Epithelial–Mesenchymal Transition (EMT) was first discovered during the transition of cells from the primitive streak during embryogenesis in chicks.
- Epithelial–Mesenchymal Transition and Aging
1. Introduction
Aging is associated with a range of related diseases. As humans age, they are more prone to developing multiple diseases. This has led researchers to further investigate the relationship between aging and age-related diseases. Does disease occur because the body is regressing due to the decay in the organ system and the decline of normal physiological functions?Disease and aging are closely related to epithelial–mesenchymal transition (EMT) and telomeres. A link between EMT and aging has been established, in that EMT has been found to play a crucial role in the age-related development of fibrosis in the heart and lungs [1]. Genes associated with EMT, such as transforming growth factor-β (TGF-β), have also been found to increase in the brain of patients with Alzheimer’s Disease (AD), which causes chronic neuroinflammation [2]. Aging also increases TGF-β expression, which induces EMT [3][4]. The upregulation of TGF-β could contribute to cell degeneration, tissue fibrosis, inflammation, decreased regeneration capacity, and metabolic malfunction, which will, in turn, lead to the development of various types of diseases [5][6] including cancer [7][8]. Telomeres, on the other hand, have been long associated with aging since they were first discovered [9][10]. The relationship between telomere length and aging as well as age-related diseases has been broadly explored, whereby many previous studies have reported that short telomere length is the cause of various diseases, such as dyskeratosis congenita [11][12][13], pulmonary fibrosis [14][15], and even cancer [16][17][18][19].The relationship between telomeres and EMT has not yet been established; in fact, it has not been examined by any research studies so far. However, the interconnecting function that links both aging and cancer development may indicate a possible correlation between the two, which might enable further understanding of the mechanism to develop future therapies. As shown in
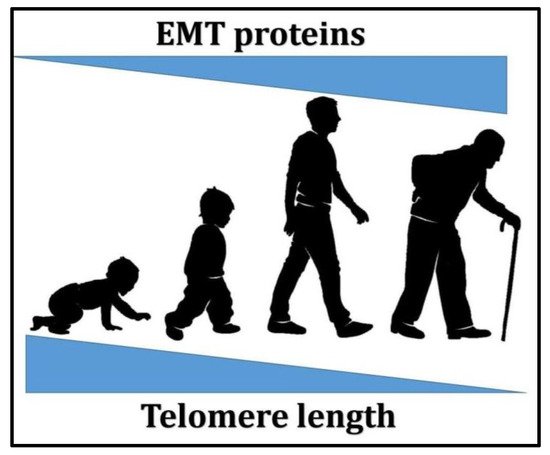
Figure 1, through aging, telomere length decreases while EMT-associated proteins levels increase, which shows a possible correlation between the two. Understanding this relationship might enable the discovery of potential therapies for various age-related diseases involving EMT and telomere length maintenanc.
Figure 1. A schematic representation of the relationship between aging, telomere length, and epithelial–mesenchymal transition (EMT)-associated proteins levels.
2. EMT
A vital aspect in embryogenesis is the cell conversion from epithelial to mesenchymal cells, especially in tissue and organ growth. The biological sequence of EMT involves the differentiation of epithelial cells into mesenchymal phenotype cells with a greater capacity for migration and an invasive character. EMT-induced cells resist apoptosis in an enhanced manner and significantly increase extracellular matrix (ECM) component production. In addition to its role in embryogenesis, the EMT process may be activated to heal wounds and regenerate tissue. The key aspects of epithelial cell characteristics are the adhesion between the cells and the apical–basal polarity. These are created due to the way tight junctions, adherens junctions, gap junctions, and desmosomes are arranged [20]. Cells of this type are located on a basement membrane, forming at least one layer that functions as a barrier to the outlines of the tissues and organs.EMT is induced by numerous transcription factors and signaling pathways, depending on both physiological and pathological circumstances. As the most potent EMT activator, TGF-β leads signaling pathways to become activated. This process culminates in the expression of genes with the function of encoding EMT transcription factors (EMT-TFs) [4][21][22]. In EMT, three major groups of EMT-TFs play vital roles, including SNAI (Snail and Slug), ZEB (ZEB1 and ZEB2) and TWIST (TWIST1 and TWIST2) [23][24][25][26], which repress the expression of E-cadherin, thus causing the disassembly of cell–cell junctions and inducing EMT [27][28]. As the main component, TGF-β is also involved in many signaling pathways contributing to the development of EMT, such as the TGF-β signaling pathway [29], the WNT signaling pathway [4][30], and the Smad Signaling pathway [31][32], which regulates the EMT process. Furthermore, growth factors such as insulin-like growth factor (IGF), fibroblast growth factor (FGF) and epidermal growth factor (EGF) can also trigger EMT via the previously-mentioned transcription factors [33][34].Although the specific roles and triggers of EMT remain unclear, it can be classified into three different types according to biological function. EMT is associated with implantation, embryo formation, and organ development, and is organized to generate diverse cell types that share common mesenchymal phenotypes. This class of EMT, which has the proposed term ‘type 1,′ neither causes fibrosis nor induces an invasive phenotype resulting in systemic spread via circulation. This type of EMT occurs during embryonic gastrulation that gives rise to the mesoderm and endoderm. The primitive epithelium during this stage could generate the primary mesenchyme (mesenchymal cells) through EMT, and could also be reverted to secondary epithelial via mesenchymal–epithelial transition (MET) [35].The second type of EMT is associated with wound healing, tissue regeneration, and organ fibrosis. Type 2 EMT involves tissue reconstruction, required due to trauma or inflammatory injury, which induces a repair cascade that normally generates fibroblasts and other related cells. Unlike type 1 EMT, type 2 EMT is not naturally occurring and is linked to inflammation. As inflammation is attenuated, EMT type 2 reduces, as observed in wound healing and tissue regeneration. Type 2 EMT can provide a continual response to ongoing inflammation in organ fibrosis. However, extended expression of EMT factors may cause organ damage. Persistent inflammation causes prolonged wound healing, known as tissue fibrosis.Type 3 EMT occurs in neoplastic cells that undergo a genetic and epigenetic change; this refers specifically to genes in which clonal outgrowth is favored and localized tumors are developed. Such alterations, which particularly affect oncogenes and tumor suppressor genes, when acting in cooperation with the regulatory circuitry of EMT, will produce vastly dissimilar results from those witnessed with the two other forms of EMT. If the cells undergo a type 3 EMT, carcinoma cells metastasize and invasion might occur, thus increasing the progression of cancer. Significantly, the extent to which cancer cells might traverse EMT differs. Certain cells retain numerous epithelial attributes as they acquire certain mesenchymal aspects, while different cells lose all remnants of their epithelial origins and become completely mesenchymal. The exact signals that induce type 3 EMT in carcinoma cells remain unexplained. Numerous primary carcinomas may be linked to various signals originating from tumor stroma.These EMT classifications could enable further comprehension of EMT function in biological processes and assist in investigations related to the mechanisms involved in distinguishing each type of EMT. Most studies have only focused on type 1 and type 3 EMTs, as the function in these cases is crucial in embryogenesis as well as cancer development. However, an exploration of type 2 EMT potentially offers a greater in-depth understanding of its role in aging and age-related diseases, since this type has been associated with aging, where EMT has the potential to regenerate and replace damaged or dead cells.
3. EMT and Aging
Aging can be distinguished by the decline in cell, tissue, and organ function, which is connected to a greater risk of developing age-related diseases and is also influenced by environmental and lifestyle factors [36][37]. Some research has shown that aging impacts the regenerative capabilities of tissues [38]. However, work on animal models with strong regenerative capabilities, such as zebrafish and newts, suggests that aging does not affect regeneration [39][40]. Repair and regeneration of tissues are vital processes in the maintenance of an organism’s integrity and ability to survive. When aging occurs, the reliability of these mechanisms reduces, resulting in a lower capacity for repair and an ongoing reduction in the structural and functional capacity of the tissue [41][42]. A decline in the normal function of cells is considered concomitant with aging progression and the development of age-related diseases. It has been shown that advanced age is connected to pathological fibrosis, and is a fibrotic disorder risk factor [43]. Pathological fibrosis is mediated by fibroblasts that are responsible for the deposition of ECM components. The accumulation of fibroblast cells leads to excess production of fibrotic tissues, thus compromising the normal physiological function of the vital organs [44]. It has been proven that certain fibroblasts derive from epithelial cells that have undergone EMT, indicating that this procedure plays a key role in tissue fibrosis [45]. E-cadherin is less expressed in mesenchymal fibroblasts, whereas alpha-smooth muscle actin (α-SMA) is highly expressed in mesenchymal fibroblasts of aged rats [46]. Additionally, cardiac fibrosis could be caused by an occurrence of EMT in the heart, particularly in a patient suffering from advanced cardiac failure [47]. Furthermore, fibrosis forms one feature of cardiovascular pathology in syndromes where aging is accelerated, as in for example Hutchinson–Gilford progeria syndrome (HGPS). This can be compared to the cardiovascular pathologies noted among geriatric patients [48]. In addition, older cells have been discovered with altered differential TGF-β, which bears a close resemblance to the profiles of patients suffering from HGPS [49].In Idiopathic Pulmonary Fibrosis (IPF) patients, the nucleus has been shown to contain β-catenin [50]. This indicates that the Wnt/β-catenin pathway is involved in EMT-related fibrosis. Moreover, IPF, a characteristic of which is lost respiratory functionality caused by ECM being excessively deposited, could induce EMT, since an abnormal Wnt/β-catenin signaling pathway is exhibited in such cases [51].Diminished function in the blood–brain barrier (BBB) properties is a major occurrence in a number of conditions, such as multiple sclerosis (MS) [52] or in normal human aging [53]. According to Troletti et al. (2016), EMT may play a role during blood–brain barrier dysfunction in neurological disorders, as EMT interacts with factors that give rise to MS pathogenesis. The collapse in the BBB has been reported to be involved in several neurodegenerative diseases such as Alzheimer’s disease [54]. EMT may also play a potential role in Alzheimer’s disease, where certain areas of the affected brain were found to have a high expression of genes associated with EMT [2].Aging is also associated with the development of senescent cells. Senescent cells exhibit metabolic and functional changes, including cell cycle arrest. They also acquire a senescent-associated secretory phenotype (SASP) characterized by increased secretion of the pro-inflammatory phenotype secreting cytokines, growth factors, metalloproteinases, and reactive oxygen species [55]. The increase in SASP in senescent cells creates an environment that is harmful to other cells, which may contribute to age-related diseases such as arteriosclerosis [56] and cancer [57]. The induction of EMT might be influenced by senescent cells. Secretory phenotypes linked to these cells that derive from senescent fibroblasts have the capacity to initiate EMT induction in the surrounding epithelial cells [58]. The latter displayed low levels of cell membrane-associated β-catenin, E-cadherin, and cytokeratin, while the vimentin protein level rose. These represent key indicators of EMT following treatment with a conditioned medium from senescent cells. It is interesting to note that stimulation by the senescent cells-conditioned medium is reduced by inhibiting inflammatory cytokines, namely Interleukin-6 (IL-6) and Interleukin-8 (IL-8). Meanwhile, addition of IL-6 and IL-8 to the pre-senescent cell-conditioned medium appeared to result in the promotion of cancer cell invasion [59]. Inflammatory cytokines IL-6 and IL-8 as well as chemokines CXCL-1 and human growth regulated α protein (human GROα) are among the highly secreted human SASP factors [59][60]. Consequently, it seems that SASP and inflammation promote EMT. Larger amounts of hepatocyte growth factor (HGF) were identified in the senescent fibroblast-conditioned medium compared to pre-senescent prostate fibroblasts. An association exists between HGF and cell–cell junction disintegration, epithelial cell morphogenesis disruption, as well as migration and invasion stimulation; hence, in the neighboring epithelia, EMT is promoted [61][62]. Furthermore, a higher level of HGF has been identified within skin fibroblasts among aged individuals, which responds to the increased level of insulin-like growth factors (IGFs) [63]. A further alteration linked to age is the level of various growth factors, such as TGF-β, epithelial growth factor (EGF) and IGFs, which may increase EMT and fibrosis progression [64]. However, the regulatory function of EMT transcription factors, along with several senescent key players in different conditions, is still unclear.Cancer and aging are interconnected since through the latter, the risk factor for cancer development also increases. An important tumor suppressor, p53, has been reported to decline with age [65]. However, SASP may also be targeted by p53, as it has been reported that the loss of p53 caused higher levels of SASP components, such as IL-6 and IL-8, to be secreted in the cells [60][66]. These have the ability to induce EMT, suggesting an important association of p53 with senescence [67]. The function of p53 was shown to be significantly more important in preventing cancer in older organisms; a study found a higher incidence of tumors in mice at 12 months compared to mice at 3 months after p53 deletion [68]. This is because SASP prevention by p53 becomes less effective as the levels of p53 declines with age [69]. This would, in turn, cause an accumulation of SASP components that may induce EMT, thus leading to development of tissue fibrosis or cancer.