When presented with an animal exhibiting signs of keratitis (inflammation of the cornea), such as impaired vision, mucoid discharges, redness, swelling, and corneal oedema, most veterinarians would think of bacteria, viruses, or fungi as the potential causative agent(s). Evidence has arisen in recent years of a possible connection between the protozoan Acanthamoeba and keratitis in animals. Acanthamoeba infection is underdiagnosed, but potentially common, in animals.
- Acanthamoeba keratitis
- animals
- epidemiology
- diagnostics
- treatment
1. Introduction
Acanthamoeba keratitis (AK) is a rare parasitic disease caused by acute infection with trophozoites of the opportunistic protozoan Acanthamoeba castellanii (Figure 1), which can have serious adverse health effects on infected individuals. Other Acanthamoeba spp., such as A. culbertsoni, A. hatchetti, A. griffini, A. rhysodes, A. mauritaniensis, and A. lugdunensis of the T4 genotype, as well as genotypes T1, T2, T3, T5, T6, and T11, can be also associated with ocular infections [1]. Clinical symptoms generally include eye pain and photophobia, however severe disease can lead to blindness if left untreated [2]. Acanthamoeba spp. are distributed throughout the world and can be established in a wide variety of environmental habitats including soil, mud water reservoirs and hospitals, providing numerous opportunities for contacts with animals and humans. Human exposure to these organisms can be very high, as shown by antibody titers in surveyed populations. For example, a London-based study found that more than 87.7% of the study participants (11 out of 114 healthy asymptomatic individuals) were positive for the presence of secretory anti-A. castellanii IgA antibody in their saliva [3]. However, having antibodies to A. castellanii does not necessarily indicate an active infection, as antibodies develop in individuals who have been exposed to A. castellanii previously. In developed countries, most cases of AK are related to contact lens use. However, in developing countries, AK can also be associated with corneal trauma [4].
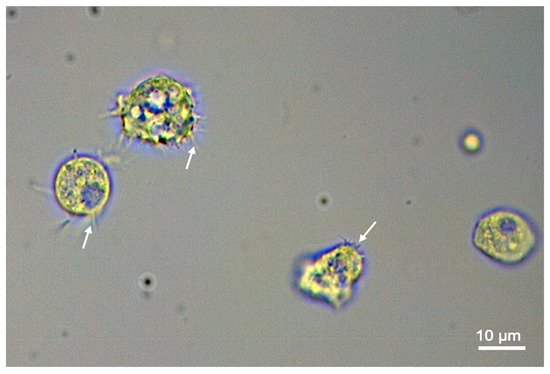
Figure 1.
Acanthamoeba castellanii
Acanthamoeba spp. have been reported in a variety of animal species, including monkeys, dogs, a kangaroo, and an Indian buffalo [5] and even in aquatic animals, such as fish, amphibian and reptiles [6][7][8][6,7,8]. An earlier report showed that Acanthamoeba can kill mice and monkeys [9]. Several animal models, such as rat, hamster, rabbit and micropig, have been developed to investigate the pathogenesis of AK by infecting these animals with Acanthamoeba via intrastromal injections or scratching the corneal surface before placing contaminated contact lenses over the eye [10][11][10,11]. Other free-living amoebae with neuropathogenic potential exist including Balamuthia mandrillaris [12], which has been detected in dogs, a horse, a sheep and non-human primates [13].
Although evidence for zoonotic transmission is lacking, given the limited host specificity, the similarity between humans and some animals in the binding affinity of Acanthamoeba to corneal tissue [14], and the proximity between humans and animals in many settings, it is essential to be aware of the epidemiology, pathogenesis, diagnosis and treatment options for this condition in animals. However, the current status of animals infected with Acanthamoeba is unknown and the literature exploring the significance of AK in animals is very limited.
2. Can Animals Be Affected by AK?
Factors such as being a stray or having a compromised immune status are more likely to predispose animals to Acanthamoeba, because it is an opportunistic organism. A previous study found 2 out of 55 (3.6%) cats examined to be Acanthamoeba positive when corneal scrapings were cultured on non-nutrient agar (NNA) plates. No Acanthamoeba cases were detected using direct molecular (18S rRNA gene-based Polymerase Chain Reaction [PCR]) analysis. However, genotyping of the culture-positive samples revealed that one of the positive cats had the T2 genotype, while the other cat had the T4 genotype, highlighting the potential risk of infection for humans and animals. Both of these cats were immunocompromised; one tested positive for feline immunodeficiency virus (FIV) and the other for feline leukaemia virus (FeLV) [15]. None of the infected cats showed any of the characteristic clinical signs of AK seen in humans. However, Acanthamoeba is detected in cats with ocular pathologies in Switzerland [16] and Malaysia [17]. A previous study reported that 14.6% (27 out of 184) of the tested conjunctival swab samples from the eyes of stray dogs were positive for Acanthamoeba [18]; although 7 out of 27 dogs with evidence of Acanthamoeba did show signs of ocular disease such as conjunctivitis and keratitis. Neither of the studies conducted by Montoya et al. [15] or Karakuş et al. [18] obtained a representative sample of the studied population; both obtained their candidates from local organisations which had animals available. Montoya et al. [15] took advantage of a health check and neutering programme where stray cats were collected and registered every Spring; whereas Karakuş et al. [18] used a previous study sample of stray dogs’ conjunctival swabs taken from eight villages where canine leishmaniasis was endemic. Given the nature of both studies, this sampling method seems to be appropriate and the most practical option for sampling animals, including strays which are at greater risk of AK.
Acanthamoeba infections have also been detected in wild predatory birds in Turkey [19]. Corneal samples were acquired from 18 dead wild birds and 16.6% (3 out of 18) were identified as positive for Acanthamoeba using PCR, while 5.5% (1 out of 18) tested positive using the agar plate method. Two strains, T4 and T5, were identified from the DNA sequencing data. While the sample size of this study was not large enough to make the data about prevalence and importance of AK in birds conclusive, it did prove that Acanthamoeba exists in wild bird populations. These studies (Table 1) provide evidence of the possibility that animals can develop AK or extraocular disseminated acanthamoebiasis. Infection rates with Acanthamoeba may be higher in animals prone to fighting and acquiring corneal injuries; foreign particles contaminated with Acanthamoeba can become lodged in the cat’s eye leading to AK, as has been suggested previously [17].
Table 1. Representative reports of natural Acanthamoeba infections in animals.
Animal Species | Samples (n) | Country | Diagnostic Method | Infection Rate | Reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Stray cats | 307 | Spain | Culturing on non-nutrient agar (NNA) plates and | 18S rRNA gene-based PCR | 3.6% positive by culture and 0% by PCR |
[15] |
|||||||||||
Stray dogs | 184 | Turkey | 18S rRNA gene-based PCR | 14.6% by PCR |
[18] |
||||||||||||
Deceased wild birds | 18 | Turkey | Culturing on NNA plates and 18S rRNA gene-based PCR | 5.5% positive by NNA culture and 16.6% by PCR |
[19] |
3. Clinical Signs
AK may be the main presentation of Acanthamoeba in humans, although other multi-systemic manifestations have been reported in two dogs including encephalitis, ascites and peritonitis, related to the T4 genotype strain infection [20]. Other studies reported a range of symptoms including anorexia, pyrexia, secretions from nose and eyes, limb stiffness and neurological manifestations that suggest Acanthamoeba infections are not confined to the eye and can indeed lead to disease in other body systems in dogs [21][22][23][21,22,23].
Many biological, immunological and ecological factors define the ability of Acanthamoeba species to infect a particular host. Of these, an ability to bind to, and interact with, the corneal epithelial tissue can be a key factor in dictating the level of pathogenicity of Acanthamoeba species. Acanthamoeba does not interact in the same way with corneal epithelium of humans, hamsters and pigs as it does with a range of other species such as chickens, rabbits, rodents, guinea pigs, dogs and horses [14]. This differential selectivity to binding corneal tissue may be attributed to species-specific differences in the mannose residues on the surface of the cornea [24]; however, the precise mechanisms remain unknown. As a result, the clinical signs of AK in animals may differ from those in humans, which may explain the under-reporting of Acanthamoeba-infected animals. However, remarkable similarities in the clinical and histopathologic features of contact lens-induced AK have been demonstrated in a micropig model and humans [11]. If Acanthamoeba cannot be detected by clinical examination, many individuals who come into contact with animals, either for occupational or pet ownership reasons, may not be aware that their animal is infected with the pathogenic T4 genotype which could, potentially, be transmitted to the individual and other animals.
4. Available Diagnostics
Currently, diagnosis of AK in humans is based on history and initial clinical examination. A characteristic sign of Acanthamoeba is the prominent white ring around the cornea [25]. Nonetheless, diagnosis is difficult due to the rarity of the disease and the non-specific nature of clinical signs [26]. Differential diagnoses often include herpes keratitis in the earlier stages and fungal keratitis in the later stages [27]. Misdiagnosis has detrimental outcomes for the patient; with inappropriate treatment leading to an increased severity of infection and, in extreme cases, surgical intervention, keratoplasty, is required [25]. A 2-year case report study found that 69% (73/106) of individuals with AK were not initially diagnosed [28]. A patient’s history (e.g., management of their contact lens or corneal injury) can provide important clues to help distinguish between differential diagnoses, and to choose the most suitable laboratory methods for the detection and identification of the causative agent [25]. In the context of veterinary medicine, it would be important to take note of the animal’s recent travel destinations, immune- and feral-status.
Diagnostic tools include direct microscopic examination of corneal scrapings [15], molecular tests and culturing [18][19][18,19]. Optimal specimens for laboratory diagnostic tests include corneal scrapings [15] or conjunctival swabs [18] of the infected eye. Wet microscopic mounts on a warmed slide are useful for examining the specimen [20][23][25][20,23,25]. However, a subsequent confirmatory laboratory diagnosis is required as microscopic diagnosis can be subjective [29] with the possibility of human error in confirming that the observed rounded structures are indeed Acanthamoeba trophozoites and cysts. Isolation of Acanthamoeba can be achieved using a non-nutrient agar plate seeded with Escherichia coli [15][18][25][15,18,25] or, in axenic cultures, in protease peptone-yeast extract-glucose media [12]. However, these conventional techniques may lack sensitivity and the true Acanthamoeba prevalence may be underestimated [29]. Culturing may also take several days before results can be analysed [29] and an ideal diagnostic tool needs to provide results relatively quickly to address appropriate treatment options.
Molecular biology methods, such as PCR, that amplify the nucleic acids of Acanthamoeba have been developed to enhance the management of amoebic infections. Although PCR can detect the presence of Acanthamoeba, a positive PCR result is not necessarily indicative of an active infection as genetic information from viable and nonviable organisms can both show up as positive. The fact that Acanthamoeba can be present in the eyes of cats with [16][17][16,17] or without [15] AK, and in the corneas of wild birds with or without keratitis [19], further substantiates this assumption. Future research should explore the correlation between the presence of Acanthamoeba organisms in the eye of a given animal and the risk of developing AK. PCR kits are often used only by referral and research laboratories to identify the amoeba at species level [25], rather than commercially. A previous study, which compared all the published molecular methods, found that real time quantitative PCR (qPCR) had the highest sensitivity at 89.3% and the lowest limit of detection of 0.1 organism/microliter [30]. While qPCR is more expensive than traditional methods, the benefits of high sensitivity outweigh the costs of unnecessary treatments and the complications involved with misdiagnosed AK. Molecular diagnostic tools in animals include screening methods such as PCR and 18S rRNA sequencing [15][18][19][15,18,19]. In another study, involving a rabbit model of human AK infection, confirmation of the infection was established by microscopically examining corneal tissue samples, culturing and measuring the serum IgG titre using an enzyme-linked immunosorbent assay [10].
5. Treatment Options
The standard treatment for AK in humans involves the administration of biguanide antiseptic eyedrops, for example 0.02% chlorhexidine (CHX) and polyhexamethylene biguanide (PHMB). The diamidines (e.g., propamidine and hexamidine) are also often used to treat AK [1]. The usefulness of CHX and PHMB has been investigated [31]. However, treatment was highly demanding with human patients being expected to administer eye-drops hourly for the first two days. This meant waking up every hour throughout the night to administer eye-drops. The dosage was then reduced to hourly within the day for the next 5 days with a further reduction to four times per day over the succeeding weeks. CHX and PHMB are not normally administered alone and other drugs are also used, such as topical steroids (dexamethasone, 0.1%) for treating corneal inflammation, cycloplegics (atropine) for photophobia and oral nonsteroidal anti-inflammatories for patients suffering with sclerokeratitis. This rigorous treatment routine is often necessary as AK is usually diagnosed when the disease has become more severe. The use of topical steroids in AK treatment remains contentious. Topical dexamethasone has been shown to increase the pathogenicity of Acanthamoeba infection in Chinese hamsters [32] and to exacerbate AK in the rabbit cornea [33]. In contrast, using topical corticosteroids after the initiation of anti-acanthamoebic therapy, does not seem to worsen the clinical outcomes of AK [34].
Acanthamoeba presents a challenge when the active (trophozoite) form is exposed to stressful conditions and becomes encysted (Figure 2). In a nationwide 7-year survey in the Netherlands, 39% of patients’ treatment failed [26]. Common factors associated with treatment failure include age, delayed diagnosis, and corticosteroid use prior to diagnosis [26]. There is currently no exclusive drug that can eradicate Acanthamoeba cysts and trophozoites in humans or animals. Successful treatment relies on a combination of the anti-amoebic agents biguanide and diamidine [35].
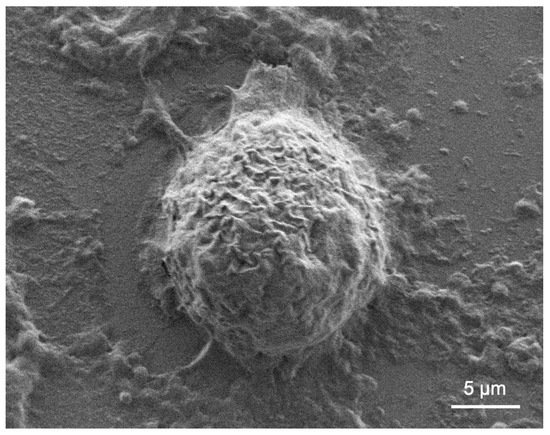
Figure 2.
Acanthamoeba castellanii
Based on current veterinary knowledge there are no successful treatments for systemic or central nervous system amoebiasis in animals [36]. Some examples of effective treatment against systemic Acanthamoeba infections in humans include antibiotics (e.g., trimethoprim-sulfamethoxazole, rifampin) and anti-fungals (e.g., ketoconazole) [13][37][13,37]. More research into such drugs should be conducted in order to treat animals suffering from multi-systemic Acanthamoeba infections. In a dog with a multi-systemic infection, the attempted treatments included corticosteroid and anti-metabolite therapy [36]. The dog’s condition continued to deteriorate, suggesting that the pharmacological treatment employed was ineffective. Unfortunately, this dog died of respiratory arrest associated with infiltration of the lung interstitial tissue by Acanthamoeba.
Cows and bulls are also susceptible to Acanthamoeba [5]. Research on a triple therapy of CHX, Na2EDTA and poloxamer (a polymer used to enhance drug delivery) ocular gel, using bovine eyes as test material, showed successful anti-amoebic activity when compared with single drugs [38]. Although this experimental research was on the feasibility of this approach to the treatment of AK in humans, there is evidence that this drug regimen was effective as an anti-amoebic agent in bovine eyes. Therefore, it is reasonable to assume that the same treatment plan can be effective in live cattle and, potentially, other animals affected with AK. In a similar pharmacological study for the treatment of AK, rats infected with Acanthamoeba were treated with nine different drug compounds/combinations for 28 days. Cultures were then taken to investigate the existence and extent of Acanthamoeba development. One drug combination—miltefosine + PHMB—was the most effective combination, with approximately 86% of rat eye cultures showing no evidence of amoeba growth after treatment [35]. A topical treatment using miltefosine for AK in Syrian hamsters has been investigated but was proved to be ineffective [39].
Chronic amoebic keratitis has also been modeled in rats undergoing treatment and investigated both in vivo and in vitro. In the in vivo model, the amalgamation of PHMB and hexamidine diisethionate was more potent at destroying Acanthamoeba when compared with the single drugs: PHMB, hexamidine diisethionate and miltefosine [40]. This study again reinforces that drug combination therapies have shown the most success against AK. Rabbits have also been used as AK models to determine the risk factor of using corticosteroids as a treatment option [33]. The rabbits’ eyes were co-inoculated with Acanthamoeba and the bacterium P. aeruginosa, in either high or low concentrations. Treatment involved eye-drops containing the steroid Betamethasone sodium phosphate. The topical corticosteroids were found to worsen AK in two scenarios: firstly, when the cornea was already infected with Acanthamoeba and high numbers of the bacteria; secondly, when the administration of the corticosteroid was delayed and the Acanthamoeba infection was allowed to become established—even in the presence of a small number of bacteria. In this case, the steroids only aggravated the AK and provided no therapeutic benefit.
