Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
Ubiquitin (Ub) is a highly conserved small protein of about 76 amino acids that is present in practically all eukaryotic cells. During evolution, its sequence, and even more so, its fold has been extremely conserved
[1]
Ub-like proteins have also been identified in prokaryotes, providing clues on the origin of this ubiquitous protein
[2]
The structure of Ub revealed a unique fold formed by a β-sheet with five antiparallel β-strands and a single helical segment, which is shared by other Ub-like proteins and domains, known as the β-grasp fold (
).
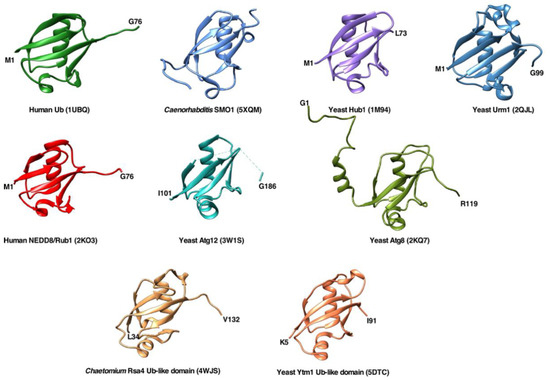
Figure 1.
Overall structures of ubiquitin and different ubiquitin-like proteins or domains shown as ribbon diagrams. The figures were prepared using the UCSF Chimera program using the structural data provided by NMR, X-ray crystallography, and cryo-EM studies deposited in the Protein Data Bank (PDB). The following structures are shown:
Homo sapiens
ubiquitin (PDB code 1UBQ),
Caenorhabditis elegans
SUMO homologue SMO-1 (PDB 5XQM),
S. cerevisiae
Hub1 (PDB 1M94),
S. cerevisiae
Urm1 (PDB 2QJL),
H. sapiens
NEDD8/Rub1 (PDB 2KO3),
S. cerevisiae
Atg12 (PDB 3W1S),
S. cerevisiae
Atg8 (PDB 2KQ7), ubiquitin-like domain of
Chaetomium thermophilum
Rsa4 (taken from PDB 4WJS), and ubiquitin-like domain of
S. cerevisiae
Ytm1 (PDB 5DTC).
Ub functions as a reversible post-translational modifier of proteins to regulate many different cellular processes. Since its discovery, a role of Ub in proteasome-dependent protein degradation has been emphasized (e.g.
[3]
), but beyond this function, Ub participates in many other cellular processes, such as DNA repair, chromatin dynamics, cell cycle regulation, membrane and protein trafficking, endocytosis, autophagy, and transcriptional and translational control. Accordingly, Ub is a very abundant cellular protein that is used to modify a large number of different proteins in yeast (>1000) and human (>9000) cells
[4]
. The enzymatic conjugation of Ub to other cellular proteins is referred to as ubiquitination or ubiquitylation and requires specific Ub ligases, while, conversely, removal of Ub from modified targets is called deubiquitination and involves the activity of specific proteases. As a result of ubiquitination, the Ub moiety is attached via an isopeptide linkage to its substrate protein, involving the carboxyl group of Ub’s terminal glycine residue (G76) and an epsilon-amino group of a lysine residue in its substrate or in another Ub molecule (K6, K11, K27, K29, K33, K48, and K63)
[5]
. Occasionally, the carboxyl group of G76 can be covalently linked to the N-terminal methionine residue (M1) of another Ub molecule and, less frequently, to non-lysine residues within substrate proteins. Thus, ubiquitination comes in different flavors: substrate proteins can be modified at one (mono-Ub) or multiple (multimono-Ub) site(s) by a single Ub or several Ubs, forming structurally distinct homo- or heterotypic linear, or even branched, chains; moreover, the attached Ub chains can also be modified by acetylation or phosphorylation, leading to a further expansion of the repertoire of functional consequences of ubiquitination
[6]
. The best understood role of Ub is probably the targeting of substrate proteins carrying single K48-linked polyUb chains to the proteasome for degradation
[7]. However, some types of ubiquitination, especially the ones containing mixed or branched heterotypic Ubs, still await the assignment of a specific function. In any case, it is clear that the different modes of Ub conjugation offer new possibilities and fates to the substrates, including the alteration of their inter- and intramolecular interactions, which could affect their localization, complex formation or dissociation, or activity, a concept that it is not limited to Ub but can also be applied to Ub-like modifiers (see below).
. However, some types of ubiquitination, especially the ones containing mixed or branched heterotypic Ubs, still await the assignment of a specific function. In any case, it is clear that the different modes of Ub conjugation offer new possibilities and fates to the substrates, including the alteration of their inter- and intramolecular interactions, which could affect their localization, complex formation or dissociation, or activity, a concept that it is not limited to Ub but can also be applied to Ub-like modifiers (see below).
The Ub fold is widely distributed across eukaryotes and is even present in prokaryotes
[1]. Basically, proteins containing the Ub fold can be classified, based on their ability to be covalently attached to substrate proteins or other molecules, into two categories, comprising either Ub-like or Ub-related modifiers (class I) or proteins harboring as part of their larger structures a Ub-like domain (class II) and, therefore, also being referred to as Ub-domain proteins.
. Basically, proteins containing the Ub fold can be classified, based on their ability to be covalently attached to substrate proteins or other molecules, into two categories, comprising either Ub-like or Ub-related modifiers (class I) or proteins harboring as part of their larger structures a Ub-like domain (class II) and, therefore, also being referred to as Ub-domain proteins.
(i) Proteins belonging to the first category share with Ub the presence of one to two glycine residues at their C-terminal end and internal lysine residues. As Ub (see later), these proteins are normally translated as immature precursors that must be processed by a specific protease to generate their mature forms, bearing an active carboxyl group on the exposed terminal glycine, which can be attached to a protein, normally via a lysine residue, or, exceptionally, a phospholipid substrate. However, there are Ub-like modifiers that are not synthetized as precursors (e.g., Urm1 proteins). Most Ub-like conjugating enzymes and pathways resemble those involved in ubiquitination. Prominent members among a larger list of Ub-like modifiers are SUMO (yeast Smt3), ISG15, NEDD8 (yeast Rub1), and ATG8 family proteins (yeast Atg8)
[8]. Similarly, as observed upon ubiquitination, the covalent modification of substrates with Ub-like proteins enables functional regulation by altering their inter- or intramolecular interactions, thereby transforming and modulating their properties, including their structure, activities, or localization.
. Similarly, as observed upon ubiquitination, the covalent modification of substrates with Ub-like proteins enables functional regulation by altering their inter- or intramolecular interactions, thereby transforming and modulating their properties, including their structure, activities, or localization.
(ii) The second category of Ub-related proteins comprises proteins containing a Ub-like domain as an integral part of a longer protein from which it is not dislodged by a protease. The Ub-like domain of these proteins provides a specific important function, such as, for instance, a binding site for another protein
[9]
2. The Ubiquitin Genes in Eukaryotes
Practically all Ub-like modifiers, as well as Ub itself, are synthesized by proteolytic maturation from precursor proteins. This maturation is required to release and activate the C-terminal glycine residue, which is essential for the conjugation onto its targets via a cascade of three different enzymes
[10]
.
To study how Ub genes are arranged along the eukaryotic kingdom, we used the protein family database PFAM. For this, we searched for the well-defined Ub domain (PF00240 entry), as eukaryotes that are evolutionarily far apart show a high degree of Ub conservation (e.g., human and yeast Ub proteins share 96% identity). However, and as expected due to the high structural homology among Ub and Ub-like proteins, this entry, in addition to the bona fide Ub domain, also contains a large number of diverse Ub-like and incomplete Ub domains. Thus, to properly perform this analysis, we first downloaded all 38,884 Ub domains comprised within the PF00240 entry of PFAM and compared them by BLAST to the yeast Ub domain, which corresponds to the Ub moiety present in any of the four
UBI
genes present in yeast (see below). Two main groups of identity were found; for further analyses, we focused on the group most similar to the yeast Ub domain, thereby discarding all proteins belonging to the PF00240 entry whose Ub domain had less than 80% identity in at least 80% of its length with the yeast Ub. This procedure yielded a total of 5349 proteins. Then, using Perl scripts developed by us, we cross-referenced this list of proteins with the collection of protein families of PFAM, which are organized as architectures and, thus, composed of one or more domains in diverse combinations. In such a way, we evaluated the combination of the Ub domain with other domains, taking additionally into account the taxonomic information for each studied protein. As a result, we found that the Ub domain combines with 114 domains in 147 different architectures. Amongst these, the 10 most common ones comprise about 92% of all Ub-containing proteins in three distinct architectural arrangements (
):
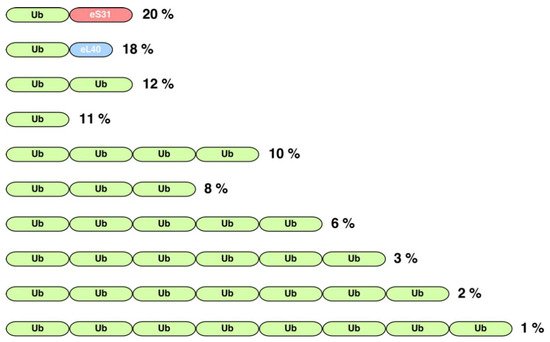
Figure 2.
Schematic representation of the organization of the 10 most common protein architectures containing the ubiquitin domain (PFAM entry PF00240). Green blocks represent the ubiquitin sequence. Red and blue blocks represent the characteristic domains of the eS31 (PFAM PF01599) and eL40 (PFAM PF01020) family of ribosomal proteins, respectively. Note that the shown architectures comprise about 92% of all ubiquitin-containing proteins. Numbers on the right indicate the percentage of a particular architecture among all analyzed proteins. As described in the text, this analysis was carried out with all those proteins of the PF00240 entry that showed more than 80% identity over at least 80% of their length with the Ub domain of
S. cerevisiae
.
(i) First, there are genes consisting of only a single copy of the Ub coding sequence; thus, the translation of the corresponding mRNA generates a monoUb precursor protein (11% of all architectures). Although it has been considered in the literature that this arrangement has only exceptionally been found in a small group of eukaryotes, such as two primitive single-celled intestinal parasites,
Giardia lamblia
and
Entamoeba histolytica
, the true situation seems to be quite different as our analysis reveals that a variety of different animals, fungi, and plants also possess genes encoding a monomeric Ub domain (
).
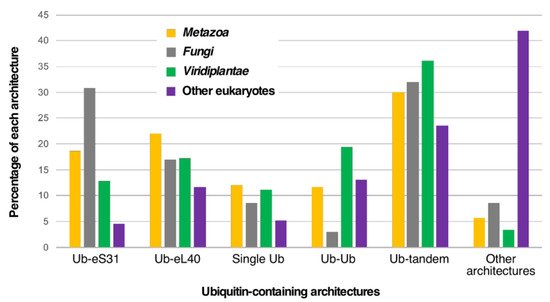
Figure 3.
Taxonomic distribution of the most frequent ubiquitin-containing architectures among eukaryotes. Out of all possible architectures, only Ub-eS31 and Ub-eL40 fusions, monogenic Ub, dimeric Ub, and multiple Ub tandem repeats are shown. Analysis was done with different groups of eukaryotes from which we have extracted the
Metazoa
or animals,
Viridiplantae
or green algae and plants, and fungi. The percentages corresponding to each architecture category is shown for each group.
(ii) The other abundant architectures include two different gene arrangements leading either to a polyUb precursor protein as the result of a spacer-less tandem fusion of several Ub monomers (42% of all architectures) or to a single Ub protein fused to a distinct protein (
). Regarding polyUb tandem genes, the most frequent architecture corresponds to that of a dimer (12% of all architectures), followed by a tetramer (10%) (
). In the particular case of
S. cerevisiae
, but also in mammals, Ub is encoded by four different genes
[11]
. The
UBI4
gene encodes a polyUb precursor protein consisting of five tandem head-to-tail Ub repeats. In the case of humans, the equivalent of the
UBI4
gene corresponds to the
UBB
and
UBC
genes, which encode poly-Ub precursors consisting of three and nine tandem Ub moieties, respectively. The number of Ub coding repeats is usually below 10 in most eukaryotes; indeed, architectures containing more than nine Ub repeats are very rare (less than 2% of all architectures). However, in some organisms, such as the protozoa
Trypanosoma cruzi,
a polyUb gene with more than 40 tandemly linked Ub coding sequences has been described
[12]
. From our analysis, we found that the longest polyUb fusion (46 Ub domains in tandem) in PFAM appears in the cichlid
Astatotilapia calliptera
. In addition to tandem repeats, there are examples of exceptional hybrid architectures where the tandem Ub moieties are also combined with other domains.
(iii) Ub can be fused to other proteins. Strikingly, Ub is almost exclusively fused to the eS31 and eL40 ribosomal proteins (
), although fusions with other proteins can be identified in different eukaryotes. Normally, a single Ub moiety, which is positioned as the N-terminal part of the precursor protein, is fused to a single eS31 or eL40 ribosomal protein; however, Ub-eS31 or Ub-eL40 fusions combined in other different architectures can also be found. Curiously, the combinatorial capability of the Ub-eS31 fusion with other domains is much more restricted than that of the Ub-eL40 fusion. In the particular case of
S. cerevisiae
, the paralogous
UBI1
and
UBI2
genes (human
UBA52
gene) encode a single Ub moiety fused to the 60S ribosomal proteins eL40A and eL40B, respectively. The
UBI3
gene (human
RPS27A/UBA80
gene) encodes a single copy of Ub fused to the 40S ribosomal protein eS31
[13]
. Although exceptions have been identified, it can be generally said that, in most eukaryotes, the amino acid sequence of the Ub proteins originating from the different genes are identical, suggesting that the different loci have undergone concerted evolution, which homogenizes their sequences by gene conversion
[14]
. Our analysis confirms that the Ub-eL40 and Ub-eS31 fusions are universally distributed amongst model eukaryotes (
). Only rarely, Ub is fused to other proteins in different architectures, although these arrangements are widely represented in the eukaryotic groups of animals, fungi, and plants (
). In this sense, in a small group of mixotrophic algae, it has been reported that one or several N-terminal Ub moieties are fused in-frame inter alia to ribosomal protein P1, actin, a zinc-finger protein, a nickel superoxide dismutase, or a protein with similarity to bacterial integral membrane proteins
[15]
. A more careful study indicates that the Ub fusion to the latter three proteins is found in other eukaryotes, such as different types of algae and diverse fungi
[15]
. Indeed, our analysis shows that the zf-AN1 (PF01428) and zf-RRN7 (PF11781) domains, which correspond to zinc-finger domains, are the most abundant ones in these unusual architectures (ca. 1.2% abundance). We have also identified a few cases of Ub fusions with other ribosomal proteins different from eS31 or eL40, such as eS8, eS19, uL1, or eL41. Interestingly, in some cases, the domain arrangement in the fusion proteins is different to the “classical” N-terminal position of Ub, as the Ub moiety can also occupy a C-terminal or even an internal position relative to its fusion partner (e.g.,
[15]
).
Free monoUb is generated by proteolytic cleavage of the last C-terminal residues (single Ub gene) or at the Ub-protein junction (Ub fused genes) by specific proteases or deubiquitinases (e.g.,
Figure S2
)
[16]
, an aspect that has, however, only been experimentally validated in few organisms. In addition, given the large redundancy found for these enzymes in model eukaryotes, the responsible deubiquitinase(s) could so far not be unambiguously revealed. In yeast, human, and other model eukaryotes, no intact Ub precursors can be detected unless cleavage-retarding or -inhibitory mutations were introduced at the C-terminus of the Ub moiety in the precursors (e.g.,
[17][18]
); this indicates that processing is a fast, likely co-translational, event. An interesting in vitro rabbit reticulocyte lysate-based translation system has been developed to tackle Ub maturation by deubiquitinases
[19]
. The results obtained by this system have allowed suggesting that processing of human UBA52 and UBA80 precursors occurs mostly post-translationally while that of UBB or UBC precursors probably occurs through a combination of co- and post-translational mechanisms
[19]
; however, we believe that these results are far from the real physiological situation and therefore must be validated by in vivo studies. Further biochemical analyses upon fractionation of a mouse liver cytosolic extract have allowed the identification of distinct deubiquitinases as the enzymes responsible for the processing of either human Ub-eL40, Ub-eS31, or polyUb precursors
[19]
, but again, these experiments do not exactly reflect the in vivo situation. One notable exception to proteolytic maturation of a Ub precursor has been found in
G. lamblia
; in this organism, the Ub-fused to eS31 is not cleaved, presumably because an additional alanine is present between the two glycine residues at the junction between the Ub-like domain and the eS31 protein
[20]
.
3. The Significance of Ubiquitin Fusion Genes
Why evolution has selected unusual gene fusions to produce de novo Ub by proteolytic cleavage of precursor proteins in practically all eukaryotes is a mystery. It has been speculated that a polyUb precursor consisting of tandem head-to-tail Ub repeats could allow the prompt synthesis of large amounts of Ub as a cellular response to special environmental conditions, such as a variety of sudden insults
[21]
. In this sense, the yeast Ubi4 precursor is the main source of Ub when cells enter the stationary phase or when they are subjected to different stress conditions, including high temperature, oxidative stress, or starvation
[21][22]
. In agreement with this, a
ubi4∆
yeast strain is not only hypersensitive to different stresses, but also prematurely induces apoptosis and shows decreased replicative lifespan
[23]
. Interestingly, a recent study shows that, in laboratory and industrial yeasts, the variation in the number of Ub moieties derived from a polyUb gene modulates Ub-dependent proteasome activity following a heat shock and suggests a positive correlation between the number of Ub moieties and cell survival, with different repeat numbers being optimal for coping with different stress conditions
[24]
. Similar conclusions can be obtained from the interpretation of the transcriptional regulation of polyUb genes in different animals and plants or from the consequences of the deletion of the mouse
UBC
gene for proliferation and stress tolerance of embryogenic fibroblasts during fetal development
[25]
.
As mentioned above, Ub is also expressed from fusion genes containing a single Ub coding unit combined in frame with the coding region of another protein that is unrelated to Ub. In a vast majority of eukaryotes, these proteins are the ribosomal proteins eS31 and eL40. In microorganisms, such as yeast, most cellular Ub originates from the
UBI1
,
UBI2
, and
UBI3
genes under physiological growth conditions
[11][22]
. This fact invites us to consider the attractive idea that fusing the Ub moiety to a ribosomal protein could have evolved to couple the synthesis and degradation of proteins to maintain proteostasis in eukaryotes, this way explaining the selective advantage of having fused Ub to either eL40 and eS31 in most eukaryotes and even to eS30, eS19, eL41, or P1 in those organisms where these fusions have arisen. However, this reasoning is unable to sufficiently explain why eL40 and eS31, apart from the latter anecdotic fusions to other ribosomal proteins, have been specifically selected in eukaryotes and suggests a precise and important role for Ub in eS31 and eL40 expression, ribosome assembly, or ribosome function, or, vice versa, a specific relevance of these ribosomal proteins for the fused Ub.
(i) However, the Ub moiety of Ub-fused eL40 and eS31 is not strictly required for the function of these two ribosomal proteins. Examples of stand-alone genes encoding either eS31 or eL40 without N-terminal Ub fusions can naturally be found in a large variety of organisms, mainly archaea but also algae, plants, fungi, or animals, when searching the eS31 and eL40 domains (PF01599 and PF01020, respectively) in the protein family database PFAM. Moreover, as demonstrated in yeast, constructs expressing eL40 and eS31 proteins lacking their N-terminal Ub moieties can fully complement
ubi1
,
ubi2
, and
ubi3
null mutants, respectively; nevertheless, this is only possible as long as these unfused constructs are overexpressed from high-copy-number plasmids, suggesting that indeed the N-terminal ubiquitin moiety of the Ubi1 and Ubi3 precursors contributes to the efficient expression (synthesis and/or folding) of eL40 and eS31, respectively, or to their assembly into ribosomes. However, when the sole cellular source of eL40 or eS31 originates from a single-copy allele of
UBI1
or
UBI3
lacking the Ub-coding sequence (
ubi1∆ub
and
ubi3∆ub
alleles, respectively), integrated at the native loci, cells showed a pronounced slow-growth phenotype due to the shortage of the corresponding ribosomal proteins and respective ribosomal subunits
[13][17][26]
. Due to these observations, it has been suggested that the N-terminal Ub moiety fused to eL40 and eS31 could act as a cis-acting chaperone to facilitate the correct folding and hence the efficient production and accumulation of these ribosomal proteins
[13][26]
, thus performing what Varshavsky and co-workers had proposed to be the primordial Ub function
[27]
. In agreement with this hypothesis, a minor but significant aggregation of a C-terminally HA-tagged eS31 protein could be observed when it was produced from a Ub-free Ubi3∆ub-HA precursor, a tendency that was, however, not observed when eL40A-HA was generated from the
ubi1∆ub
-HA allele
[26]
. Moreover, the replacement of the Ub moiety of Ubi1 by the yeast Ub-like SUMO protein (yeast Smt3, see below), which has been proven as an N-terminal fusion partner to augment the production of recombinant heterologous proteins through significant improvement of protein stability and solubility (e.g.,
[28][29]
), was able to modestly increase the steady-state levels of the mature eL40A-HA protein and, therefore, to slightly but significantly suppress the growth defect of a
ubi1∆ub ubi2∆
mutant strain
[26]
. Thus, altogether, these observations provide adequate experimental evidence to suggest that Ub (or Smt3) has indeed a minor but positive role as a cis-acting chaperone for the correct folding of eL40 and eS31
[26]
. Accordingly, it has been directly demonstrated that heterologous gene expression in yeast can be considerably increased by expressing particular proteins as Ub fusions
[30]
. This function is unlikely to occur in trans, as the expression of free Ub molecules in
ubi1∆ub ubi2∆
or
ubi3∆ub
cells did not result in the suppression of the growth defects of these mutants (
[26]
and S. M.-V., unpublished results).
(ii) In addition to the specific function for Ub in eL40 and eS31 expression, we have also addressed whether the Ub moiety of the yeast Ubi1 and Ubi3 precursors has a role in the assembly and function of the respective eL40A and eS31 ribosomal proteins. We considered this question to be very pertinent, especially given the strategical location of eL40 and eS31 on both sides of the binding site of translational GTPases (
). First, different experimental evidence suggests that the Ub moiety of Ubi1 and Ubi3 seems not to directly contribute to the assembly of the respective eL40A and eS31 proteins. Thus, under wild-type conditions, the Ubi1/2 and Ubi3 precursor proteins have so far never been detected, indicating a very rapid proteolytic maturation. Therefore, it is very improbable that the Ub molecule fused to eS31 may directly participate in the assembly of this ribosomal protein into pre-40S ribosomal particles as this occurs in the nucleus
[31][32]
. The ribosomal protein eL40 assembles in the cytoplasm
[33]
; thus, a direct role of its fused Ub molecule could be theoretically possible but would require that this should practically happen in an almost co-translational manner. Still, it is possible that the Ub moiety of a Ubi1/2 or Ubi3 precursor forms a non-covalently linked complex with the respective ribosomal proteins once cleaved; thus, these Ub molecules would operate as a kind of dedicated chaperone for the assembly of these ribosomal proteins. Interestingly, such a molecular complex between Ub and eL40 has been suggested to form upon cleavage of the mouse UBA52 precursor
[18]
, but whether Ub is acting as a dedicated chaperone for eL40 assembly or eL40 is working as a carrier to bring Ub to the nascent 60S ribosomal subunits has not yet been elucidated. It is now clear that, at least in yeast, the presence of Ub obstructs the assembly of eL40 or eS31 into pre-ribosomal particles
[17][34]
. While cleavage-resistant Ubi1 or Ubi3 precursor variants, generated by mutating the terminal region of Ub to partially or totally impair Ub removal by specific deubiquitinases, are able to incorporate into nascent pre-ribosomal particles, this occurs much less efficiently than in the case of the processed eL40 or eS31 proteins derived from wild-type Ubi1 or Ubi3 precursors, respectively; consequently, non-cleaved Ubi1 and Ubi3 are also highly instable
[17][34]
. Besides being due to the generally observed rapid degradation of unassembled ribosomal proteins (see next section), degradation of these precursors could also be specifically facilitated by the fact that a non-cleavable Ub moiety, when fused to the N-terminus of another protein, can function as a degradation signal in the so-called Ub fusion degradation pathway, as demonstrated for artificially engineered, non-cleavable Ub fusion proteins
[35]
. Forcing the assembly of non-cleaved Ubi1 and Ubi3 proteins has important functional consequences. The incorporation of Ubi3 into nascent 40S ribosomal subunits does not impair their biogenesis but leads to translation initiation defects and hypersensitivity to antibiotics targeting translation
[17]
. Strikingly, as mentioned above, a natural non-cleaved form of Ubi3 is present in all ribosomes from
G. lamblia
without apparent functional consequences
[20]
. In turn, the incorporation of Ubi1 into 60S ribosomal subunits affects their biogenesis, due to the assembly factor Tif6 not being properly recycled, and impairs their function during translation elongation; most likely, these defects are the consequence of the interference of the unprocessed Ubi1 with the binding and function of the GTPases that bind to the GTPase-associated center of the ribosome, such as Efl1, involved in the recycling of Tif6, and eEF1A and eEF2, which are general translation elongation factors
[34]
.
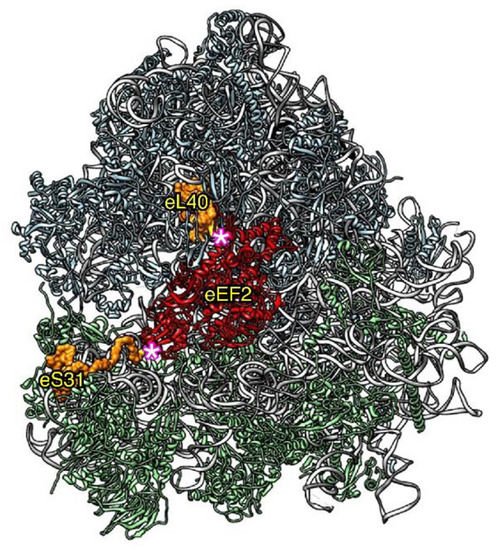
Figure 4.
Relative position of ribosomal proteins eL40 and eS31 (orange) within the large and the small ribosomal subunit, respectively, with respect to the binding site of the GTPase eEF2 (red). The remaining ribosomal proteins are colored in blue (large subunit) or green (small subunit) and the 25S and 18S rRNA in pale gray. The 5.8S and the 5S rRNA are shown in dark gray and black, respectively. The representation was generated with the UCSF Chimera program, using the atomic model of the cryo-EM structure V of the yeast 80S ribosome bound to the Taura syndrome virus IRES (not shown) and sordarin-stalled eEF2·GDP (PDB 5JUU). Note that eL40 could be modelled from its third residue (E79) and eS31 from its sixth residue (K82); these are highlighted by a pink asterisk. Due to the positions of eL40 and eS31 within 60S and 40S ribosomal subunits, respectively, the assembly of non-cleaved Ubi1/2 or Ubi3 is expected to sterically interfere with the binding of eEF2 to the ribosomal GTPase-associated center.
The biological significance of Ub fusions to other proteins, including actin, nickel superoxide dismutase, zinc-finger-containing proteins, or proteins with similarity to bacterial integral membrane proteins, is, if there is any, even more intriguing and currently unknown.