, 107, 504–522.
- Karwowska, M.; Kononiuk, A. Nitrates/nitrites in food—Risk for nitrosative stress and benefits. Antioxidants 2020, 9, 241.
- Alidadi, M.; Jamialahmadi, T.; Cicero, A.F.G.; Bianconi, V.; Pirro, M.; Banach, M.; Sahebkar, A. The potential role of plant-derived natural products in improving arterial stiffness: A review of dietary intervention studies. Trends Food Sci. Technol. 2020, 99, 426–440.
- Baião, D.S.; da Silva, D.V.T.; Paschoalin, V.M.F. Beetroot, a remarkable vegetable: Its nitrate and phytochemical contents can be adjusted in novel formulations to benefit health and support cardiovascular disease therapies. Antioxidants 2020, 9, 960.
- Anjana, S.U.; Muhammad, I.; Abrol, Y.P. Are nitrate concentrations in leafy vegetables within safe limits? Curr. Sci. 2007, 92, 355–360. Available online: (accessed on 5 March 2021).
- Blekkenhorst, L.C.; Sim, M.; Bondonno, C.P.; Bondonno, N.P.; Ward, N.C.; Prince, R.L.; Devine, A.; Lewis, J.R.; Hodgson, J.M. Cardiovascular health benefits of specific vegetable types: A narrative review. Nutrients 2018, 10, 595.
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10.
- Santamaria, P.; Elia, A.; Serio, F.; Todaro, E. A survey of nitrate and oxalate content in fresh vegetables. J. Sci. Food Agric. 1999, 79, 1882–1888.
- European Food Safety Authority (EFSA). Opinion of the scientific panel on contaminants in the food chain on a request from the European Commission to perform a scientific risk assessment on nitrate in vegetables. EFSA J. 2008, 689, 1–79.
- Tamme, T.; Reinik, M.; Roasto, M.; Meremäe, K.; Kiss, A. Nitrate in leafy vegetables, culinary herbs, and cucumber grown under cover in Estonia: Content and intake. Food Addit. Contam. Part B 2010, 3, 108–113.
- van Velzen, A.G.; Sips, A.J.; Schothorst, R.C.; Lambers, A.C.; Meulenbelt, J. The oral bioavailability of nitrate from nitrate-rich vegetables in humans. Toxicol. Lett. 2008, 181, 177–181.
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012, 108, 2066–2074.
- Hobbs, D.A.; Goulding, M.G.; Nguyen, A.; Malaver, T.; Walker, C.F.; George, T.W.; Methven, L.; Lovegrove, J.A. Acute ingestion of beetroot bread increases Endothelium-Independent vasodilation and lowers diastolic blood pressure in healthy men: A randomized controlled trial. J. Nutr. 2013, 143, 1399–1405.
- Jajja, A.; Sutyarjoko, A.; Lara, J.; Rennie, K.; Brandt, K.; Qadir, O.; Siervo, M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014, 34, 868–875.
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327.
- Baião, D.S.; Conte-Junior, C.A.; Paschoalin, V.M.F.; Alvares, T.S. Beetroot juice increase nitric oxide metabolites in both men and women regardless of body mass. Int. J. Food Sci. Nutr. 2016, 67, 40–46.
- Bezerra, A.D.L.; Costa, E.C.; Pacheco, D.A.; Souza, D.C.; Farias-Junior, L.F.; Ritti-Dia, R.M.; Grigolo, G.B.; Júnior, P.I.H.B.; Krause, M.; Fayh, A.P.T. Effect of acute dietary nitrate supplementation on the post-exercise ambulatory blood pressure in obese males: A randomized, controlled, crossover trial. J. Sports Sci. Med. 2019, 18, 118–127.
- Silva, D.V.T.; Silva, F.O.; Perrone, D.; Pierucci, A.P.T.R.; Conte-Junior, C.A.; Alvares, T.S.; Del Aguila, E.M.; Paschoalin, V.M.F. Physicochemical, nutritional, and sensory analyses of a nitrate-enriched beetroot gel and its effects on plasmatic nitric oxide and blood pressure. Food Nutr. Res. 2016, 60, 29909.
- Baião, D.S.; d’El-Rei, J.; Alves, G.; Neves, M.F.; Perrone, D.; Del Aguila, E.D.; Paschoalin, V.M.F. Chronic effects of nitrate supplementation with a newly designed beetroot formulation on biochemical and hemodynamic parameters of individuals presenting risk factors for cardiovascular diseases: A pilot study. J. Funct. Foods 2019, 58, 85–94.
- Liu, A.H.; Bondonno, C.P.; Croft, K.D.; Puddey, I.B.; Woodman, R.J.; Rich, L.; Ward, N.C.; Vita, J.A.; Hodgson, J.M. Effects of a nitrate-rich meal on arterial stiffness and blood pressure in healthy volunteers. Nitric Oxide 2013, 35, 123–130.
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 95–102.
- Jonvik, K.L.; Nyakayiru, J.; Pinckaers, P.J.; Senden, J.M.; van Loon, L.J.; Verdijk, L.B. Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults. J. Nutr. 2016, 146, 986–993.
- Haun, C.T.; Kephart, W.C.; Holland, A.M.; Mobley, C.B.; McCloskey, A.E.; Shake, J.J.; Pascoe, D.D.; Roberts, M.D.; Martin, J.S. Differential vascular reactivity responses acutely following ingestion of a nitrate rich red spinach extract. Eur. J. Appl. Physiol. 2016, 116, 2267–2279.
- Ashor, A.W.; Jajja, A.; Sutyarjoko, A.; Brandt, K.; Qadir, O.; Lara, J.; Siervo, M. Effects of beetroot juice supplementation on microvascular blood flow in older overweight and obese subjects: A pilot randomised controlled study. J. Hum. Hypertens. 2015, 29, 511–513.
- Baião, D.S.; Silva, F.O.; d’El-Rei, J.; Neves, M.F.; Perrone, D.; Del Aguila, E.M.; Paschoalin, V.M.F. A new functional beetroot formulation enhances adherence to nitrate supplementation and health outcomes in clinical practice. SDRP J. Food Sci. Technol. 2018, 3, 484–496.
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790.
- Ormesher, L.; Myers, J.E.; Chmiel, C.; Wareing, M.; Greenwood, S.L.; Tropea, T.; Lundberg, J.O.; Weitzberg, E.; Nihlen, C.; Sibley, C.P.; et al. Effects of dietary nitrate supplementation, from beetroot juice, on blood pressure in hypertensive pregnant women: A randomized, double-blind, placebo-controlled feasibility trial. Nitric Oxide 2018, 80, 37–44.
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Shinde, S.; Moodley, Y.; Lundberg, J.O.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 368–375.
- Lara, J.; Ashor, A.W.; Oggioni, C.; Ahluwalia, A.; Mathers, J.C.; Siervo, M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: A systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 451–459.
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent. Food Agric. 2016, 2, 1–14.
- Appeldoorn, M.M.; Venema, D.P.; Peters, T.H.F.; Koenen, M.E.; Arts, I.C.W.; Vincken, J.P.; Gruppen, H.; Keijer, J.; Hollman, P.C.H. Some Phenolic Compounds Increase the Nitric Oxide Level in Endothelial Cells in Vitro. J. Agric. Food Chem. 2009, 57, 7693–7699.
