Bacterial biofilm is a group of cooperative and coordinated unicellular microbes which are associated with physiological and structural complexity, and they are analogous to multicellular microorganisms.
- biofilm
- polymicrobial infections
- nosocomial infections
- superhydrophobic
- nanomaterials
- anti-biofilm surfaces
1. Introduction
Bacterial biofilm is a group of cooperative and coordinated unicellular microbes which are associated with physiological and structural complexity, and they are analogous to multicellular microorganisms. Bacterial biofilms can exist on a vast range of biotic surfaces, such as the skin, connective tissues, bones, airways, vascular endothelium, and intestinal mucosa, resulting in multiple types of tissue-associated chronic infections. Biofilms can also be associated with the development of infections from indwelling medical devices, such as catheters, sutures, orthopaedic implants, heart valves, intrauterine devices, and vascular grafts. Some examples of the biofilm pathogens that commonly result in medical device-associated bacterial infections are Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae (Table 1) [1][2][3][4,11,12]. Specifically, the formation of biofilms on medical devices used within the healthcare setting enables pathogens to persist as reservoirs which can be easily spread in patients [4][13]. The formation of bacterial biofilms can be attributed to the alternative dynamic and multifaceted lifestyle of microbials cells within the biofilm that confers them remarkable capability to survive in diverse environmental niches [4][5][13,14]. Generally, a diverse array of microbial cells can form biofilm that often consists of various species under normal conditions, whereby the maintenance of such a biofilm is regulated by intra- and intercellular communications via autoinducers [6][15]. During the process of biofilm formation, the microbes which are present in the host establish contact with a surface as part of a probabilistic process, driven by hydrodynamic force, gravitational force, as well as Brownian movement. Such surface attachment of microbials can either be reversible or irreversible, which is often dependent on the surrounding biological environment. The adhered microbial cells then proceed to penetrate host tissue for deriving nutrients and to prepare themselves for cellular division, forming a bacterial biofilm [5][7][14,16]. In the following sections, we discuss the basic structure and composition of a bacterial biofilm and provide a general overview on the steps and mechanisms leading to biofilm formation.
Table 1.
Examples of medical device-associated bacterial infections and their common causative pathogens.
| Type of Medical Device-Associated Bacterial Infections | Common Causative Pathogens | Reference(s) |
|---|---|---|
| Central line-associated bloodstream infection | Coagulase-negative Staphylococci Staphylococcus aureus Enterococcus spp. Pseudomonas spp. |
[2][4][11,13] |
| Catheter-associated urinary tract infection | Escherichia coli Pseudomonas spp. Enterococcus spp. Staphylococcus aureus Coagulase-negative Staphylococci Enterobacter spp. |
[3][8][12,17] |
| Ventilator-associated pneumonia | Pseudomonas spp. Klebsiella spp. Enterococcus spp. Staphylococcus aureus Pseudomonas aeruginosa Acinetobacter baumannii |
[2][8][11,17] |
| Prosthetic heart valve infection | Staphylococcus aureus Staphylococcus epidermidis Streptococcus spp. |
[2][11] |
| Surgical site infection | Staphylococcus aureus Enterococcus spp. Acinetobacter spp. Pseudomonas spp. Escherichia coli |
[8][17] |
2. Structure and Composition of Bacterial Biofilm
Biofilm can be defined as structured communities of microorganisms that are adherent to a biotic or abiotic surface, which are embedded in a matrix of self-producing extracellular polymeric substance (EPS). Typically, approximately 35% of the biofilm volume is made up of microorganisms, while the remaining volume is constituted by EPS [9][18]. As such, the production of EPS is the hallmark of biofilm formation, in which the EPS facilitates the attachment of microbial cells to surfaces and promotes cell-to-cell adhesion and aggregation [1][3][4,12]. At the same time, the matrix of EPS functions as a three-dimensional protective barrier that shields the microbial cells against external threats, which may include the host defence mechanisms and antimicrobial therapeutics. In addition, through the modulation of chemical and nutrient gradients, the EPS matrix can lead to the formation of a harsh biological environment that is essential for major virulence attributes [3][12]. Nevertheless, the function of EPS within the biofilm is vast and it has a variable composition between different microbial species. For example, cellulose produced by Escherichia coli contributes to increased resistance of the microbial communities to desiccation, whereas BslA, a bacterial hydrophobin of Bacillus subtilis, forms a water-resistant coat over the microbial communities [10][19]. In general, water constitutes the major part which accounts for approximately 97% of the EPS, with the remaining constituents such as exopolysaccharides (1–2%), proteins (more than 2%), DNA and RNA molecules (less than 1%), as well as ions making up the composition of biofilm (Figure 1) [9][10][18,19]. Certain host derived components including platelets, fibrin, as well as immunoglobulins may also be present in biofilms within complex host environments [3][12]. In terms of its architecture, the layout of biofilm comprises two major components, namely an area of closely packed microbial cells lacking eminent pores, and water channels that act as a simple circulatory system for the efficient transport of nutrients [11][12][8,20]. As biofilm is polymorphic in nature, its structure can also be altered in response to changes in the amount of nutrients, in which microcolonies grow faster in the presence of high glucose concentration, leading to increased biofilm thickness. On the contrary, low glucose concentration leads to reduced biofilm biomass, thereby restoring its former structure. Hydrodynamic condition is another factor that could affect the structure of biofilm, for instance, bacterial microcolonies become round in a laminar flow environment, whereas in a turbulent flow environment, bacterial microcolonies extend in downstream, having various phenotypes [12][20].
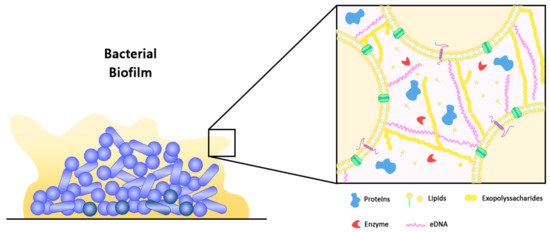
Figure 1. Schematic representation of a bacterial biofilm and its extracellular polymeric substance (EPS). Microcolonies of a mature biofilm are typically characterized by the presence of an EPS matrix that is composed of exopolysaccharides, proteins, extracellular DNA (eDNA), lipids, and enzymes. The EPS matrix acts as a protective barrier to shield the microbial community from external threats, including those of host defense mechanisms and antimicrobial therapeutics [3][12].
(References would be added automatically after the entry is online)
