2. Continuous Low-Substrate Assay of PPi-Hydrolyzing Activity
The low value of the Michaelis constant of PPases makes it necessary to use highly sensitive assays in kinetic studies conducted at sub-
Km
substrate concentrations. The assay currently used in our labs combines two approaches to achieve high sensitivity: first, it measures the formation of the intensively colored complex of 12-molibdophosphoric acid with a triphenylmethane dye, methyl green, and, second, it is continuous. The PPase reaction mixture and two-color reagents are continuously pumped off by a peristaltic pump and mixed, and the absorbance of the resulting solution is measured at 620 or 660 nm in a flow photometer. Even though the background absorbance is relatively high, it is automatically subtracted in this continuous assay mode, allowing the precise recording of much smaller changes in absorbance (P
i
concentration) compared to a fixed-time assay. The principal advantage of the methyl green dye in this application is its low tendency to deposit on the optical cuvette [12].
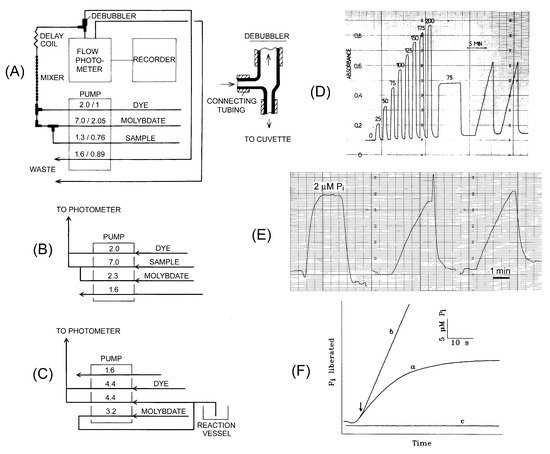
The phosphate analyzer consists of three main parts: a four-channel peristaltic pump, flow photometer, and paper recorder (
A). Alternatively, the photometer’s output can be directed to a computer, which calculates initial velocity from the absorbance time-course. However, we found that the manual procedure, using paper recordings, is less sensitive to signal fluctuations and provides more accurate data. We have used a four-channel Gilson Minipuls pump equipped with Tygon tubings. Three pump channels are used to deliver a sample, acid/molybdate, and dye/Triton X-305 solutions. Their initial mixing occurs in tubing T-joints and is completed in a mixing device consisting of a glass tube of 1.3 mm inner diameter with 12–15 bubbles of 3.5 mm inner diameter. The fourth pump channel eliminates any air bubbles from the final mixed solution before it is directed to the flow photometer. To prevent excessive air from entering the flow system, the pump is stopped when the sample inlet tubing is transferred between the samples and water. To ensure that the color reaction proceeds to completion, the time intervals between the two mixing events and between the second mixing event and entry to the photometer cuvette should be adjusted to 12 and 32 s, respectively, by using connecting tubings of appropriate diameter and length. The small nonlinearity of the calibration plot at low phosphate content is eliminated by adding a small amount of phosphate to the stock acid/molybdate solution. We have been using ISCO UA-5 and 229 flow photometers. These instruments are no longer produced but can be replaced by any flow photometer operating at 620–660 nm wavelengths and a flow rate of approximately 10 mL/min. Alternatively, one can use a standard spectrophotometer with a flow cuvette, such as a Helma model 178-010-10-40 (10 mm pathlength, 80 µL internal volume).
Figure 1.
The phosphate analyzer is used to assay PPase activity in a continuous way. (
A
) Flow diagram for the phosphate analyzer in standard mode; (
B
) Tubing connections on the pump in the high-sensitivity mode; (
C
) tubing connections in the low dead-time mode. Numbers on the pump refer to flow rate in mL/min (before the slash) and tubing diameter in mm (panel A) or flow rate in mL/min (panels B and C). (
D
) Actual P
i
accumulation recordings in setup A with photometer sensitivity of 1 absorbance unit per recorder scale. The calibration data shown at the beginning of the recording was obtained by adding 0–200 µM P
i
to the reaction buffer. (
E
) Actual P
i
accumulation recordings in setup B with photometer sensitivity of 0.1 absorbance unit per recorder scale. The assay mixture of 40 mL volume contained 140 µM PP
i
, 5 mM MgCl
2
, 50 mM MOPS–KOH, pH 7.2, and 0.03 nM
Streptococcus gordonii
PPase with a specific activity of 480 s
−1
. (
F
) Actual recordings of P
i
accumulation in the setup C for rat liver PPase in the presence (a) or absence (b) of slow-binding inhibitor (10 mM fluoride). The arrow marks the moment of enzyme addition. Part of the figure was taken with permission from references [13] (panels A and D) and [14] (panels C and F).
Three setups of the phosphate analyzer were found useful for standard, high-sensitivity, and low dead-time measurements (
A–C). A five-fold increase in sensitivity was achieved in version B by interchanging the sample and molybdate tubings on the pump (
B). As the response is linear up to approximately 0.5 absorbance unit, the photometer sensitivity is adjusted to 0.5 absorbance unit per recorder scale in the standard mode (version A) and down to 0.1 unit in the high-sensitivity mode (version B). These settings correspond to approximately 100 and 4 µM P
i
, respectively, per recorder scale. As each PP
i
molecule yields 2 molecules of P
i
, this sensitivity is sufficient for a reliable measurement of initial velocities at PP
i
concentrations down to 0.5 µM (see
Appendix A
for further details). PPase concentration in the assay is typically in the pM range when activity is determined at saturating substrate concentrations and optimal pH and temperature values. We have successfully used the phosphate analyzer to determine PPase reaction kinetics over a broad temperature range (reaction mixture temperature 20–60 °C).
The dead-time between withdrawing the sample and mixing it with acid/molybdate is 10 s in the standard mode but can be decreased to 1 s by shifting the first mixing point to the pump inlet (
C). In this version, the sample is withdrawn from the reaction vessel at a rate of 1.2 mL/min due to differences in the two lowest pump tubes’ flow rates. This setup helps monitor reactions demonstrating nonlinear progress curves because of enzyme activation or inactivation during the reaction. Reagent concentrations were adjusted in versions B and C as indicated in
to ensure the same optimal final concentrations in the photometer cuvette.
Table 1.
Assays measuring PP
i
hydrolysis. Instrument setups for the continuous assay versions A–C are shown in
A–C.
The continuous P
i
assay is robust and tolerates the presence of many biochemical compounds, including magnesium ions at high concentrations (up to 40 mM). In contrast, Mg
2+
interferes with the P
i
assay based on 12-molibdophosphoric acid reduction [17]. Nevertheless, it is wise to calibrate the instrument by measuring phosphate standard against background each time when a new component is added to the sample assayed, especially when working in the high-sensitivity mode (version B). Importantly, PP i
at high concentrations was found to suppress the P
i
signal (by 25% for 1.5 mM PP
i
) in this mode. This should be taken into consideration in measurements of PPase activity at a variable substrate concentration. The effect is proportional to PP
i
concentration and likely results from the competitive formation of 12-molibdopyrophosphoric acid, detected by Raman spectroscopy [18].
Other known continuous assays of PPase activity monitor, depending on pH, uptake or release of hydrogen ions during PP
i
conversion to P
i [19][20] or monitor PP
i
concentration using synthetic PP
i sensors [21][22][23][24][25]. These assays are less sensitive and less convenient in that the signal is not proportional to the degree of conversion.
sensors [21,22,23,24,25]. These assays are less sensitive and less convenient in that the signal is not proportional to the degree of conversion.
Substances that modulate PPase activity will interfere with any assay. Among them, Tris and other amine buffers were found to dramatically increase the Michaelis constant for family I and membrane PPases [26][27][28]. Accordingly, zwitterionic buffers, such as Tes, Mops, and HEPES, are preferable for work at sub-
Substances that modulate PPase activity will interfere with any assay. Among them, Tris and other amine buffers were found to dramatically increase the Michaelis constant for family I and membrane PPases [26,27,28]. Accordingly, zwitterionic buffers, such as Tes, Mops, and HEPES, are preferable for work at sub- Km
substrate concentrations. Tetramethylammonium hydroxide was found to be useful as the second component of the zwitterionic buffers in experiments measuring K
+
and Na
+
effects on K
+
-dependent membrane PPase. However, some batches of this strongly basic compound supplied in glass containers were found to nonspecifically inactivate this enzyme when used as a buffer component instead of KOH.
3. Sensitive Fixed-Time Assay of PPi-Hydrolyzing Activity
Fixed-time assays measuring the product P
i
are perhaps most suitable for high-throughput screening of compound libraries for the effects on PPase activity. Most published procedures determine P
i
as reduced 12-molybdophosphoric acid [29] and perform well with 10 −5
–10
−4
M P
i
concentrations. Although this sensitivity suffices for most applications, the identification of samples with low PPase activity or assays performed at low PP
i
concentrations (<10 µM) would require a more sensitive P
i
assay, such as the assay based on the shift of malachite green spectrum upon binding to 12-molybdophosphoric acid. However, low dye solubility, leading to its precipitation, was a serious drawback of the original malachite green-based procedure [30] and its many later variants.
A solution to this problem was finding that increased acid concentration (2.5 M H
2
SO
4
) in stock dye solution surprisingly increases dye solubility and stability [15]. This finding allowed the formulation of a single stable color reagent that stops the enzymatic reaction and produces a green-blue color (630 nm), whose intensity is proportional to P i
concentration. The color reagent is made daily by adding the nonionic detergent Tween-20 (color stabilizer) to the stock dye/molybdate solution (
). The signal produced by 2 µM P
i
(corresponding to 1 µM PP
i
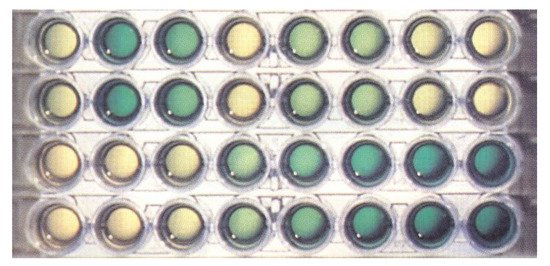
hydrolyzed) is 0.15 absorbance units in a 1 cm cuvette. The assay can be performed in a 96-well plate (
) and quantified with a plate reader.
Figure 2.
PPase assay using the malachite green procedure. Two bottom rows show a duplicate series of phosphate dilutions from 0 µM (left) to 10 µM (right). Two top rows show typical results of a duplicate screening test of a library of potential inhibitors of
Escherichia coli
PPase. The yellow color indicates strong inhibition, dark green color—no inhibition.
4. Continuous and Fixed-Time Assays of PPi-Synthesizing Activity
Like any catalyst, PPase accelerates the attainment of thermodynamic equilibrium PP
i
⇆ 2P
i
in both directions. Although the equilibrium is shifted to the right (∆G
0
= −20–25 kJ/mol), it allows the formation of measurable amounts of PP
i
under physiological conditions. For instance, the concentration of PP
i
at equilibrium with 10 mM P
i
(pH 7, 25 °C, 1 mM Mg
2+
) is approximately 1 µM [31], which is below the detection limits of most analytical methods for PP i
determination. However, micromolar sensitivity has been claimed for several assays based on fluorogenic and electrochemical PP
i sensors [21][22][23][24][25]. Some of them are commercially available. However, they are of limited use in measurements of initial velocities of PP
sensors [21,22,23,24,25]. Some of them are commercially available. However, they are of limited use in measurements of initial velocities of PP i
synthesis, which require that PP
i
concentration in the medium does not exceed 10% of the equilibrium concentration to prevent product inhibition.
This problem is elegantly obviated in the coupled-enzyme luminescent procedure of Nyrén and Lundin [32], which later became a core element of “pyrosequencing”, a well-established DNA sequencing method [33]. In their procedure, PP i
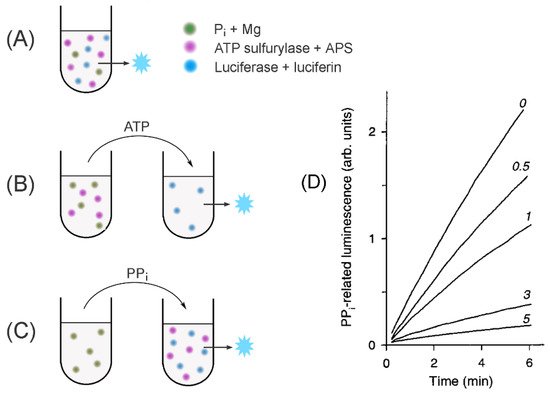
is converted to ATP by reaction with adenosine-5′-phosphosulfate (APS), catalyzed by ATP sulfurylase (E.C. 2.7.7.4), and the ATP formed is detected with a firefly luciferase/luciferin system. This method is used in three versions in PPase studies (
and
). In the first version, all components of the detection system, including ATP sulfurylase, luciferase, APS, and luciferin, are added to the PPase assay mix, and PP
i
formation is recorded continuously. This became possible because neither APS nor luciferin affects PPase activity, and both ATP sulfurylase and PPase need Mg
2+
as a cofactor. The synthesized PP
i
is continuously converted into ATP at such a high rate that the steady-state PP
i
level is not inhibitory for PPase and luciferase activities. This is ensured by maintaining high ATP sulfurylase concentration, such that its doubling does not increase the measured rate of ATP accumulation. In the second assay version, the PPase reaction mix contains ATP sulfurylase/APS, which continuously converts the formed PP
i
into ATP, whose concentration is determined at fixed time points with luciferase/luciferin in a separate tube. Importantly, PPase does not hydrolyze ATP in the presence of Mg
2+
. Both assay versions combine the high sensitivity of the luciferase ATP determination with the possibility to accumulate detectable amounts of ATP while keeping the steady-state concentration of PP
i
at a sub-equilibrium, non-inhibitory level. ATP sulfurylase and luciferase act thus as amplifiers of the PP
i
-generated signal and allow the measurement of the initial velocities of PP
i
synthesis from P
i
.
Figure 3.
Schematics of the assays to measure PPase-catalyzed PP
i
synthesis. (
A
) A continuous assay of the medium PP
i
synthesis; (
B
) fixed-time assay of the medium PP
i
synthesis; (
C
) determination of enzyme-bound PP
i
. The assayed PPase is added to all far-left tubes. Three other major components are shown as colored spots. The principal analytes transferred between the tubes are indicated above the arrows. The blue star refers to the luminescence signal. (
D
) Actual PP
i
accumulation recordings in the assay version A for baker’s yeast PPase in the presence of slow-binding inhibitor (fluoride; its concentrations in mM are indicated on the curves). Panel D was taken with permission from reference [34].
Table 2.
Assays measuring PP
i
formation.
The third version of the PP
i
assay accesses the equilibrium of the PP
i
⇆ 2P
i reaction in the active site of PPase. This information is required to estimate the rate constants for individual steps of PPase catalysis [37][38]. This equilibrium is markedly shifted to the left by comparing the equilibrium in solution—up to 20% of enzyme-bound P
reaction in the active site of PPase. This information is required to estimate the rate constants for individual steps of PPase catalysis [37,38]. This equilibrium is markedly shifted to the left by comparing the equilibrium in solution—up to 20% of enzyme-bound P i
is converted to PP
i [37][38]. To assay, the enzyme-bound PP
[37,38]. To assay, the enzyme-bound PP i
, the enzyme (20–100 µM) is incubated with P
i
and Mg
2+
, inactivated by trifluoroacetic acid to release enzyme-bound PP
i
into solution, and an aliquot is taken to assay PP
i
using the coupled ATP sulfurylase/luciferase procedure.
Several comments on the assay procedure are appropriate. First, phosphoric acid and its salts are often contaminated with PP
i
, leading to high background luminescence. A low-PP
i
potassium phosphate can be prepared in the following way: phosphoric acid is diluted to 0.2 M with water, boiled for 3 h, and neutralized with KOH [39]. Second, low but significant background luminescence results from APS being a poor substrate for luciferase. Third, as P i
is added in high concentrations, care should be taken to ensure the assay mixture’s desired pH. It is not enough to adjust to this value the pH of the stock P
i
solution because the formation of the MgHPO
4
complex from H
2
PO
4−
upon mixing stock P
i
and Mg
2+
solutions would cause acidification of the medium (by 0.10 pH unit with 50 mM P
i
, 5 mM Mg
2+
in 0.1 M MOPS–KOH buffer, pH 7.2). This effect is compensated by adding an appropriate amount of KOH to the assay mixture. An Excel subroutine to calculate this amount, which depends on P
i
, Mg
2+
, and H
+
concentrations and reaction volume, is available (
Supplementary Material
). Alternatively, one can adjust the pH of the stock P
i
solution used at each Mg
2+
concentration to the value (above the desired assay pH) determined in a trial titration.
A different coupled-enzyme assay with similar characteristics has been described [40]. In this assay, PP i
is converted to adenosine 5’-triphosphate (ATP) by pyruvate phosphate dikinase in the presence of its substrates, pyruvate phosphate and AMP. The ATP formed is similarly determined by the firefly luciferase reaction. The detection limit for PP
i
is approximately 10
−8
M. The use of this assay in PPase studies has not yet been reported.