Zearalenone (ZEA) is an estrogenic fusariotoxin, being classified as a phytoestrogen, or as a mycoestrogen. ZEA and its metabolites are able to bind to estrogen receptors, 17β-estradiol specific receptors, leading to reproductive disorders which include low fertility, abnormal fetal development, reduced litter size and modification at the level of reproductive hormones especially in female pigs. ZEA has also significant effects on immune response with immunostimulatory or immunosuppressive results. This review presents the effects of ZEA and its derivatives on all levels of the immune response such as innate immunity with its principal component inflammatory response as well as the acquired immunity with two components, humoral and cellular immune response. The mechanisms involved by ZEA in triggering its effects are addressed.
- zearalenone
- cell immunity
- humoral immunity
1. Zearalenone
Fusariotoxins are secondary metabolites originate from fungi of the
and
species which represent the largest group of mycotoxins (more than 140). Of these the most widespread and also of primary concern are the trichothecenes, fumonisins, and zearalenone [1][2]. Despite the mitigation efforts, exposure of crops to mycotoxins is indeed inevitable and decontamination is very difficult [3][4]. Even though numerous studies concerning the effect of mycotoxins are reported in the specific literature, the contamination of cereals with fusariotoxins, the impact on animal health and the economic losses they imply [5][6][7], the transmission of fusariotoxins into the target organism and the potential existence of toxic components in meat and dairy products remain unknown and need further investigations [8].
Zearalenone (ZEA) is a fusariotoxin that belongs to the class of xenoestrogens due to its structural similarity to 17β-estradiol (
), but also due to the binding affinity to estrogen receptors [9]. It is a resorcyclic acid lactone [10], produced by
[11] which binds to estrogen receptors in mammalians target cells leading to reproductive disorders especially in female pigs [12]. In terms of physicochemical properties, ZEA has been shown to have high stability, being resistant to high temperatures and UV radiation, making it almost impossible to decontaminate crops on a large scale [13].
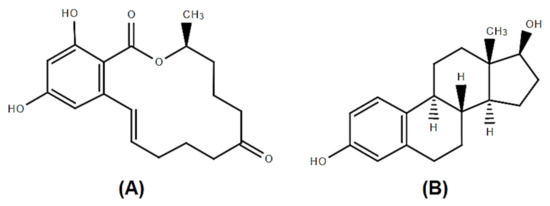
Chemical structures of Zearalenone (ZEA) (
) and 17β-estradiol (
).
ZEA is metabolized mainly at the intestinal and hepatic level and transformed in several metabolites by hydroxylation, glucuronidation or conjugation reactions [14]. Biotransformation of ZEA lead primarily to the formation of two metabolites, α-zearalenol (α-ZOL) and β-zearalenol (β-ZOL) which can be further reduced to α-zearalanol (α-ZAL) and β-zearalanol (β-ZAL) [15].
After oral contamination, zearalenone is rapidly absorbed in mammals (rabbits, rats, humans and pigs, 80–85%) [16][17]. As mentioned above, structurally, ZEA has a strong similarity with 17 β-estradiol, and is able to bind to estrogen receptors, being also classified as a phytoestrogen, or as a mycoestrogen [18]. From this reason ZEA intoxication most often leads to disorders of the reproductive system. The changes involve low fertility, abnormal fetal development, reduce litter size and modification in the level of specific reproductive hormones: Estradiol and progesterone [19]. But, ZEA manifests its toxicity on many other systems besides the reproductive one. Studies show that the liver and spleen are also affected when exposed to this mycotoxin. It has been demonstrated in an experiment on female piglets that excessive amounts of ZEA raises the key liver enzymes level such as glutathione peroxidase and decreases spleen weight [20]. ZEA exerts significant effects on immune response, the major defense mechanism against pathogens, toxins and other antigens in all mammals with immunostimulatory or immunosuppressive results [21]. The cellular mechanisms activated by zearalenone in triggering its different effects are not yet well understood.
2. Effect of ZEA on Gut Immunity
The gut represents a physical barrier that ensure the body’s protection against environmental agents, mycotoxins included [22]. This barrier involves intestinal epithelial cells (IECs), but also components of both innate and adaptive immunity, as IgA and pro-inflammatory cytokines or molecules involved in the inhibition of bacterial colonization (mucins and antimicrobial peptides) [23].
A recent study of Lahjouji et al. [24] reported that the intestine is a target of ZEA which cause pathological intestinal changes. Depending on different factors as specie, sex, dose or time of exposure, ZEA can affect or not the normal anatomic structure of the intestine. For example, ZEA (40 μg/kg b.w.) did not change the mucosa thickness, the height of villi or the number of goblet cells in swine [25][26], but the ingestion of ZEA (0.3–146 mg/kg feed) in pregnant rats modified the structure of villi and decrease the junction proteins expression [27]. Also, in the first week of a chronic exposure of pigs to ZEA (40 µg/kg b.w.) produced transitory morphological modifications of small intestine and an increase in Paneth cell numbers in the crypt [24].
Exposure to ZEA was associated with the impairment of cell viability, apoptosis and necrosis in different organs including gut [28][29]. For example, recent studies have shown that treatment with ZEA at 10–100 µM for 24h decreased viability of porcine intestinal epithelial cells IPEC-J2 [30] and IPEC-1 [31][32] and increase LDH activity [30]. Apoptosis is one of the main mechanisms involved by ZEA induced toxicity in the gut and it was shown that ZEA can cause apoptosis in various intestinal epithelial cells as IPEC-J2 [33], MODE-K [34] and HCT116 [35]. Sub-chronic exposure of female Polish Large White pigs to low doses of ZEA increased apoptosis and decreased the ileal Peyer’s patches lymphocytes proliferation [36].
Some recent studies have shown that ZEA exposure alters or not the T- and B-cell subtype populations in small intestine. The administration of ZEA (5 or 20 mg/kg b.w.) to female BALB/C mice increased the percentages of CD8+ and decreased the percentages of CD4+ cells in the lamina propria and intra-epithelium lymphocytes, while no effect on CD8+ and CD19+ lymphocytes was observed in the Peyer’ patches [37]. Also, the exposure of prepubertal gilts to 100mg ZEA/kg feed decreased the percentage of CD21+ lymphocytes in pig ileum [36].
Endoplasmic reticulum stress (ERS) pathway seems to be the main signaling pathway involved in ZEA induced apoptosis. In MODE-K mouse cells exposed to zearalenone, the toxin increased the gene expression and protein synthesis of molecules involved in ERS-induced apoptosis pathway as: c-Jun N-terminal kinase, C/EBP homologous protein, GRP78, caspase-1 and the anti-apoptotic protein Bax, while decreasing the levels of the pro-apoptotic related protein Bcl-2 [34]. Similarly, an increased number of apoptotic cells were observed in the Peyer’ patches of the 20 mg/kg ZEA-treated mice associated with a significantly increase of Bax gene expression and of the ratio of Bax:Bcl-2 [37].
Mucosal IgA protects the intestinal epithelium from xenobiotics, viruses and bacteria and maintains also the homeostasis of the intestinal environment [38]. Indeed, previous studies have linked the increase of IgAs to repeated exposure to environmental toxins or to chronic infections [39]. The effect of ZEA on IgAs synthesis is controversial. ZEA administered by oral gavage to female BALB/c mice for two weeks at a dose of 5 or 20 mg/kg b.w. provokes a significantly decrease of the mucosal IgA antibody level in mice duodenum [37]. On the other side, the administration of 20 mg of ZEA/kg b.w. for only one week to male BALB/c mice significantly increased fecal IgA levels in the jejunum (1.5-fold higher that in the control group) [40]. Because the same concentration of ZEA (20 mg/kg b.w.) and the same way of administration (oral gavage) were used in both experiments, the different results could be related to the experiment duration (one week vs. two weeks) or could be influenced by the sex of the animals (male vs. female) and the intestinal segment.
Mucin glycoproteins are produced by epithelial or submucosal mucus-producing cells and represents the principal constituent of mucus [41]. The mucus structure is permanently renewed and could be rapidly adjusted to respond to the changes of the environment, as for example in response to microbial infection or to exposure to contaminants [29]. Recent studies have shown that ZEA (20 mg/kg b.w.) is responsible for intestinal mucosa abnormalities [40] as mice exposure for a short period (one week) to ZEA significantly increased the expression of mucin 1 and mucin 2 genes in the gut. Results on in vitro cell cultures also indicates that ZEA interferes with the mucin synthesis since the toxin increased the concentration of these molecules in Caco-2/HT29-MTX cells [42]. In vitro exposure of swine IPEC-J2 cells to 40 µM ZEA [43], and in vivo exposure of mice to 20 mg ZEA/kg b.w. [40] resulted in the upregulation of β-defensin, an important antimicrobial peptide, with important consequences for the defense against intestinal infection.
The effect of ZEA on the intestinal inflammatory response is not very clear, and this fact is due probably to the different experimental models and various ZEA concentrations used in several studies. Thus, in vitro studies showed that the incubation of porcine intestinal cells with 10 and 40 µM of zearalenone respectively lead to the increased expression of genes encoding for molecules involved in inflammation such as TLRs and the cytokines:
in IPEC-1 [44] and of
and
in IPEC-J2 cells [43]. The increase in the expression of pro-inflammatory cytokines (IL-1β and TNF-α) was also registered in the in vivo studies, in jejunum of mice intoxicated with 20 mg ZEA/kg b.w. Also, prepubertal gilts exposed to 100 mg ZEA/kg feed for 42 days had elevated ileum concentrations of IL-12/23 40p and IL-1β [36]. By contrast, other researchers found that ZEA had no effect on proinflammatory IL-8 cytokine synthesis by IPEC-1 cells [31] or decreased the gene expression of
,
and
in the jejunum of rats when administered in different doses (0.3–146.0 mg/kg feed) [27]. As well, a microarray study performed on IPEC-1 cells exposed to ZEA (25 μM) found a decrease of the expression of pro-inflammatory cytokines
,
and
[45]. The inflammatory effect produced by ZEA can be transmitted from mother to offspring. Piglets derived from sows fed 300 ppb zearalenone one week before farrowing and during the lactation period developed gut inflammation [46].
3. Effect of ZEA on Innate Immune Response
Innate or nonspecific immunity is the first form of defense for multicellular organisms. The innate immune response is triggered by receptors that recognize the pathogen and activate a series of signaling pathways that control the immune response [47]. Neutrophils, NK and NKT cells, monocytes/macrophages and dendritic cells that mediate interactions with pathogens are the innate immune system components able to form networks with key role in the initial immune response to infection and tissue damages [48]. They are phagocytic cells that when stimulated can produce reactive oxygen species (ROS), important in cell signaling and homeostasis [49][50]. An imbalance between ROS production and its inefficient elimination drives to a dramatic increase in ROS levels leading to cells damage, known as oxidative stress [49]. Recently, Wang et al., reported that ZEA (5, 10, 20 µM) increased ROS production in bovine neutrophils and decreased antioxidant enzymes (SOD and CAT) activity by involving NADPH, ERK and p38 activation followed by the formation of neutrophil extracellular traps (NETs), a network of DNA extracellular fibbers which help neutrophil cells to kill extracellular pathogens [51]. This could have significant cytotoxic and pro-inflammatory consequences.
According with the study of Marin et al., zearalenone and its metabolites decreased cells viability of porcine polymorphonuclear cells, interleukine-8 synthesis and increased superoxide production, ZEA metabolites being more immunotoxic than ZEA [52]. Moreover, Murata et al., found that the effect of ZEA and its metabolites on bovine neutrophiles depend on their chemical structure [53]. Thus, these authors found that zearalenone and its derivatives α- and β-zearalenol suppressed luminol dependent PMA chemiluminescence in neutrophils due to their C1’-2’ double bonds while, zearalanone, α- and β-zearalanols did not exert this effect because they possess a hydrogenated C1’-2’ bond instead of the double bond. In vivo experimentation conducted on Ross 308 hybrid broilers fed with two concentration of deoxynivalenol and zearalenone after hatching showed that the toxins induced oxidative stress and inhibited significantly the blood cells phagocytic activity [54]. In combination with other mycotoxins (alternariol, deoxynivalenol), ZEA affect innate immune functions by inhibited for example the differentiation of monocyte into macrophages (THP-1 cell line). Moreover, the combination of ZEA with alternariol at low concentration lead to synergistic effect on CD14 expression [55]. The combination of the two toxins altered the macrophages functions by the inhibition of TNF-α secretion. The primary function of RAW 264.7 macrophages such as proliferation was reduced and apoptosis was induced in a dose-dependent manner by zearalenone (from 0 to 100 µM) via the ERS pathway [56]. Three milligrams of ZEA /kg b.w. altered in in vivo treated rats the hydrogen peroxide release by peritoneal macrophages [57]. Also in vivo, the low concentrations of ZEA (40 μg/kg b.w. per day) alone or in combination with deoxynivalenol (DON, 12 μg/kg b.w. per day), another
mycotoxin, produced changes in the morphology of pig Kuppfer cells (stellate macrophages) with consequences on their activity [58].
Inflammation represents a rapidly nonspecific immune response through which the phagocytic cells are activated and produce bioactive molecules (inflammatory cytokines, prostaglandins and leukotrienes) as well as oxygen and nitrogen metabolites [59]. Being an agonist of the estrogenic receptors, ZEA can modulate similarly the in vitro and in vivo inflammatory response depending on its concentration, time of exposure and immune indices investigated.
In vitro studies highlight an increase or a decline of inflammatory response induced or not by ZEA depending on the immune cell type. Thus, the in vitro study of Marin et al., in which swine PBMCs were exposed to ZEA and its metabolites for 48h showed that ZEA decreased significantly at 5 and 10 µM the TNF-α response of these cells and had no significantly effect on IL-1β and IL-8, while zearalanone decreased also the production of IL-8 at 10 µM [19]. Interleukin 8 (IL-8) is a common inflammatory cytokine important in the recruitment of the immune cells, a key parameter in localized inflammation which induced after that an increase of oxidative stress mediators [60]. The same authors reported later [31] that ZEA derivatives, alpha-zearalenol (α-ZOL), beta-zearalenol (β-ZOL), and zearalanone (ZAN) decreased in a dose dependent manner the IL-8 synthesis in polymorphonuclear cells (PMN), significantly at 10 µM (−49.2% for α-ZOL; −45.6% for β-ZOL and −45.1% for ZAN respectively) after 3 h of exposure. No effect of ZEA on IL-8 concentration registered. Similarly, ZEA metabolites were more potent than ZEA itself in decreasing the IL-8 synthesis in porcine epithelial cells (IPEC-1) [31]. By contrast, Ding e al. [61] found that the level of IL-1β and IL-18 increased significantly in peritoneal mouse macrophages isolated from mice receiving ZEA by gavage (4.5 mg/kg b.w.) once a day for 9 days and treated in vitro with 8 µg/mL for 24h. In a model monocytic cell line, hER + IL-1β-CAT+, the exposure of the cells to low level 50 ng/mL of zearalenone and α-zearalenol resulted in a pro-inflammatory effect as 17β-estradiol by modulating and promoting IL-1β synthesis. The toxins manifested full agonist activity with 17β-estradiol but at lower potency [62] exposure of human placental choriocarcinoma (BeWo) cells to different concentrations of ZEA (2–16µM) increased the IL-6 production [63].
In vivo studies confirmed the biphasic effect of zearalenone on inflammation. Experiments performed on piglets found that in spleen and blood, ZEA increased the gene expression of pro-inflammatory
and
, while in liver the toxin decreased dramatically the expression of these cytokine which might have consequences on immune homeostasis taking into account that liver is considered a key organ for immune homeostasis [64]. It seems that the pro- or anti-inflammatory effect of ZEA depend on the organ involved. The earlier results of Salah-Abbes [65][66] showed a significant reduction of TNF-α, IL-1β and IL-12 in plasma of mice treated with 40 mg ZEA /kg b.w. as well as an increase of the cytokines (TNF-α, IL-6, IL-10) in kidney of mice fed the same concentration of toxin [67]. The inflammatory reaction in kidney is produced through the activation of macrophages which, once activated, will further produce inflammatory cytokines and chemokines subsequently responsible for different reactions and effects such as apoptosis and necrosis, the up- or down regulation of pro- and anti- apoptotic genes [67]. In testicular tissue of mice exposed for 48h to ZEA (40mg/kg b.w.) the toxin increased the level of TNF-α, IL-1β, IL-6 and decreased the level of anti-inflammatory IL-10 cytokine [68].
4. Effect of Zearalenone on Adaptive Immune Response
4.1. Effect of Zearalenone on Humoral Immune Response
Animal exposure to different doses of zearalenone has resulted in an alteration of humoral immunity as can be seen in
. Literature studies show that ZEA leads to a decrease in serum IgG levels regardless the animal species (mice, rat or swine), toxin concentration or the duration of the exposure. Similarly, most studies have shown that the level of IgM in serum decreases no matter the species (mice or rats), time of exposure (12–36 days) and mycotoxin concentration (5–30 mg/kg b.w.). However, sub-chronic exposure (3–4 weeks) of rats to lower concentrations of ZEA (2–4 mg/kg b.w.) was associated with an increase in serum IgM concentration [69][70]. It seems that the effect of ZEA on IgM concentration is correlated with the sex of animal, since male piglets exposed to low concentration of toxin (0.8 mg/kg feed) resulted in a decreased in IgM level, while no significant changes were observed in serum IgM concentration of gilts receiving ZEA higher concentrations (1.1–3.2 mg/kg feed). Serum IgA concentration was not affected in mice, rats or swine after the exposure to low and medium concentration of the toxin (0.08–30 mg/kg feed) as resulted from most studies (
) and it is not related to the time of exposure (18–42 days). BALB/c female mice fed higher concentration of ZEA (40 mg/kg feed) for 48h showed a decrease of IgA concentration [71].
Effect of ZEA on the humoral immune response.
| Effect (s) | Species/Cell Type | Dose/Time of ZEA Administration |
References |
|---|
| ↑ IgE, ↓ IgM no changes of IgG and IgA |
BALB/c mice female | 20 mg/kg b.w. 14 days |
[38][37] |
| ↓ IgG, IgA ↑ IgM |
Pregnant rats | 100,150 mg/kg feed 7 days |
[70] |
| ↓ IgG, IgM, IgE no changes of IgA |
Wistar rats female |
0, 1, 5, 30 mg/kg b.w. 36 days |
[72] |
| ↓ Ig A, Ig G | BALB/c mice, female | 40 mg/kg b.w. 48h |
111].
Other examples concerning the effect of zearalenone on cellular immune response are illustrated in
.
5. Effect of Zearalenone on Immune Organs
Zearalenone is responsible for the increase of reproductive organs’ weight such as the uterus [112]. By contrast, the weight of the immune organs seems to be less affected by the exposure to ZEA as resulted from the literature data (
), but the toxin has been responsible for immune organs atrophy and depletion as well as for other histopathological modifications in immune organs. ZEA caused also a decrease of B cell percentage in the spleen or swelling of red pulp [57][96][99].
The effect of zearalenone on immune organs weight and structure.
| Effect (s) | Species/Cell Type | Dose/Time of ZEA Administration |
References |
|---|
| Thymic atrophy with histological and thymocyte phenotype changes and decrease in the B cell percentage in the spleen |
Wistar rats | 3.0 mg/kg b.w. 28 days |
[57] | ||||
| Atrophy of white pulp and swelling of red pulp | post-weanling gilts | 2.0, 3.2 mg/kg feed 18 days |
[110][98] | ||||
| No effect on spleen and bursa of Fabricius weights | one-day-old broiler chicks | 10–800 mg/kg feed 21 days |
[113] | ||||
| [ | 71 | ] | |||||
| No effect on spleen and bursa of Fabricius weights | one-day -old broiler chicks | 50–800 mg/kg feed 3 weeks |
[114] | ↓ IgG ↑ Ig M |
Wistar rats male |
2 mg/kg b.w./week 3 weeks |
[69] |
| Enlargement of the spleen in males | Sprague Dawley rats | 1.25, 3.75 mg/kg b.w. 8 weeks |
[ | ↓ Ig G no changes of IgA, IgM |
Prepubertal gilts | 200, 800, 1600 μg/kg feed 14 days |
[73] |
| 115 | ] | winter time | [106 | ||||
| No effect on spleen weight | Sprague Dawley rats | 0.5, 0.9, 1.8, 3.6 mg/kg b.w. 4 weeks | ][94] | ||||
| [ | 116 | ] | ↓ IgG, IgM no changes of IgA |
ZEA 40 µM: Inhibit T cell-chemotaxis by CCL19 ↑ CD4+ T cells induced by CCL19 chemotaxis ZEA 20 µM: ↑ CD8+ T cells induced by CCL21 chemotaxis | Piglets male |
0.8 mg/kg feed 4 weeks |
[74] |
| ↓ IgG, IgM in serum ↑ IgA in serum |
Kunming mice, female |
30 mg/kg b.w. 12 days |
[75] | ||||
| ↓ IgG in serum no changes on IgA, IgM |
post-weaning female piglets |
1.1-3.2 mg/kg feed 18 days |
[76] | ||||
| no effect of serum IgG, IgM, IgA | B6C3F1 mice | 10 mg/kg feed 6 weeks |
[77] | ||||
| ↓ IgG, IgA, IgM in cell SN | Swine PBMC | 10 mM 7 days |
[19] | ||||
| ↓ IgG ↓ IgM |
BALB/c mice | 5, 10, 15 mg/kg b.w./day 2 weeks |
[66] |
Moreover, ZEA metabolites interfere with immunoglobulin synthesis. As it was shown in an in vitro study using swine peripheral blood mononuclear cells, both ZEA and its metabolites (α-ZOL, β-ZOL and ZAN) significantly decreased the immunoglobulins IgG, IgM and IgA synthesis at concentrations higher that 5 µM [19].
It was observed that the consumption of contaminated feed led to an increase of toxin concentration in the serum of intoxicated animals before farrowing and during lactation suggesting that ZEA or its metabolites can interfere with immunoglobulin secretion in colostrum/milk and in offspring. Also, α-zearalenol metabolite was found in the colostrum and milk of the sows [46], but in our knowledge no data concerning this interference are available until now in the literature. However, feeding sows with a dietary mixture of mycotoxins containing DON, ZEA and fusaric acid resulted in a decrease of the concentration of IgA in the colostrum and of IgA and IgG in serum of their offspring [78].
A common method for the assessment of the T-cell-dependent antibody responses is represented by the sheep red blood cells (SRBCs) assay. Few data (
) concerning this type of immune response related to ZEA exposure are available. For example, exposure to 10 mg ZEA /kg b.w. of female B6C3F1 mice had no effect on the splenic plaque forming cells in response to SRBC [79] while a decrease of the B cells producing immunoglobulin M antibody to SRBC was observed in female Wistar rats exposed for 28 days to 3 mg of ZEA/kg b.w. [57].
Humoral immunity with specific antibody/in vaccination.
| Effect (s) | Species/Cell Type | Dose/Time of ZEA Administration | References |
|---|
| ↓ B cells producing IgM to SRBC 1 | Wistar rats female |
3 mg/kg b.w. 28 days |
[57] |
| No differences in humoral immune response against SRBC 1 | prepubertal gilts | 0.75 mg/kg feed 21 days |
[81][80] |
| ↓ Ab titer to porcine parvovirus | Wistar rats | 5 mg/kg b.w. 36 days |
[72] |
| ↓ Ab titer to swine plague | post-weaning female piglets | 1.1–3.2 mg/kg feed 18 days |
[76] |
| No effect on the splenic PFC 2 response to SRBC 1 Delayed hypersensitivity response to keyhole limpet hemocyanin |
B6C3F1 female mice | 10 mg/kg b.w. 2–8 weeks |
[79] |
In a recent review concerning the impact of
mycotoxins on human and animal host susceptibility to infectious diseases, it was shown that in contrast to other fusariotoxins, the interaction between zearalenone and infectious disease was less studied [81]. Pestka and collaborators showed that mice fed 10 mg ZEA/kg feed for 2 weeks and infected with
, registered a decreased resistance to Listeria and an increase of the bacterial count in spleen as compared with control animals [79]. It can be claimed that ZEA exposure interferes with the capacity of organism to realize an adequate immune response to vaccination and that the toxin can alter the specific antibody synthesis. Indeed, several studies have shown a decrease of antibody titer to porcine parvovirus [72] or to swine plague [76] in zearalenone intoxicated animals, but however more studies are needed in order to better understand the relation between zearalenone and the response to infectious disease.
4.2. Effect of Zearalenone on Cellular Immune Response
Beside its effect on humoral immune response, ZEA cause negative effects on cellular immune response (e.g., cell viability and proliferation, apoptosis and necrosis, and cytokine production) due to the fact that most of the cells involved in the immune response have estrogenic receptors on their surface [82]. A disturbance of cell proliferation and apoptosis was reported in a number of studies investigating ZEA toxicity. As proved by many studies zearalenone is an inductor of apoptosis and necrosis in different type of immune cells. B and T lymphocytes are among the immune cells affected by the action of ZEA. It seems in fact that the immunosuppression produced by ZEA is caused by the decrease in B and T lymphocytes viability and proliferation [66][83]. These authors reported that ZEA (0.2–1800 ng/mL) produced a reduction of peripheral lymphocytes and this was a consequence of apoptosis and cell death triggered by ZEA at the spleen level knowing that spleen is one of the most important organs for maturing lymphocytes [66][71]. The death of spleen lymphocytes leads to the decrease of the peripheral lymphocytes. Zearalenone metabolites also reduced the lymphocytes proliferation. EFSA Scientific Opinion (2011) [84] cited the work of Forsell [85] in which the proliferation of human lymphocytes stimulated with different mitogens was reduced with 50% by 3.5 μg/mL zearalenone, 6.3 μg/mL α-zearalenol, 36 μg/mL β-zearalenol, 3.8 μg/mL α-zearalanol and 33 μg/mL β-zearalanol. The inhibition of proliferation was not related to the estrogenic potential of ZEA and its derivatives, but to their structure and the presence of a single or double bond. The presence in C-6’ of a keto parent molecule (ZEA) or alpha-hydroxyl substituents (alpha-ZEL and alpha-ZAL) led to a 10-fold higher toxicity [86]. Comparing the in vitro effect of zearalenone and is derivatives α-zearalenol and β-zearalenol with that of trichothecenes on proliferation of human peripheral mononuclear cells (PBMC) [83] observed that only the high concentration of these toxins had significant immunosuppressive effect. Indeed, in vitro investigation of Vlata et al. [87] on freshly human PBMC using increased concentration of zearalenone (0.1, 1, 5, 10, 30 µg/mL) showed that the highest concentration of ZEA (30 µg/mL) inhibited the proliferation of B and T lymphocytes and induced also a necrotic effect. A clear necrotic effect was also found irrespective of cells stimulation. The study of Zhang et al. [88] demonstrated that ZEA at 10–50 μg/mL had a time and dose dependent inhibitory action on mouse thymic epithelial cells proliferation and arrested thymic cells in G2/M phase of cellular cycle. Studies performed on TM3 cells shows that low doses of ZEA increases cell proliferation [89].
ZEA decrease not only the lymphocytes viability and proliferation but also lymphocytes phenotype number. A decreased expression of T (CD3+, CD4+, CD8+), NK and B lymphocytes was observed by Salah-Abbes et al. [66], when BALB c male mice were treated with ZEA 40 mg/kg. In the same line, Swamy et al. [90] pointed out that a diet naturally contaminated with
mycotoxins, ZEA (0.4 mg/kg and 0.7mg/kg) among them decreased linearly the number of B-cells, CD3+, CD4+, CD8+ lymphocytes and NK cells in broiler chickens via the reduction of interferon-β levels and IL-2 expression. Studies in mice, rats or pigs indicated a decrease in splenic coefficients, including proliferation and cell viability, the most affected cell populations being CD4+ and CD8+.
CDs (Clusters of Differentiation) are glycoproteins expressed on the surface of the immune cells. T cells are characterized by the expression of CD3, CD4 and CD8 markers [91][92] which are involved in in the transduction of signals from T cell surfaces (CD4 and CD8), while CD3 markers activate both the cytotoxic and helper T cells. As can be seen also in
, there are conflicting data concerning the effect of ZEA on T cells subpopulations. While, most studies indicate a decrease in CD4+, CD8+ and CD3+ expression under the influence of ZEA, regardless of the animal species, other studies suggest an increase in CD4+ and CD8+ expression. However, any change in the CD4/CD8 ratio may indicate an immune dysfunction [91][92].
Effect of ZEA on cellular immune response.
| Effect (s) | Species/Cell Type | Dose/Time of ZEA Administration | References |
|---|
| ↓ CD4+, CD8+, CD11c+ in spleen ↓ CD4+, CD8+, F4/80+ and ↑ CD19+ and CD11c+ in the mesenteric lymph nodes ↑TNFα and apoptosis, ↓ IL-6 |
BALB/c mice (female, 7-week-old) |
5,20 mg/kg b.w.2 weeks |
[38][37] | ||||
| ZEA 100 and 150 mg/kg: ↓ viability of splenocytes ↓ T-cell proliferation Induce histopathological damage in spleen ZEA 150mg/kg: ↑ interleukin IL-6, IL-18 and IL-1β ↓ interferon-γ, TNFα and IL-10 in spleen |
Sprague Dawley Pregnant Rats | 50, 100, 150 mg/kg b.w. 7 days |
[70] | ||||
| disrupt the proliferation of CD4+8+ in peripheral blood cells | Polish Landrace x Polish Large White crossbreeds |
0.5 mg/kg 6 weeks |
[105][93] | ||||
| ↓ IL-1 in thymus and spleen ↓ IFN-γ in serum ↓ IL-2, IL-6, IL-10 in thymus ↓ IL-10 and IFN-γ in the spleen |
Wistar rat | 1, 5, 30 mg/kg b.w. 36 days |
[72] | ||||
| ↓ CD3+, CD4+, CD8+, CD56+ cells | BALB/c mice | 40 mg/kg b.w. 48h |
[71] | ||||
| ↓ CD4+, CD8+ cells in peripheral blood |
BPC and SPC Sheep | 3.07– 14.49 μg/kg feed | |||||
| ↓ expression of chemokine receptor CCR7 and CCR2 | BALB/cmouse splenic lymphocytes |
10, 20, 40 µM 48h |
|||||
| No effect on spleen weight | BALB/c mice female, 7-weeks-old |
5, 20 mg/kg b.w. | [107][95] | ||||
| 14 days | [ | 38 | ][37] | ↓ CD3+CD4+ T cells ↑ CD3+CD8+ T cells |
Female Kunming Mice | 20, 30 mg/kg b.w. 12 days |
[108][96] |
| No effect on spleen weight | White Leghorn female chickens, 2-weeks-old |
50, 200, 400, 800 mg/kg b.w. 7 days |
[117] | ↓ CD21+B, CD2+T, CD4+CD8−T ↑ CD8+CD4− and TCRγδ+ T |
Polish Large White female | 0.1 mg/kg 42 days |
[109][97] |
| ↓ CD4+CD8+, CD4+, CD4+/CD8+(2 mg/kg) ↑ CD8+ (3.2 mg/kg) |
Landrace × Yorkshire × Duroc Piglets |
1.1, 2, 3.2 mg/kg feed 18 days |
[76] | ||||
| ↑ IL-1β and IL-6, ↓ IFN-γ cytoplasmic edema chromatin deformation splenic damages |
Yorkshire × Landrace × Duroc Piglets |
1.1, 2.0, 3.2 mg/kg feed 18 days |
[110][98] | ||||
| ↓ IFN-γ, IL-10↓ proliferation |
kidneys of piglets | 0.8 mg/kg 4 weeks |
[111][99] | ||||
| ↑ IL-2, ↓ IL-6 | Isa Brown chicken splenic lymphocytes |
0.1–25 μg/mL 48 h |
[82] |
Induction of cellular death and proliferation inhibition was also found on other type of immune cells than lymphocytes. Viability of polymorphonuclear cells was decreased after 24h by 50 µM of ZEA and its metabolites α-ZOL, β-ZOL and ZAN action [52] and the exposure of RAW 264.7 macrophages to 10 to 100 μM ZEA for 24h diminished the cell viability in a dose dependent manner through apoptosis and necrosis [56][100].
By its estrogenic like-effects, ZEA impacts the development of reproductive organs irrespective of animal species, but with a different sensitivity of cells.
In weaned piglets, ZEA (0.5, 1.0 and 1.5 mg/kg) induces ovarian development by accelerating ovarian follicles proliferation through the activation of ERs/GSK-3β-dependent Wnt-1/β-catenin signaling pathway [101]. Also, ZEA (0.5–1.5 mg/kg) determined an abnormal uterine proliferation through TGF signaling pathway [102]. Investigating other regulatory pathways involved by ZEA in uterine hypertrophy, these authors exposed porcine endometrial epithelial cells to ZEA 0, 5, 20 and 80 μmol/L for 24 h and cell cycle was analyzed. A significant lower proportion of cells in S and G2 phases and an increase in the phase of G1 was found at ZEA 80 μmol/L [102]. The related mechanism involved also the activation of Wnt/β-catenin signaling pathway.
In mouse ovarian granulosa cells Chen et al. [103] and Zhang et al. [104] demonstrated by MTT, EdU and flow cytometry that ZEA suppressed in vitro cell viability at 30–150 μM and increased apoptosis at 15–60 μM after 24 or 72h of exposure. Close to the results recorded by Song et al. [102] in pig (a decreased of cell proportion in the S and G2 phase at ZEA 80 µmol/L), Zhang et al. [104], found that mouse granulosa cells were arrested in G1 phase of cell cycle and the cells proportion decreased in phase S and G2 after 30 μM ZEA treatment. However, in another study this author [105] found species specific ZEA effect, pig being more sensitive than mouse. Thus, ZEA 10 μM significantly increased the percentage of TUNEL porcine positive cells while the TUNEL percentage of granulosa mouse cells increased only at 30 μM.
Also, it has been observed that the metabolite of ZEA, α-ZOL at 9.4 µM concentration induces an increase in porcine granulosa cell proliferation and in progesterone levels [106].
In rats, ZEA perturb cell proliferation in both female and males. In Sprague Dawley males receiving by gavage 10 or 20 mg ZEA/kg b.w., the toxin significantly decreased the numbers of Leydig cells (adjacent cells to the testicle seminiferous tubules) which could produce anomalies of the male reproductive tract [107]. Similar results on Leydig cells were found by Wang et al. [108], with ZEA 50 μM. By contrast, Zheng et al. [89], found that low doses of zearalenone (0.01, 0.02, 0.03, 0.04, and 0.05 μmol/L) stimulated cell viability of TM3 cells (Leydig cells) measured by using the xCELLigence real-time cell analysis. Also, under the action of ZEA (20μM), cell viability of Sertoli cells derived from Male Wistar, which are important for male reproductive system, increased over control [109].
In human, study of Marton et al. [110], on ovarian epithelial cells investigating the effect of several compounds, ZEA among them on miRNA expression in correlation with cells estrogenic sensitivity observed that ZEA (1, 10, 100, 1000 nM) increased the rate of cell proliferation in direct proportion to ZEA concentration and depending on the presence of ER-α. By contrast, 30 µM of ZEA in prostate cancer cells induced G2/M cell cycle arrest and decreased cell viability compared to control [
| No macroscopic changes and no histopathologic effect on lymph nodes | |||
| 32-day-old gilts | |||
| 0.75 mg/kg feed | |||
| 21 days | |||
| [ | 81 | ] | [80] |
| No effect on thymus and spleen weights No histopathologic changes |
B6C3F1 weanling female mice | 10 mg/kg feed 56 days |
[77] |
| Decreased immune organ weight and lymphocyte counts, lymphoid atrophy and depletion in the spleen | BALB/c female mice |
40, 80 mg ZEN/kg b.w. 28 days |
[118] |
References
- Bakker, M.G.; Brown, D.W.; Kelly, A.C.; Kim, H.-S.; Kurtzman, C.P.; Mccormick, S.P.; O’Donnell, K.L.; Proctor, R.H.; Vaughan, M.M.; Ward, T.J. Fusarium mycotoxins: A trans-disciplinary overview. Can. J. Plant Pathol. 2018, 40, 161–171.
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729.
- Cavret, S.; Lecoeur, S. Fusariotoxin transfer in animal. Food Chem. Toxicol. 2006, 44, 444–453.
- Ünüsan, N. Systematic review of mycotoxins in food and feeds in Turkey. Food Control 2019, 97, 1–14.
- Aiko, V.; Mehta, A. Occurrence, detection and detoxification of mycotoxins. J. Biosci. 2015, 40, 943–954.
- Eriksen, G.S.; Pettersson, H.; Lindberg, J.E. Absorption, metabolism and excretion of 3-acetyl DON in pigs. Arch. Tierernahr. 2003, 57, 335–345.
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328.
- Rodríguez-Blanco, M.; Ramos, A.J.; Sanchis, V.; Marín, S. Mycotoxins occurrence and fungal populations in different types of silages for dairy cows in Spain. Fungal Biol. 2019.
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56.
- Maragos, C. Zearalenone occurrence and human exposure. World Mycotoxin J. 2010, 3.
- Złoch, M.; Rogowska, A.; Pomastowski, P.; Railean-Plugaru, V.; Walczak-Skierska, J.; Rudnicka, J.; Buszewski, B. Use of Lactobacillus paracasei strain for zearalenone binding and metabolization. Toxicon 2020, 181, 9–18.
- Jia, R.; Liu, W.; Zhao, L.; Cao, L.; Shen, Z. Low doses of individual and combined deoxynivalenol and zearalenone in naturally moldy diets impair intestinal functions via inducing inflammation and disrupting epithelial barrier in the intestine of piglets. Toxicol. Lett. 2020, 333, 159–169.
- Zhou, J.; Zhu, L.; Chen, J.; Wang, W.; Zhang, R.; Li, Y.; Zhang, Q.; Wang, W. Degradation mechanism for Zearalenone ring-cleavage by Zearalenone hydrolase RmZHD: A QM/MM study. Sci. Total Environ. 2020, 709, 135897.
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149.
- Busk, Ø.L.; Ndossi, D.; Verhaegen, S.; Connolly, L.; Eriksen, G.; Ropstad, E.; Sørlie, M. Relative quantification of the proteomic changes associated with the mycotoxin zearalenone in the H295R steroidogenesis model. Toxicon 2011, 58, 533–542.
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18.
- Rogowska, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Złoch, M.; Walczak, J.; Buszewski, B. A study of zearalenone biosorption and metabolisation by prokaryotic and eukaryotic cells. Toxicon 2019, 169, 81–90.
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497 LP–516.
- Marin, D.E.; Taranu, I.; Burlacu, R.; Manda, G.; Motiu, M.; Neagoe, I.; Dragomir, C.; Stancu, M.; Calin, L. Effects of zearalenone and its derivatives on porcine immune response. Toxicol. Vitr. 2011, 25, 1981–1988.
- Chang, S.; Su, Y.; Sun, Y.; Meng, X.; Shi, B.; Shan, A. Response of the nuclear receptors PXR and CAR and their target gene mRNA expression in female piglets exposed to zearalenone. Toxicon 2018, 151, 111–118.
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68.
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20.
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834.
- Lahjouji, T.; Bertaccini, A.; Neves, M.; Puel, S.; Oswald, I.P.; Soler, L. Acute Exposure to Zearalenone Disturbs Intestinal Homeostasis by Modulating the Wnt/β-Catenin Signaling Pathway. Toxins 2020, 12, 113.
- Lewczuk, B.; Przybylska-Gornowicz, B.; Gajęcka, M.; Targońska, K.; Ziółkowska, N.; Prusik, M.; Gajęcki, M. Histological structure of duodenum in gilts receiving low doses of zearalenone and deoxynivalenol in feed. Exp. Toxicol. Pathol. 2016, 68, 157–166.
- Gajęcka, M.; Tarasiuk, M.; Zielonka, Ł.; Dąbrowski, M.; Gajęcki, M. Risk assessment for changes in the metabolic profile and body weights of pre-pubertal gilts during long-term monotonic exposure to low doses of zearalenone (ZEN). Res. Vet. Sci. 2016, 109, 169–180.
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic Effects of Maternal Zearalenone Exposure on Intestinal Oxidative Stress, Barrier Function, Immunological and Morphological Changes in Rats. PLoS One 2014, 9, 1–14.
- Zheng, W.-L.; Wang, B.-J.; Wang, L.; Shan, Y.-P.; Zou, H.; Song, R.-L.; Wang, T.; Gu, J.-H.; Yuan, Y.; Liu, X.-Z.; et al. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24.
- Johansson, M.E.V.; Hansson, G.C. Mucus and the Goblet Cell. Dig. Dis. 2013, 31, 305–309.
- Wang, X.; Yu, H.; Fang, H.; Zhao, Y.; Jin, Y.; Shen, J.; Zhou, C.; Zhou, Y.; Fu, Y.; Wang, J.; et al. Transcriptional profiling of zearalenone-induced inhibition of IPEC-J2 cell proliferation. Toxicon 2019, 172, 8–14.
- Marin, D.; Motiu, M.; Taranu, I. Food Contaminant Zearalenone and Its Metabolites Affect Cytokine Synthesis and Intestinal Epithelial Integrity of Porcine Cells. Toxins 2015, 7, 1979–1988.
- Taranu, I.; Marin, D.E.; Pistol, G.C.; Motiu, M.; Pelinescu, D. Induction of pro-inflammatory gene expression by Escherichia coli and mycotoxin zearalenone contamination and protection by a Lactobacillus mixture in porcine IPEC-1 cells. Toxicon 2015, 97, 53–63.
- Fan, W.; Lv, Y.; Ren, S.; Shao, M.; Shen, T.; Huang, K.; Zhou, J.; Yan, L.; Song, S. Zearalenone (ZEA)-induced intestinal inflammation is mediated by the NLRP3 inflammasome. Chemosphere 2018, 190, 272–279.
- Long, M.; Chen, X.; Wang, N.; Wang, M.; Pan, J.; Tong, J.; Li, P.; Yang, S.; He, J. Proanthocyanidins Protect Epithelial Cells from Zearalenone-Induced Apoptosis via Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis Pathways in Mouse Small Intestines. Molecules 2018, 23, 1508.
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. Crocin and Quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress Chaperones 2015, 20, 927–938.
- Obremski, K.; Gonkowski, S.; Wojtacha, P. Zearalenone-induced changes in the lymphoid tissue and mucosal nerve fibers in the porcine ileum. Pol. J. Vet. Sci. 2015, 18, 357–365.
- Islam, M.R.; Kim, J.W.; Roh, Y.-S.; Kim, J.-H.; Han, K.M.; Kwon, H.-J.; Lim, C.W.; Kim, B. Evaluation of immunomodulatory effects of zearalenone in mice. J. Immunotoxicol. 2017, 14, 125–136.
- Kato, L.M.; Kawamoto, S.; Maruya, M.; Fagarasan, S. Gut TFH and IgA: Key players for regulation of bacterial communities and immune homeostasis. Immunol. Cell Biol. 2014, 92, 49–56.
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5.
- Wang, X.; Yu, H.; Shan, A.; Jin, Y.; Fang, H.; Zhao, Y.; Shen, J.; Zhou, C.; Zhou, Y.; Fu, Y.; et al. Toxic effects of Zearalenone on intestinal microflora and intestinal mucosal immunity in mice. Food Agric. Immunol. 2018, 29, 1002–1011.
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197.
- Wan, L.-Y.M.; Allen, K.J.; Turner, P.C.; El-Nezami, H. Modulation of Mucin mRNA (MUC5AC and MUC5B) Expression and Protein Production and Secretion in Caco-2/HT29-MTX Co-cultures Following Exposure to Individual and Combined Fusarium Mycotoxins. Toxicol. Sci. 2014, 139, 83–98.
- Wan, M.L.-Y.; Woo, C.-S.J.; Allen, K.J.; Turner, P.C.; El-Nezami, H. Modulation of Porcine β-Defensins 1 and 2 upon Individual and Combined Fusarium Toxin Exposure in a Swine Jejunal Epithelial Cell Line. Appl. Environ. Microbiol. 2013, 79, 2225–2232.
- Taranu, I.; Braicu, C.; Marin, D.E.; Pistol, G.C.; Motiu, M.; Balacescu, L.; Beridan Neagoe, I.; Burlacu, R. Exposure to zearalenone mycotoxin alters in vitro porcine intestinal epithelial cells by differential gene expression. Toxicol. Lett. 2015, 232, 310–325.
- Braicu, C.; Selicean, S.; Cojocneanu-Petric, R.; Lajos, R.; Balacescu, O.; Taranu, I.; Marin, D.E.; Motiu, M.; Jurj, A.; Achimas-Cadariu, P.; et al. Evaluation of cellular and molecular impact of zearalenone and Escherichia coli co-exposure on IPEC-1 cells using microarray technology. BMC Genomics 2016, 17, 576.
- Benthem de Grave, X.; Saltzmann, J.; Laurain, J.; Rodriguez, M.A.; Molist, F.; Dänicke, S.; Santos, R.R. Transmission of Zearalenone, Deoxynivalenol, and Their Derivatives from Sows to Piglets during Lactation. Toxins 2021, 13, 37.
- Medzhitov, R.; Janeway, C.J. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97.
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513.
- Edreva, A. Generation and scavenging of reactive oxygen species in chloroplasts: a submolecular approach. Agric. Ecosyst. Environ. 2005, 106, 119–133.
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxid. Med. Cell. Longev. 2016, 2016, 1580967.
- Wang, J.; Wei, Z.; Han, Z.; Liu, Z.; Zhu, X.; Li, X.; Wang, K.; Yang, Z. Zearalenone Induces Estrogen-Receptor-Independent Neutrophil Extracellular Trap Release in Vitro. J. Agric. Food Chem. 2019, 67.
- Marin, D.E.; Taranu, I.; Burlacu, R.; Tudor, D.S. Effects of zearalenone and its derivatives on the innate immune response of swine. Toxicon 2010, 56, 956–963.
- Murata, H.; Sultana, P.; Shimada, N.; Yoshioka, M. Structure-activity relationships among zearalenone and its derivatives based on bovine neutrophil chemiluminescence. Vet. Hum. Toxicol. 2003, 45, 18—20.
- Borutova, R.; Faix, S.; Placha, I.; Gresakova, L.; Cobanova, K.; Leng, L. Effects of deoxynivalenol and zearalenone on oxidative stress and blood phagocytic activity in broilers. Arch. Anim. Nutr. 2008, 62, 303–312.
- Solhaug, A.; Karlsøen, L.M.; Holme, J.A.; Kristoffersen, A.B.; Eriksen, G.S. Immunomodulatory effects of individual and combined mycotoxins in the THP-1 cell line. Toxicol. Vitr. 2016, 36, 120–132.
- Chen, F.; Li, Q.; Zhang, Z.; Lin, P.; Lei, L.; Wang, A.; Jin, Y. Endoplasmic Reticulum Stress Cooperates in Zearalenone-Induced Cell Death of RAW 264.7 Macrophages. Int. J. Mol. Sci. 2015, 16, 19780–19795.
- Hueza, I.; Raspantini, P.; Raspantini, L.; Latorre, A.; Górniak, S. Zearalenone, an Estrogenic Mycotoxin, Is an Immunotoxic Compound. Toxins 2014, 6, 1080–1095.
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajęcka, M.; Gajęcki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463.
- Oswald, I.I.; Bouhet, S.; Marin, D.E.; Pinton, P.P.; Taranu, I. Mycotoxin effects on the pig immune system. Feed Compd. 2003, 09, 16–20.
- Peveri, P.; Walz, A.; Dewald, B.; Baggiolini, M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J. Exp. Med. 1988, 167, 1547–1559.
- Ding, J.; Yeh, C.-R.; Sun, Y.; Lin, C.; Chou, J.; Ou, Z.; Chang, C.; Qi, J.; Yeh, S. Estrogen receptor β promotes renal cell carcinoma progression via regulating LncRNA HOTAIR-miR-138/200c/204/217 associated CeRNA network. Oncogene 2018, 37, 5037–5053.
- Ruh, M.F.; Bi, Y.; Cox, L.; Berk, D.; Howlett, A.C.; Bellone, C.J. Effect of environmental estrogens on IL-1beta promoter activity in a macrophage cell line. Endocrine 1998, 9, 207–211.
- Seyed Toutounchi, N.; Hogenkamp, A.; Varasteh, S.; van’t Land, B.; Garssen, J.; Kraneveld, A.D.; Folkerts, G.; Braber, S. Fusarium Mycotoxins Disrupt the Barrier and Induce IL-6 Release in a Human Placental Epithelium Cell Line. Toxins 2019, 11, 665.
- Pistol, G.C.; Gras, M.A.; Marin, D.E.; Israel-Roming, F.; Stancu, M.; Taranu, I. Natural feed contaminant zearalenone decreases the expressions of important pro- and anti-inflammatory mediators and mitogen-activated protein kinase/NF-κB signalling molecules in pigs. Br. J. Nutr. 2014, 111, 452–464.
- Abbès, S.; Salah-Abbès, J.B.; Sharafi, H.; Noghabi, K.A.; Oueslati, R. Interaction of Lactobacillus plantarum MON03 with Tunisian Montmorillonite clay and ability of the composite to immobilize Zearalenone in vitro and counteract immunotoxicity in vivo. Immunopharmacol. Immunotoxicol. 2012, 34, 944–950.
- Salah-Abbès, J.; Abbes, S.; Houas, Z.; Abdel-Wahhab, P.M.; Oueslati, R. Zearalenone induces immunotoxicity in mice: Possible protective effects of Radish extract (Raphanus Sativus). J. Pharm. Pharmacol. 2008, 60, 761–770.
- Ben Salah-Abbès, J.; Belgacem, H.; Ezzdini, K.; Abdel-Wahhab, M.A.; Abbès, S. Zearalenone nephrotoxicity: DNA fragmentation, apoptotic gene expression and oxidative stress protected by Lactobacillus plantarum MON03. Toxicon 2020, 175, 28–35.
- Del Fabbro, L.; Jesse, C.R.; de Gomes, M.G.; Borges Filho, C.; Donato, F.; Souza, L.C.; Goes, A.R.; Furian, A.F.; Boeira, S.P. The flavonoid chrysin protects against zearalenone induced reproductive toxicity in male mice. Toxicon 2019, 165, 13–21.
- Virk, P.; Al-mukhaizeem, N.A.R.; Bin Morebah, S.H.; Fouad, D.; Elobeid, M. Protective effect of resveratrol against toxicity induced by the mycotoxin, zearalenone in a rat model. Food Chem. Toxicol. 2020, 146, 111840.
- Yin, S.; Zhang, Y.; Gao, R.; Cheng, B.; Shan, A. The immunomodulatory effects induced by dietary Zearalenone in pregnant rats. Immunopharmacol. Immunotoxicol. 2014, 36, 187–194.
- Abbès, S.; Salah-Abbès, J.B.; Ouanes, Z.; Houas, Z.; Othman, O.; Bacha, H.; Abdel-Wahhab, M.A.; Oueslati, R. Preventive role of phyllosilicate clay on the Immunological and Biochemical toxicity of zearalenone in Balb/c mice. Int. Immunopharmacol. 2006, 6, 1251–1258.
- Choi, B.-K.; Cho, J.-H.; Jeong, S.-H.; Shin, H.-S.; Son, S.-W.; Yeo, Y.-K.; Kang, H.-G. Zearalenone affects immune-related parameters in lymphoid organs and serum of rats vaccinated with porcine parvovirus vaccine. Toxicol. Res. 2012, 28, 279–288.
- Wu, F.; Cui, J.; Yang, X.; Liu, S.; Han, S.; Chen, B. Effects of zearalenone on genital organ development, serum immunoglobulin, antioxidant capacity, sex hormones and liver function of prepubertal gilts. Toxicon 2021, 189, 39–44.
- Reddy, K.; Lee, W.; Lee, S.; Jeong, J.; Kim, D.; Kim, M.; Lee, H.; Oh, Y.; Jo, H. Effects of dietary deoxynivalenol and zearalenone on the organ pro-inflammatory gene expressions and serum immunoglobulins of pigs. J. Anim. Sci. 2017, 95, 203.
- Ren, Z.H.; Zhou, R.; Deng, J.L.; Zuo, Z.C.; Peng, X.; Wang, Y.C.; Wang, Y.; Yu, S.M.; Shen, L.H.; Cui, H.M.; et al. Effects of the Fusarium toxin zearalenone (ZEA) and/or deoxynivalenol (DON) on the serum IgA, IgG and IgM levels in mice. Food Agric. Immunol. 2014, 25, 600–606.
- Yang, L.; Yang, W.; Feng, Q.; Huang, L.; Zhang, G.; Liu, F.; Jiang, S.; Yang, Z. Effects of purified zearalenone on selected immunological measurements of blood in post-weaning gilts. Anim. Nutr. 2016, 2, 142–148.
- Forsell, J.H.; Witt, M.F.; Tai, J.-H.; Jensen, R.; Pestka, J.J. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem. Toxicol. 1986, 24, 213–219.
- Jakovac-Strajn, B.; Vengušt, A.; Pestevšek, U. Effects of a deoxynivalenol-contaminated diet on the reproductive performance and immunoglobulin concentrations in pigs. Vet. Rec. 2009, 165, 713–718.
- Pestka, J.J.; Tai, J.-H.; Witt, M.F.; Dixon, D.E.; Forsell, J.H. Suppression of immune response in the B6C3F1 mouse after dietary exposure to the fusarium mycotoxins deoxynivalenol (vomitoxin) and zearalenone. Food Chem. Toxicol. 1987, 25, 297–304.
- Teixeira, L.C.; Montiani-Ferreira, F.; Locatelli-Dittrich, R.; Santin, E.; Alberton, G.C. Effects of zearalenone in prepubertal gilts. Pesqui. Veterinária Bras. 2011, 31, 656–662.
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The Impact of Fusarium Mycotoxins on Human and Animal Host Susceptibility to Infectious Diseases. Toxins 2014, 6.
- Wang, Y.C.; Deng, J.L.; Xu, S.W.; Peng, X.; Zuo, Z.C.; Cui, H.M.; Wang, Y.; Ren, Z.H. Effects of Zearalenone on IL-2, IL-6, and IFN-? mRNA Levels in the Splenic Lymphocytes of Chickens. Sci. World J. 2012, 2012, 567327.
- Berek, L.; Petri, I.B.; Mesterházy, Á.; Téren, J.; Molnár, J. Effects of mycotoxins on human immune functions in vitro. Toxicol. Vitr. 2001, 15, 25–30.
- Committee, E.S. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA J. 2011, 9, 2379.
- Forsell, J.H.; Kateley, J.R.; Yoshizawa, T.; Pestka, J.J. Inhibition of mitogen-induced blastogenesis in human lymphocytes by T-2 toxin and its metabolites. Appl. Environ. Microbiol. 1985, 49, 1523 LP–1526.
- Forsell, J.H.; Pestka, J.J. Relation of 8-ketotrichothecene and zearalenone analog structure to inhibition of mitogen-induced human lymphocyte blastogenesis. Appl. Environ. Microbiol. 1985, 50, 1304 LP–1307.
- Vlata, Z.; Porichis, F.; Tzanakakis, G.; Tsatsakis, A.; Krambovitis, E. A study of zearalenone cytotoxicity on human peripheral blood mononuclear cells. Toxicol. Lett. 2006, 165, 274–281.
- Zhang, K.; Tan, X.; Li, Y.; Liang, G.; Ning, Z.; Ma, Y.; Li, Y. Transcriptional profiling analysis of Zearalenone-induced inhibition proliferation on mouse thymic epithelial cell line 1. Ecotoxicol. Environ. Saf. 2018, 153, 135–141.
- Zheng, W.; Fan, W.; Feng, N.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Bai, J.; Bian, J.; et al. The Role of miRNAs in Zearalenone-Promotion of TM3 Cell Proliferation. Int. J. Environ. Res. Public Health 2019, 16, 1517.
- Swamy, H.V.L.N.; Smith, T.K.; Karrow, N.A.; Boermans, H.J. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on growth and immunological parameters of broiler chickens1. Poult. Sci. 2004, 83, 533–543.
- Delves, P.J.; Martin, S.J.; Burton, D.R.; Roitt, I.M. Roitt’s Essential Immunology, 13th ed.; Wiley-Blackwell: London, UK, 2017; ISBN 978-1-118-41577-1.
- Roitt, I. Roitt‘s Essential Immunology; Blackwell Publishing: London, UK, 2006.
- Dąbrowski, M.; Obremski, K.; Gajęcka, M.; Gajęcki, M.; Zielonka, Ł. Changes in the Subpopulations of Porcine Peripheral Blood Lymphocytes Induced by Exposure to Low Doses of Zearalenone (ZEN) and Deoxynivalenol (DON). Molecules 2016, 21, 557.
- Kostro, K.; Gajęcka, M.; Lisiecka, U.; Majer-Dziedzic, B.; Obremski, K.; Zielonka, Ł.; Gajęcki, M. Subpopulation of lymphocytes CD4 + and CD8 + in peripheral blood of sheep with zearalenone mycotoxicosis. Bull. Vet. Inst. Pulawy 2011, 55, 241–246.
- Cai, G.; Pan, S.; Feng, N.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Zearalenone inhibits T cell chemotaxis by inhibiting cell adhesion and migration related proteins. Ecotoxicol. Environ. Saf. 2019, 175, 263–271.
- Ren, Z.H.; Deng, H.D.; Wang, Y.C.; Deng, J.L.; Zuo, Z.C.; Wang, Y.; Peng, X.; Cui, H.M.; Fang, J.; Yu, S.M.; et al. The Fusarium toxin zearalenone and deoxynivalenol affect murine splenic antioxidant functions, interferon levels, and T-cell subsets. Environ. Toxicol. Pharmacol. 2016, 41, 195–200.
- Obremski, K.; Wojtacha, P.; Podlasz, P.; Żmigrodzka, M. The influence of experimental administration of low zearalenone doses on the expression of Th1 and Th2 cytokines and on selected subpopulations of lymphocytes in intestinal lymph nodes. Pol. J. Vet. Sci. 2015, 18, 489–497.
- Chen, P.; Liu, T.; Jiang, S.; Yang, Z.; Huang, L.; Liu, F. Effects of purified zearalenone on selected immunological and histopathologic measurements of spleen in post-weanling gilts. Anim. Nutr. 2017, 3, 212–218.
- Reddy, K.E.; Lee, W.; Jeong, J.Y.; Lee, Y.; Lee, H.-J.; Kim, M.S.; Kim, D.-W.; Yu, D.; Cho, A.; Oh, Y.K.; et al. Effects of deoxynivalenol- and zearalenone-contaminated feed on the gene expression profiles in the kidneys of piglets. Asian-Australas. J. Anim. Sci. 2018, 31, 138–148.
- Yu, J.-Y.; Zheng, Z.-H.; Son, Y.-O.; Shi, X.; Jang, Y.-O.; Lee, J.-C. Mycotoxin zearalenone induces AIF- and ROS-mediated cell death through p53- and MAPK-dependent signaling pathways in RAW264.7 macrophages. Toxicol. Vitr. 2011, 25, 1654–1663.
- Yang, L.-J.; Zhou, M.; Huang, L.-B.; Yang, W.-R.; Yang, Z.-B.; Jiang, S.-Z.; Ge, J.-S. Zearalenone-Promoted Follicle Growth through Modulation of Wnt-1/β-Catenin Signaling Pathway and Expression of Estrogen Receptor Genes in Ovaries of Postweaning Piglets. J. Agric. Food Chem. 2018, 66, 7899–7906.
- Song, T.; Yang, W.; Huang, L.; Yang, Z.; Jiang, S. Zearalenone exposure affects the Wnt/β-catenin signaling pathway and related genes of porcine endometrial epithelial cells in vitro. Asian-Australas J. Anim. Sci. 2020.
- Chen, F.; Wen, X.; Lin, P.; Chen, H.; Wang, A.; Jin, Y. HERP depletion inhibits zearalenone-induced apoptosis through autophagy activation in mouse ovarian granulosa cells. Toxicol. Lett. 2019, 301, 1–10.
- Zhang, G.-L.; Song, J.-L.; Zhou, Y.; Zhang, R.-Q.; Cheng, S.-F.; Sun, X.-F.; Qin, G.-Q.; Shen, W.; Li, L. Differentiation of sow and mouse ovarian granulosa cells exposed to zearalenone in vitro using RNA-seq gene expression. Toxicol. Appl. Pharmacol. 2018, 350, 78–90.
- Zhang, R.Q.; Sun, X.F.; Wu, R.Y.; Cheng, S.F.; Zhang, G.L.; Zhai, Q.Y.; Liu, X.L.; Zhao, Y.; Shen, W.; Li, L. Zearalenone exposure elevated the expression of tumorigenesis genes in mouse ovarian granulosa cells. Toxicol. Appl. Pharmacol. 2018, 356, 191–203.
- Cortinovis, C.; Caloni, F.; Schreiber, N.B.; Spicer, L.J. Effects of fumonisin B1 alone and combined with deoxynivalenol or zearalenone on porcine granulosa cell proliferation and steroid production. Theriogenology 2014, 81, 1042–1049.
- Pan, P.; Ma, F.; Wu, K.; Yu, Y.; Li, Y.; Li, Z.; Chen, X.; Huang, T.; Wang, Y.; Ge, R. Maternal exposure to zearalenone in masculinization window affects the fetal Leydig cell development in rat male fetus. Environ. Pollut. 2020, 263, 114357.
- Wang, S.; Ren, X.; Hu, X.; Zhou, L.; Zhang, C.; Zhang, M. Cadmium-induced apoptosis through reactive oxygen species-mediated mitochondrial oxidative stress and the JNK signaling pathway in TM3 cells, a model of mouse Leydig cells. Toxicol. Appl. Pharmacol. 2019, 368, 37–48.
- Cai, G.; Si, M.; Li, X.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Zearalenone induces apoptosis of rat Sertoli cells through Fas-Fas ligand and mitochondrial pathway. Environ. Toxicol. 2019, 34, 424–433.
- Márton, É.; Varga, A.; Széles, L.; Göczi, L.; Penyige, A.; Nagy, B.; Szilágyi, M. The Cell-Free Expression of MiR200 Family Members Correlates with Estrogen Sensitivity in Human Epithelial Ovarian Cells. Int. J. Mol. Sci. 2020, 21, 9725.
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Domińska, K.; Urbanek, K.A.; Piastowska-Ciesielska, A.W. ERβ and NFκB—Modulators of Zearalenone-Induced Oxidative Stress in Human Prostate Cancer Cells. Toxins 2020, 12, 199.
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and Reproductive Function in Farm Animals. Int. J. Mol. Sci. 2008, 9, 2570–2584.
- Chi, M.S.; Mirocha, C.J.; Kurtz, H.J.; Weaver, G.A.; Bates, F.; Robison, T.; Shimoda, W. Effect of Dietary Zearalenone on Growing Broiler Chicks1,2. Poult. Sci. 1980, 59, 531–536.
- Allen, N.K.; Mirocha, C.J.; Weaver, G.; Aakhus-Allen A, S.; Bates, F. Effects of Dietary Zearalenone on Finishing Broiler Chickens and Young Turkey Poults1,2. Poult. Sci. 1981, 60, 124–131.
- Kiessling, K.-H. The Effect of Zearalenone on Growth Rate, Organ Weight and Muscle Fibre Composition in Growing Rats. Acta Pharmacol. Toxicol. (Copenh). 1982, 51, 154–158.
- Denli, M.; Blandon, J.C.; Salado, S.; Guynot, M.E.; Pérez, J.F. Effect of dietary zearalenone on the performance, reproduction tract and serum biochemistry in young rats. J. Appl. Anim. Res. 2017, 45, 619–622.
- Chi, M.S.; Mirocha, C.J.; Weaver, G.A.; Kurtz, H.J. Effect of zearalenone on female White Leghorn chickens. Appl. Environ. Microbiol. 1980, 39, 1026–1030.
- Salah-Abbès, J.; Abbes, S.; Abdel-Wahhab, P.M.; Oueslati, R. Immunotoxicity of zearalenone in Balb/c mice in a high subchronic dosing study counteracted by Raphanus sativus extract. Immunopharmacol. Immunotoxicol. 2010, 32, 628–636.
