Cancer is a multifactorial disease responsible for millions of deaths worldwide. It has a strong genetic background, as mutations in oncogenes or tumor suppressor genes contribute to the initiation of cancer development. Integrin signaling as well as the signaling pathway of
Ras
oncogene, have been long implicated both in carcinogenesis and disease progression. Moreover, they have been involved in the promotion of metastasis, which accounts for the majority of cancer-related deaths.
Ras Suppressor-1 (RSU1)
was identified as a suppressor of
Ras-induced transformation and was shown to localize to cell-extracellular matrix adhesions. Recent findings indicate that its expression is elevated in various cancer types, while its role in regulating metastasis-related cellular processes remains largely unknown. Interestingly, there is no in vivo work in the field to date, and thus, all relevant knowledge stems from in vitro studies.
induced transformation and was shown to localize to cell-extracellular matrix adhesions. Recent findings indicate that its expression is elevated in various cancer types, while its role in regulating metastasis-related cellular processes remains largely unknown. Interestingly, there is no in vivo work in the field to date, and thus, all relevant knowledge stems from in vitro studies. In this review, we summarize recent studies using breast, liver and brain cancer cell lines and highlight the role of RSU1 in regulating cancer cell invasion.
- cell-extracellular matrix adhesion
- actin cytoskeleton
- invasion
- migration
- metastasis
- breast cancer
- hepatocellular carcinoma
- glioblastoma
Although the involvement of Ras proteins as GTPases in cancer progression through intracellular signaling transmission and actin remodeling has been well-established [1][2], and RSU1 was first characterized as a
Ras
RSU1 in cancer is still vague [3]. Interestingly, while several studies have been performed in vitro using various cancer cell lines, an in vivo investigation of the role of RSU1 in cancer is still missing.
1.
RSU1 in Breast Cancer
With regard to breast cancer, a study performed in 2000 by Vasaturo et al. [4] showed that overexpression of
RSU1
RSU1
RSU1
RSU1
PINCH-1 expression was upregulated and cell proliferation was enhanced through the inhibition of p53 and upregulation of a regulator of apoptosis, namely p53 Up-regulated Modulator of Apoptosis (PUMA) [5]. Interestingly, these results were further validated in 32 human breast cancer samples with or without metastasis to the lymph nodes having respective normal adjacent tissues as controls.
RSU1 was found to be dramatically and significantly elevated in metastatic breast cancer samples compared to non-metastatic and compared to the normal adjacent tissues and, in fact, its expression was shown to be negatively correlated with PINCH-1 expression and positively with PUMA expression [5].
Since all relevant in vitro studies were performed in two-dimensional (2D) culture systems, in which, by definition, cell-matrix interactions are not taken into account, a recent study developed three-dimensional (3D) culture models to better study the role of
RSU1 in a more physiologically relevant manner. In that regard, breast cancer cells were either grown inside a 3D collagen gel of tunable stiffness (by adjusting the collagen concentration) or were left to form tumor spheroids and were then embedded in 3D collagen gels in an attempt to investigate cancer cell invasion [6][7]. It was shown that
RSU1 was significantly upregulated in increased stiffness conditions, while its silencing diminished the invasive capacity of tumor spheroids through collagen gels. In fact, this was mediated by urokinase Plasminogen Activator (uPA) and Matrix metalloproteinase 13 (MMP13) [7].
Another recent study in breast cancer cells involved transient silencing of
RSU1
GDF15), a member of the Transforming Growth Factor-β (TGF-β) family of proteins, known to be associated with actin cytoskeleton reorganization and metastasis [8][9][10].
RSU1
PARVA
RhoA
ROCK-1
Fascin-1.
RSU1 silencing on cell migration and invasion [11], further suggesting that GDF15 can compensate for
RSU1 loss.
Interestingly, regarding the alternatively-spliced
RSU1
RSU1-X1), it was shown to be expressed in human gliomas [12]. Depletion of this isoform from breast cancer cells has been also found to inhibit their migration, while inhibitor studies revealed that the MEK-ERK pathway regulates its expression [13]. This RSU-X1 isoform was also observed to be present in highly invasive MDA-MB-231 and MDA-MB-231-Lung Metastasis-2 (MDA-MB-231-LM2) breast cancer cells, but not in the less invasive MCF-7 cells [7]. In addition, a recent study [6], investigating the involvement of
RSU1
RSU1. RSU1
RSU1
RSU1-X1
RSU1.
RSU1-X1
RSU1
uPA expression [6].
Furthermore, a connection between RSU1 and miR-409-5p has also been made, as RSU1 was confirmed to be directly targeted by this miRNA in breast cancer cell lines MCF-7 and MDA-MB-231. Specifically, in cells that had been previously treated with a lentivirus that inhibited miR-409-5p, siRNA-mediated silencing of
RSU1 promoted cancer cell proliferation and migration, indicating that the regulatory effect of miR-409-5p inhibition in breast cancer is achieved through the inverse upregulation of RSU1 [14].
In conclusion, studies in breast cancer cells clearly show that both RSU1 isoforms promote breast cancer cell migration and invasion in vitro but there is also a mechanism in place by which the truncated
RSU1-X1
RSU1 when the latter is lost. Hence, ideally both isoforms should be blocked to effectively abolish the invasive and migratory potential of breast cancer cells (Figure 1).
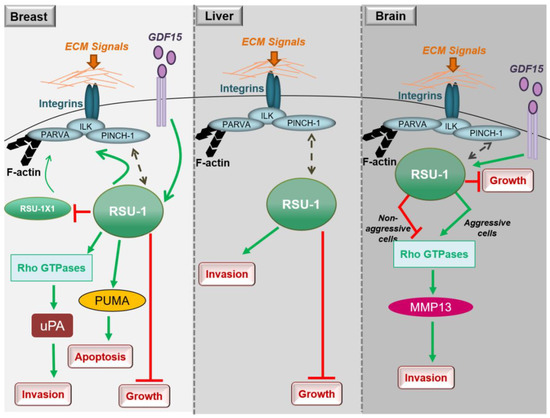
Figure 12. Diagrammatic representation of the role of RSU1 with regard to the cancer-related properties of breast, liver and brain cancer cells.
2. RSU1 in Hepatocellular Carcinoma
Little is known regarding the role of RSU1 in hepatocellular carcinoma, with the thus far available data being in agreement with what has been shown in breast cancer. More specifically,
RSU1 expression was found to be dramatically elevated in more aggressive HepG2 hepatocellular carcinoma cells compared to the non-metastatic Alexander cells and its elimination promoted cell proliferation [15]. In addition, Hepatitis C virus infection was shown to upregulate
RSU1 expression promoting a cancerous phenotype [16]. Moreover, Donthamsetty et al. [17] also showed that elimination of PINCH-1 in mouse hepatocytes resulted in reduced
RSU1
RSU1 is frequently deleted in hepatocellular carcinomas [18]. Finally, with regard to liver cancer cell invasion and similarly to breast cancer cells (Figure 1), depletion of the
RSU1 from aggressive hepatocellular carcinoma cells leads to significantly impaired cell invasion [15].
3. RSU1 in Glioblastoma
As
RSU1 has been previously linked to basic functions of the CNS [19][20], it is not surprising that it is also involved in the pathogenesis of glioblastoma, the most aggressive type of brain cancer [12][21][22]. Transient overexpression of
RSU1
RSU1
RSU1 likely acts as a tumor-suppressor gene [23]. However, no information was available on the role of
RSU1 on basic metastasis-related properties, such as cell migration and invasion, until recently.
A recent study explored the role of
RSU1
RSU1
RSU1
RSU1
RSU-1 silencing was shown to inhibit migration and invasion of aggressive cells and promote those of less aggressive cells [21], indicating that
RSU1
RSU1.
RSU1 in low levels, was also observed to take place through negative regulation of STAT6 and MMP13 [21].
Thus,
RSU1 apparently has distinct roles with regard to glioblastoma cell invasion depending on the cells’ aggressiveness as well as based on its expression level in the specific cells (Figure 1). In more aggressive glioma cells in which
RSU1
RSU1 expression, it is inhibited [21]. The molecular mechanism by which this is achieved is not yet defined, but it is in accordance with other focal adhesion proteins whose level is also associated with cell migration capacity [24]. It is also reminiscent of the TGF-β [25] and GDF15 [26] mechanisms of action, which are known to act as tumor suppressors during early stages of the disease and as oncogenes at later stages. In fact, it was recently shown that the link between RSU1 and GDF15 is active in brain cells similarly to what happens in breast cancer cells, in regulating cell aggressiveness [27], as GDF15 is known to be associated with cancer cell malignancy and is elevated in glioblastoma patients [28]. Furthermore, the correlation of the expression levels of GDF15 and RSU1 determines the aggressiveness of brain cells through the regulation of RhoA, PINCH-1 and MMP13 [27], providing the basis for future investigations towards deciphering the molecular mechanism of RSU1 action.
A summary of existing studies on the role of RSU1 in cancer development and progression is presented in Table 1 below.
Table 1. Summary of studies on the role of RSU1 in cancer.
| Cancer Type | Cell Lines | RSU1 Role | References |
|---|
| Breast |
| Reduces proliferation Induces apoptosis Induces invasion Reduces migration |
[4] [5] [6][7][11][13] | [68] [69] [48,70,74] [60] |
||
| Liver |
| Reduces proliferation | [15] | [76] | ||
| Glioblastoma |
| Reduces proliferation Reduces invasion and migration Induces invasion and migration |
[23][29][21][27] [21][27] | [46,47] [80,84] [80,84] |
4. Current Clinical Knowledge
Although there is a lack of in vivo work related to RSU1 function, there is significant evidence from clinical samples corroborating the in vitro findings. Specifically, analysis of Kaplan-Meier survival plots from human breast cancer patients revealed that high
RSU1 expression is associated with poor prognosis for distant metastasis-free survival and remission-free survival [4][7]. Furthermore, protein expression analysis data from 23 human breast cancer samples showed that RSU1 is elevated in metastatic breast cancer cells, while the levels of the truncated isoform,
RSU1-X1, are significantly reduced [6]. This is also in accordance with in vitro data in breast cancer cells lines, where more metastatic cell lines express RSU1 at higher levels [5][6], and further supports the hypothesis that RSU1 promotes a metastatic phenotype.
- Tajiri, H.; Uruno, T.; Shirai, T.; Takaya, D.; Matsunaga, S.; Setoyama, D.; Watanabe, M.; Kukimoto-Niino, M.; Oisaki, K.; Ushijima, M.; et al. Targeting Ras-Driven Cancer Cell Survival and Invasion through Selective Inhibition of DOCK1. Cell Rep. 2017, 19, 969–980. [Google Scholar] [CrossRef]
- Sahai, E.; Marshall, C.J. RHO-GTPases and cancer. Nat. Rev. Cancer 2002, 2, 133–142. [Google Scholar] [CrossRef]
- Zacharia, L.C.; Stylianopoulos, T.; Gkretsi, V. Ras Suppressor-1 (RSU-1) in Cancer Cell Metastasis: Friend or Foe? Crit. Rev. Oncog. 2017, 22, 249–253. [Google Scholar] [CrossRef]
- Vasaturo, F.; Dougherty, G.W.; Cutler, M.L. Ectopic expression of Rsu-1 results in elevation of p21CIP and inhibits anchorage-independent growth of MCF7 breast cancer cells. Breast Cancer Res. Treat. 2000, 61, 69–78. [Google Scholar] [CrossRef]
- Giotopoulou, N.; Valiakou, V.; Papanikolaou, V.; Dubos, S.; Athanassiou, E.; Tsezou, A.; Zacharia, L.C.; Gkretsi, V. Ras suppressor-1 promotes apoptosis in breast cancer cells by inhibiting PINCH-1 and activating p53-upregulated-modulator of apoptosis (PUMA); verification from metastatic breast cancer human samples. Clin. Exp. Metastasis 2015, 32, 255–265. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianou, A.; Louca, M.; Stylianopoulos, T. Identification of Ras suppressor-1 (RSU-1) as a potential breast cancer metastasis biomarker using a three-dimensional in vitro approach. Oncotarget 2017, 8, 27364–27379. [Google Scholar] [CrossRef]
- Ji, H.; Lu, H.W.; Li, Y.M.; Lu, L.; Wang, J.L.; Zhang, Y.F.; Shang, H. Twist promotes invasion and cisplatin resistance in pancreatic cancer cells through growth differentiation factor 15. Mol. Med. Rep. 2015, 12, 3841–3848. [Google Scholar] [CrossRef] [PubMed]
- Aw Yong, K.M.; Zeng, Y.; Vindivich, D.; Phillip, J.M.; Wu, P.H.; Wirtz, D.; Getzenberg, R.H. Morphological effects on expression of growth differentiation factor 15 (GDF15), a marker of metastasis. J. Cell. Physiol. 2014, 229, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Wallin, U.; Glimelius, B.; Jirstrom, K.; Darmanis, S.; Nong, R.Y.; Ponten, F.; Johansson, C.; Pahlman, L.; Birgisson, H. Growth differentiation factor 15: A prognostic marker for recurrence in colorectal cancer. Br. J. Cancer 2011, 104, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Gkretsi, V.; Louca, M.; Stylianou, A.; Minadakis, G.; Spyrou, G.M.; Stylianopoulos, T. Inhibition of Breast Cancer Cell Invasion by Ras Suppressor-1 (RSU-1) Silencing Is Reversed by Growth Differentiation Factor-15 (GDF-15). Int J. Mol. Sci 2019, 20, 163. [Google Scholar] [CrossRef]
- Yu, H.; Xing, H.; Han, W.; Wang, Y.; Qi, T.; Song, C.; Xu, Z.; Li, H.; Huang, Y. MicroRNA-409-5p is upregulated in breast cancer and its downregulation inhibits cancer development through downstream target of RSU1. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef]
- Gkretsi, V.; Bogdanos, D.P. Elimination of Ras Suppressor-1 from hepatocellular carcinoma cells hinders their in vitro metastatic properties. Anticancer Res. 2015, 35, 1509–1512. [Google Scholar]
- Aizaki, H.; Harada, T.; Otsuka, M.; Seki, N.; Matsuda, M.; Li, Y.W.; Kawakami, H.; Matsuura, Y.; Miyamura, T.; Suzuki, T. Expression profiling of liver cell lines expressing entire or parts of hepatitis C virus open reading frame. Hepatology 2002, 36, 1431–1438. [Google Scholar] [CrossRef]
- Donthamsetty, S.; Bhave, V.S.; Mars, W.M.; Bowen, W.C.; Orr, A.; Haynes, M.M.; Wu, C.; Michalopoulos, G.K. Role of PINCH and its partner tumor suppressor Rsu-1 in regulating liver size and tumorigenesis. PLoS ONE 2013, 8, e74625. [Google Scholar] [CrossRef]
- Nalesnik, M.A.; Tseng, G.; Ding, Y.; Xiang, G.S.; Zheng, Z.L.; Yu, Y.; Marsh, J.W.; Michalopoulos, G.K.; Luo, J.H. Gene deletions and amplifications in human hepatocellular carcinomas: Correlation with hepatocyte growth regulation. Am. J. Pathol. 2012, 180, 1495–1508. [Google Scholar] [CrossRef]
- Louca, M.; Stylianou, A.; Minia, A.; Pliaka, V.; Alexopoulos, L.G.; Gkretsi, V.; Stylianopoulos, T. Ras suppressor-1 (RSU-1) promotes cell invasion in aggressive glioma cells and inhibits it in non-aggressive cells through STAT6 phospho-regulation. Sci. Rep. 2019, 9, 7782. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, Y.; Gkretsi, V.; Wu, C. Migfilin interacts with vasodilator-stimulated phosphoprotein (VASP) and regulates VASP localization to cell-matrix adhesions and migration. J. Biol. Chem. 2006, 281, 12397–12407. [Google Scholar] [CrossRef] [PubMed]
- Jakowlew, S.B. Transforming growth factor-beta in cancer and metastasis. Cancer Metastasis Rev. 2006, 25, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, P.J.; Duffin, K.L.; Chintharlapalli, S.; Wu, X. GDF15 and Growth Control. Front. Physiol. 2018, 9, 1712. [Google Scholar] [CrossRef] [PubMed]
- Louca, M.; Gkretsi, V.; Stylianopoulos, T. Coordinated Expression of Ras Suppressor 1 (RSU-1) and Growth Differentiation Factor 15 (GDF15) Affects Glioma Cell Invasion. Cancers 2019, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
Regarding brain cancer, the first report on the involvement of RSU1 in glioblastoma was made as early as in 1995, showing that the
RSU1 gene is frequently deleted in high-grade gliomas [23], but no other evidence is available in human samples thus far.
