Aparna Das*a, Ajay K. Boseb and Bimal Krishna Banik*c
aDepartment of Mathematics and Natural Sciences, College of Sciences and Human Studies, Prince Mohammad Bin Fahd University, Al Khobar 31952, KSA; Email: aparnadasam@gmail.com; adas@pmu.edu.sa
bDepartment of Chemistry and Chemical Biology, Stevens Institute of Technology, Hoboken, New Jersey 07030, USA;
cDepartment of Mathematics and Natural Sciences, College of Sciences and Human Studies, Deanship of Research, Prince Mohammad Bin Fahd University, Al Khobar 31952, KSA; Email: bimalbanik10@gmail.com; bbanik@pmu.edu.sa
Abstract:
Stereoselective synthesis of cis and trans β-lactams under diverse conditions is performed. Numerous conditions are used for this study. The formation of β-lactam depends on the conditions of the experiments, structures of the imines and acid chlorides, order of addition of the reagents, reaction temperature, and solvents. A few mathematical graphs are plotted to explain the results.
- β-Lactam
- Stereoselectivity
- Microwave
1. Introduction
INTRODUCTION
β-Lactams are medicinally active molecules. Several publications have disclosed the anticancer
[1]
, antibacterial
[2]
, antifungal
[3]
, cholesterol absorption inhibitors
[4]
, anti-inflammatory
[5]
, anti-hepatitis
[6]
, analgesic activities
[7]
and antihyperglycemic
[8]
properties of β-lactams. Many methods are available for the preparation of β-lactams, such as Staudinger cycloaddition
[9]
, hydroxamate approach
[10]
, ester enolate-imine condensation
[11]
, alkene-isocyanate method
[12]
, the alkyne-nitrone reaction (Kinugasa reaction)
[13]
, catalytic asymmetric synthesis
[14]
and polymer-supported method
[15]
. Our group has also demonstrated the synthesis of β-lactams
[16]
.
Depending on the reactants and reaction conditions the stereochemistry of β-lactams may alter. Both the stereoisomers are important for medicinal applications. Thus, controlling diastereoselectivity (
cis
or
trans
) of the β-lactams is important. Stereoselective synthesis of diverse β-lactams following a variety of conditions through cycloaddition reaction of imines and acid chlorides is described. The results are also explained by plotting the ratios of the two isomeric β-lactams formed with respect to the time of the reaction.
2. Results with Acetoxy DerivativeESULTS WITH ACETOXY DERIVATIVE
The Staudinger reaction mainly required an imine, a tertiary base, and an acid chloride. The reaction of an acid chloride or equivalent with an imine in the presence of a tertiary produced
cis
and
trans
isomers of β-lactams. In this study, ten different reaction conditions including microwave-induced organic reaction enhancement (MORE) chemistry techniques and traditional synthesis/one-pot synthesis was adopted. A domestic microwave oven was used for irradiation and a large Erlenmeyer flask was used as the reaction vessel.
Experiment 1:
Experiment 1:
Microwave irradiation of a solution of imine with an acid chloride in chlorobenzene
[17]
produced a mixture of
cis
and
trans
β-lactams. N-methylmorpholine (NMM) was used as a base for this reaction instead of trimethylamine (TEA). Non-polar solvent, benzene was chosen as the reaction medium and reaction temperature was kept in between 45°C-50°C. It was observed that the reaction is not completed after 4 min and it produced a mixture of
cis
(70%) and
trans
(30%) β-lactams. The ratios of the
cis
and
trans
-isomers were determined from the coupling constants of the C
3
and C
4
protons of the β-lactam rings.
Experiment 2:
Experiment 2:
To identify the effect of the polarity of the solvent on stereoselectivity, chlorobenzene was used. Chlorobenzene being a polar solvent absorbs microwave energy efficiently. NMM was chosen as a base and reaction temperature was between 95°C-100°C. The reaction was completed within 5 min and it produced a mixture of
cis
(5-10%) and
trans
(90-95%). Thus this reaction condition was suitable for the preparation of
trans
β-lactams.
Experiment 3:
Experiment 3:
The third reaction was undertaken without any solvent. The reaction between the imine and acid chloride was conducted in a microwave oven at the temperature range of 95°C-100°C in the presence of NMM. The temperature was noted when the reaction was performed with 10 mmol of the substrates. The reaction produced a mixture of
cis
(5-10%) and
trans
(90-95%) isomers. It appeared that the solvent makes the reaction slower.
Experiment 4:
Experiment 4:
The reaction was conducted in a preheated oil bath at 90
0
C in the presence of NMM. The reaction was completed within 5 min and it gave a mixture of
cis
(5-10%) and
trans
(90-95%) β-lactams.
Experiment 5:
Experiment 5:
In another experiment, the oil bath was used, but the temperature was gradually increased from room temperature to 90
0
C. Chlorobenzene was chosen as the solvent and NMM was the base. The same reaction of the imine with acid chloride was performed and it was completed within 15 min. It produced a mixture of
cis
(50%) and
trans
(50%) β-lactams.
Experiment 6:
Experiment 6:
Another variation of this reaction was conducted using a one-pot method. In this experiment, benzaldehyde and
p
-anisidine were reacted in the presence of clay. NMM, AcOCH
2
COCl, and chlorobenzene were added to it. Irradiating the reaction mixture in a microwave for 2 min, the
trans
isomer of β-lactam was formed. The reaction produced only the
cis
isomer in the absence of microwave irradiation at room temperature.
3. Results with Acetoxy Derivative:
Results with Acetoxy Derivative:
The results obtained under different reaction conditions were extremely interesting.
Table 1
showed the ratios of the
cis
and
trans
β-lactams obtained under different conditions. The data showed that reaction conditions 2, 3, 4, and 6(a) were helpful for the synthesis of
trans
β-lactam. In contrast, reaction conditions 1 and 6(b) were good for the synthesis of
cis
β-lactam. On the other hand, reaction condition 5 was perfect for the synthesis of a mixture of
cis
and
trans
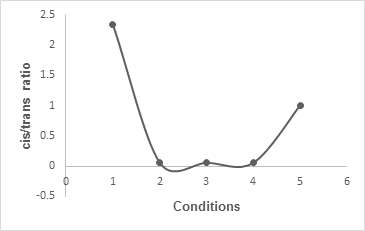
β-lactams. The graphical representation of these observations under diverse conditions is shown in
Figure 1.
.
|
Experiments |
Reaction temperature |
Time |
cis/trans ratio |
|
1 |
45°C-50°C |
4 min |
70:30 |
|
2 |
95°C-100°C |
5 min |
5:95 |
|
3 |
95°C-100°C |
3 min |
5:95 |
|
4 |
90°C |
5 min |
5:95 |
|
5 |
RT-90°C |
15 min |
50:50 |
|
6 (a)† |
95°C-100°C |
5-10 min |
0:100 |
|
6 (b) † |
0°C-RT |
Overnight |
100:0 |
Table 1:
Ratios of the cis and trans lactams under diverse conditions.
|
Experiments |
Reaction temperature |
Time |
cis/trans ratio |
|
1 |
45°C-50°C |
4 min |
70:30 |
|
2 |
95°C-100°C |
5 min |
5:95 |
|
3 |
95°C-100°C |
3 min |
5:95 |
|
4 |
90°C |
5 min |
5:95 |
|
5 |
RT-90°C |
15 min |
50:50 |
|
6 (a)† |
95°C-100°C |
5-10 min |
0:100 |
|
6 (b) † |
0°C-RT |
Overnight |
100:0 |
Figure 1:
Graphical representation of the β-lactam formation under diverse conditions.
Also, the
cis
β-lactams did not change to
trans
β-lactams when they were treated with NMM in chlorobenzene in a domestic microwave oven for 2-3 min even at 90
0
C. This experiment established that there is no isomerization of the
cis
β-lactams to the more thermodynamically stable
trans
β-lactams under microwave irradiation at a high temperature.
The reaction was performed with phenyl-substituted imine and similar ratios of the
cis
and
trans
isomers were formed.
Experiment 7:
Experiment 7:
The reaction of benzyloxyacetyl chloride with imine in the presence of dimethyl-formamide (DMF) and NMM also yielded a mixture of
cis
and
trans-
β-lactam in varying proportions.
Table 2 showed the
cis
/
trans
ratios during the time of microwave irradiation. The results showed that
cis
lactams were formed with low power radiation. On the other hand, high power radiation generated
trans lactams formation preferentially.
lactams formation preferentially.
|
Time |
Temperature |
Power |
Cis |
Trans |
cis/trans ratio |
|
1 min |
70°C |
Low |
85 |
15 |
85:15 |
|
2 min |
75°C |
Low |
80 |
20 |
80:20 |
|
3 min |
80°C |
Low |
60 |
40 |
60:40 |
|
4 min |
95°C |
Low |
56 |
44 |
56:44 |
|
5 min |
97°C |
Low |
55 |
45 |
55:45 |
|
4 min |
110°C |
High |
45 |
55 |
45:55 |
Table 2:
Ratios of the
cis
and
trans
lactams with respect to time.
|
Time |
Temperature |
Power |
Cis |
Trans |
cis/trans ratio |
|
1 min |
70°C |
Low |
85 |
15 |
85:15 |
|
2 min |
75°C |
Low |
80 |
20 |
80:20 |
|
3 min |
80°C |
Low |
60 |
40 |
60:40 |
|
4 min |
95°C |
Low |
56 |
44 |
56:44 |
|
5 min |
97°C |
Low |
55 |
45 |
55:45 |
|
4 min |
110°C |
High |
45 |
55 |
45:55 |
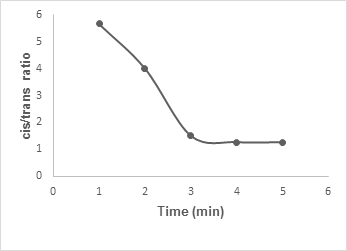
Figure 2 represented graphically the variation of the
Figure 2 represented graphically the variation of the
cis
and
trans
ratios with irradiation time up to 5 min at low power mode. The data indicated that the
cis
/
trans
ratio decreases with the progress of time and finally stabilizes at 4-5 min.
Figure 2:
Graphical representation of the
cis/trans
ratio of
β
-lactam with the time of irradiation.
Experiment 8:
Experiment 8:
Microwave irradiation of activated phthalimido acetic acid with imine in the presence of chlorobenzene and NMM produced a mixture of
cis
and
trans
β-lactams.
Experiment 9:
Experiment 9:
Microwave irradiation of acid chloride with imine (produced from D-glyceraldehyde) in the presence of chlorobenzene and NMM in 0-5 min gave
cis
β-lactam. However, irradiation of acid chloride with imine (obtained from L-glyceraldehyde) gave
cis
β-lactam with opposite absolute stereochemistry.
Experiment 10:
trans
β-Lactam was formed in 100% yield by slow addition of NMM in ethylene dichloride to a refluxing solution of imine and the acid chloride.
4. Conclusion
CONCLUSIONS
The stereochemistry (
cis
and/or
trans
) of the β-lactams under diverse conditions was analyzed. The data showed that some reaction conditions are favorable for the synthesis of
trans
β-lactams. On the other hand, some reaction conditions are favorable for the synthesis of
cis
β-lactams. A few reaction conditions are favorable for the synthesis of a mixture of
cis
and
trans β-lactam. The diastereoselectivity of the β-lactam formation strongly depends on reactants and reaction conditions. The data suggest that the β-lactam formation reaction depends on two pathways. One pathway is favored at high temperatures and or concentrated solution/microwave-mediated reaction conditions.
β-lactam. The diastereoselectivity of the β-lactam formation strongly depends on reactants and reaction conditions. The data suggest that the β-lactam formation reaction depends on two pathways. One pathway is favored at high temperatures and or concentrated solution/microwave-mediated reaction conditions.
Contributors
CONFLICT OF INTEREST
Aparna Dasa, Ajay K. Boseb and Bimal Krishna Banikc
The authors confirm that this result has no conflict of interest.
a Department of Mathematics and Natural Sciences, College of Sciences and Human Studies, Prince Mohammad Bin Fahd University, Al Khobar 31952, KSA; Email: aparnadasam@gmail.com; Email: adas@pmu.edu.sa
ACKNOWLEDGEMENTS
b Department of Chemistry and Chemical Biology, Stevens Institute of Technology, Hoboken, New Jersey 07030, USA;
AD and BKB are grateful to Prince Mohammad Bin Fahd University for support. BKB is also grateful to US NIH, US NCI, and Kleberg Foundation of Texas for finance support.
c Department of Mathematics and Natural Sciences, College of Sciences and Human Studies, Deanship of Research, Prince Mohammad Bin Fahd University, Al Khobar 31952, KSA; Email: bimalbanik10@gmail.com; bbanik@pmu.edu.sa
Acknowledgements
AD and BKB are grateful to Prince Mohammad Bin Fahd University for support. BKB is also grateful to US NIH, US NCI, and Kleberg Foundation of Texas for finance support.
References
- Indrani Banik; Frederick F. Becker; Bimal K. Banik; Stereoselective Synthesis of β-Lactams with Polyaromatic Imines: Entry to New and Novel Anticancer Agents†. Journal of Medicinal Chemistry 2003, 46, 12-15, 10.1021/jm0255825.
- Tamás Sperka; János Pitlik; Péter Bagossi; József Tőzsér; Beta-lactam compounds as apparently uncompetitive inhibitors of HIV-1 protease. Bioorganic & Medicinal Chemistry Letters 2005, 15, 3086-3090, 10.1016/j.bmcl.2005.04.020.
- Marci O’Driscoll; Kerriann Greenhalgh; Ashley Young; Edward Turos; Sonja Dickey; Daniel V. Lim; Studies on the antifungal properties of N-thiolated β-lactams. Bioorganic & Medicinal Chemistry 2008, 16, 7832-7, 10.1016/j.bmc.2008.06.035.
- Duane A. Burnett; Mary Ann Caplen; Harry R. Davis; Robert E. Burrier; John W. Clader; 2-Azetidinones as Inhibitors of Cholesterol Absorption. Journal of Medicinal Chemistry 1994, 37, 1733-1736, 10.1021/jm00038a001.
- Synthesis of new 2-chloro-phenothiazinothiadiazol-2-oxoaze tidines: Antimicrobial and antiinflammatory agents . Indian J. Chem., 2000, 39B, 464. Retrieved 2020-6-8. Indian J. Chem., 2000, 39B, 464. . Retrieved 2020-6-7
- Manjinder S. Lall; Yeeman K. Ramtohul; Michael N. G. James; John C. Vederas; Serine and Threonine β-Lactones: A New Class of Hepatitis A Virus 3C Cysteine Proteinase Inhibitors. The Journal of Organic Chemistry 2002, 67, 1536-1547, 10.1021/jo0109016.
- Carmela Saturnino; Bruno Fusco; Paola Saturnino; Giovanni De Martino; Flavio Rocco; Jean-Charles Lancelot; Evaluation of analgesic and anti-inflammatory activity of novel beta-lactam monocyclic compounds. Carmela Saturnino; Bruno Fusco; Paola Saturnino; Giovanni De Martino; Flavio Rocco; Jean-Charles Lancelot; Evaluation of analgesic and anti-inflammatory activity of novel beta-lactam monocyclic compounds.. Biological & Pharmaceutical Bulletin 2000, 23, 654-656, 10.1248/bpb.23.654.
- Rajesh Kumar Goel; Mohinder P Mahajan; Shrinivas K Kulkarni; Evaluation of anti-hyperglycemic activity of some novel monocyclic beta lactams.. Journal of Pharmacy & Pharmaceutical Sciences 2004, 7, 80.
- Hermann Staudinger; Zur Kenntniss der Ketene. Diphenylketen. European Journal of Organic Chemistry 1907, 356, 51-123, 10.1002/jlac.19073560106.
- Marvin J. Miller; Hydroxamate approach to the synthesis of .beta.-lactam antibiotics. Accounts of Chemical Research 1986, 19, 49-56, 10.1021/ar00122a004.
- David J. Hart; Deok Chan Ha; The ester enolate-imine condensation route to .beta.-lactams. Chemical Reviews 1989, 89, 1447-1465, 10.1021/cr00097a003.
- Marek Chmielewski; Zbigniew Kałuża; Bartłomiej Furman; Bart?omiej Furman; Stereocontrolled synthesis of 1-oxabicyclic β-lactam antibiotics via[2 + 2]cycloaddition of isocyanates to sugar vinyl ethers. Chemical Communications 1996, 24, 2689-2696, 10.1039/cc9960002689.
- Manabu Kinugasa; Shizunobu Hashimoto; The reactions of copper(I) phenylacetylide with nitrones. J. Chem. Soc., Chem. Commun. 1972, --, 466, 10.1039/c39720000466.
- Andrew E. Taggi; Ahmed M. Hafez; Harald Wack; Brandon Young; William J. Drury; Thomas Lectka; Catalytic, Asymmetric Synthesis of β-Lactams. Journal of the American Chemical Society 2000, 122, 7831-7832, 10.1021/ja001754g.
- Donato Donati; Costanza Morelli; Andrea Porcheddu; Maurizio Taddei; A New Polymer-Supported Reagent for the Synthesis of β-Lactams in Solution. The Journal of Organic Chemistry 2004, 69, 9316-9318, 10.1021/jo048400i.
- Banik, Bimal K. Beta Lactams: Novel Synthetic Pathways and Applications; Banik, Bimal K, Eds.; Springer: Switzerland, 2017; pp. 1-419.
- Laurence Perreux; Andre Loupy; A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 2001, 57, 9199-9223, 10.1016/s0040-4020(01)00905-x.