Ca2+ is pivotal intracellular messenger that coordinates multiple cell functions such as fertilization, growth, differentiation, and viability. Intracellular Ca2+ signaling is regulated by both extracellular Ca2+ entry and Ca2+ release from intracellular stores. Apart from working as the cellular recycling center, the lysosome has been increasingly recognized as a significant intracellular Ca2+ store that provides Ca2+ to regulate many cellular processes. The lysosome also talks to other organelles by releasing and taking up Ca2+. In lysosomal Ca2+-dependent processes, autophagy is particularly important, because it has been implicated in many human diseases including cancer.
- lysosome
- ion channel
- calcium
- autophagy
- cancer
1. Introduction
The lysosome is an acidic single-membrane organelle first discovered in 1955 by Christian de Duve while investigating the mechanism of action of insulin [1][2][3][1,2,3]. It contains more than 60 hydrolytic enzymes (e.g., nucleases, glycosidase, phosphatases, sulfatases, lipases, and proteases), which are active at the luminal acidic environment (pH 4.5–5.0) established by the vacuolar-ATPase (V-ATPase) proton pump [4][5][4,5]. Since its discovery, the lysosome has mainly been considered to be the center of waste disposal, which digests unwanted macromolecules, damaged and senescent organelles, microbes, and other particles delivered via endocytosis, autophagy, and phagocytosis [4][6][7][8][4,6,7,8]. Once degraded, some breakdown products such as free fatty acids, amino acid, monosaccharides and nucleotides are transported back to the cytosol via specific exporters in the lysosome membrane for reutilization in anabolic processes [9][10][9,10]. Lysosomes also contain more than 60 membrane proteins that are implicated in the maintenance of the lumen homeostasis, especially ionic homeostasis and membrane potential, in the control of molecular export across the lysosomal membrane, and in lysosomal membrane trafficking (i.e., fusion and fission). The functions of lysosomes in material degradation, catabolite export, or trafficking are key to maintaining cellular homeostasis, the perturbations of which often lead to lysosomal storage diseases (LSDs) [11].
Recent studies have shown that the lysosome is not only the terminal degradative compartment, but also a multifunctional signaling hub that integrates the cell’s responses to nutrient status, growth factors, and hormones. Noticeably, in order to adapt to changes in cellular environment, the lysosome has a nutrient-sensing mechanism involving mammalian/mechanistic target of rapamycin complex 1 (mTORC1) and transcription factor EB (TFEB) [12][13][14][12,13,14]. mTORC1 is capable of sensing a myriad of nutrient and energy cues, phosphorylating numerous cell growth-related substrates including TFEB, and thus governing the balance between catabolic and anabolic metabolic pathways in the cell [15]. TFEB can bind to a palindromic 10 bp nucleotide motif, named the coordinated lysosomal expression, and regulation (CLEAR) element, and activate the transcription of many genes encoding lysosomal proteins and autophagy-related proteins [16][17][16,17]. Under nutrient sufficient conditions, TFEB is sequestered away from the nucleus due to being phosphorylated by mTORC1. Conversely, under starvation, TFEB becomes dephosphorylated due to a reduction in mTORC1 and an activation of calcineurin (CaN), a Ca2+ and calmodulin (CaM) dependent serine/threonine protein phosphatase, and translocates to the nucleus to promote the transcription of the CLEAR element, which subsequently promotes autophagy-lysosome pathway as well as exocytosis and phagocytosis [8][18][19][20][8,18,19,20].
Autophagy is an evolutionarily conserved cellular degradative process that is induced by nutrient and energy starvation. It is a fundamental cellular program for cells to maintain intracellular energy and nutrient homeostasis and to protect cells against stress. During autophagy, intracellular components such as macromolecules and unwanted organelles are engulfed in autophagosomes that then fuse with lysosomes to form autolysosomes for degradation [21]. Lysosomal Ca2+ plays a key role in autophagy. For example, Transient Receptor Potential Mucolipin 1 (TRPML1, encoded by MCOLN1 gene), an important Ca2+ channel in the lysosome, governs autophagy through regulating both mTORC1 [22][23][22,23] and TFEB [24][25][24,25]. Because autophagy has an essential role in cellular homeostasis, it is implicated in various physiological processes and human diseases. Among them, the roles of autophagy in cancer have been extensively studied.
2. Lysosomal Ca2+ Homeostasis
The lysosome is a significant intracellular Ca2+ store, which coordinates cellular adaptive responses [12][15][26][27][28][29][30][12,15,26,27,28,29,30]. In the early 1990s, nicotinic acid adenine dinucleotide phosphate (NAADP) was discovered to be a potent mobilizer of Ca2+ from stores separated from those sensitive to inositol 1,4,5-trisphosphate (IP3) and cyclic ADP-ribose (cADPR) [31]. It was later shown that the NAADP-sensitive Ca2+ store is the functional equivalent of the lysosomal system, suggesting the lysosome may function as a Ca2+ store [32]. Indeed, in the same time, the lysosomal Ca2+ concentration was estimated to be ~0.5 mM, approximately 5000-fold higher than the cytosolic Ca2+ concentration (∼100 nM) [33][34][35][33,34,35]. This Ca2+ gradient across the lysosomal membrane is thought to be established by an unidentified Ca2+/H+ exchanger or Ca2+ transporter [36][37][36,37]. Multiple Ca2+ sensors including CaM, apoptosis linked gene 2 (ALG-2), and synaptotagmin 7 (Syt 7) associated with lysosomes have also been identified [22][33][38][39][40][41][22,33,38,39,40,41]. This further increases the diversity of lysosomal Ca2+-dependent processes. Recently, several groups have made new discoveries elucidating the molecular machinery underlying lysosomal Ca2+ release, Ca2+ signaling, and Ca2+ uptake (Figure 1).
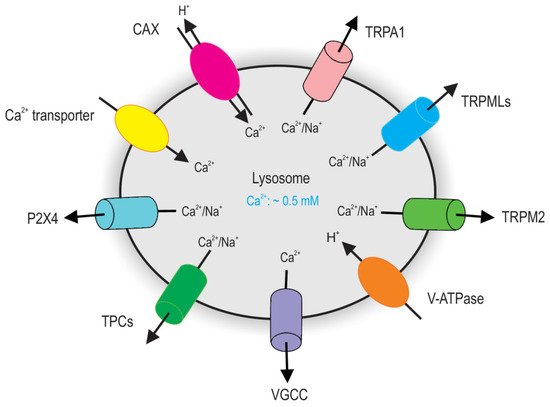
Figure 1. Major Ca2+ releasing channels and transporters on lysosomes. TRPML channels and TPC channels are major groups of Ca2+ channels on lysosomes that have been definitely defined by endolysosome-patch-clamp. Given the topology of the TRPML proteins at the endolysosomal membrane and the electrical properties of the endolysosome, TRPML opening leads to Ca2+ and Na+ release from the endolysosome to the cytosol. Activation of TPCs release lysosomal Na+ and Ca2+. Lysosomes accumulate Ca2+ using a putative Ca2+ Transporter or Ca2+/H+ exchanger (CAX).
3. Autophagy
Autophagy is a self-eating process that is important for balancing sources of energy at critical times in development and in response to nutrient stress. The cell uses an autophagy pathway to degrade and recycle cytoplasmic constituents such as protein aggregates, lipids, and complete organelles for cell survival. It is especially important in postmitotic cells, such as muscles and neurons, where accumulation of aggregated proteins and damaged organelles often results in cell death [42][43][44][91,92,93]. Indeed, suppression of autophagy causes compromised neuron and muscle differentiation [45][46][47][94,95,96] as well as neurodegeneration [42][43][44][91,92,93] and myofiber degeneration [48][49][97,98].
In most cells, autophagy is kept at a low level under nutrient rich condition. However, stressful conditions, such as nutritional deprivation, oxidative stress, Ca2+ overload, pathogen infection, and other diseases, activate autophagy. By upregulating autophagy under such conditions, cells degrade macromolecules into their building blocks for reutilization, thereby adapting to extreme conditions and maintaining cellular homeostasis.
There are three types of autophagy: macroautophagy [50][51][99,100], microautophagy [50][52][99,101], and chaperone-mediated autophagy (CMA) [53][54][102,103]. Macroautophagy is the most common form of autophagy, which is characterized by the formation of a typical double-membrane cistern (so called phagophore) that extends and engulfs part of the cytoplasm to form a whole vesicle (so called autophagosome). The autophagosome ultimately fuses with a lysosome to form an autolysosome [55][56][57][104,105,106]. In this review, we focus on macroautophagy (hereafter referred to as autophagy).
The process of autophagy is controlled by multiple complexes of proteins encoded by evolutionarily conserved, autophagy-related (ATG) genes, which were originally identified in yeast. The products of these ATG genes, together with other autophagy-related factors, regulate autophagosome formation, tethering, and fusion with lysosomes [58][59][60][61][107,108,109,110]. Autophagy is also regulated by some non-ATG proteins. For example, in the presence of nutrients, ATG1/ULK1 is phosphorylated by mTORC1 [62][111], thereby inhibiting autophagy initiation [63][112]. mTORC1 can also phosphorylate and inactivate TFEB, repressing autophagy [13][64][13,113]. In the absence of nutrients, TRPML1-metiated lysosomal Ca2+ release activates CaN, which further causes TFEB dephosphorylation and nuclear translocation, thereby promoting autophagy [25].
Studies of mammalian systems have highlighted many important roles of autophagy in health and diseases including cell growth [65][114] and differentiation [47][96], LSDs [66][67][49,115], neurodegenerative diseases [43][44][92,93], bacterial infections [68][116], and cancers [69][70][117,118].
4. Lysosomal Ca2+ in Autophagy
4.1. TRPML Channels in Autophagy
It is widely accepted that Ca2+ can regulate autophagy, while mechanisms differ depending on the conditions. The interplay between lysosomal Ca2+ signal and autophagy has also been reported. In line with this, several lysosomal Ca2+-permeable channels have been suggested to regulate autophagy [71][72][73][119,120,121].
As a key Ca2+ release channel in the lysosomal membrane, TRPML1 deficiency leads to defective autophagy including accumulation of autophagosomes and aggregation of p62 proteins [74][75][76][77][122,123,124,125]. Growing evidence suggests that TRPML1 plays multifaceted roles in autophagy. Under normal conditions, mTORC1 phosphorylates and inhibits both TRPML1 and TFEB. Cellular stress activates TRPML1 due to mTORC1 inhibition. This further activates downstream pathways including (1) CaM/CaMKKβ/AMPK-dependent autophagosome formation [78][126], (2) ALG-2-dependent lysosome centripetal movement to promote autophagosome–lysosome fusion [79][45], (3) proteolytic degradation in autolysosomes [80][127], (4) Syt7-dependent lysosomal exocytosis to remove cellular garbage [79][81][45,128], (5) CaM-dependent mTORC1 reactivation to prevent cell death by increasing protein synthesis and promote lysosome reformation [22][23][22,23], and (6) CaN/TFEB activation to continuously supply lysosome and autophagy proteins [24][25][82][24,25,47] (Figure 3). Because TRPML1 is also a target of TFEB, a positive feedback is established to largely potentiate autophagy during stress [24][25][24,25]. Thus, TRPML1 is involved in several steps of autophagy including autophagosome formation, autophagosome maturation, autolysosome degradation, and autophagic lysosome reformation.
In addition to TRPML1, TRPML3 also takes part in autophagy regulation. TRPML3 has been found in the plasma membrane and multiple intracellular compartments, including autophagosomes, early endosomes, late endosomes, and lysosomes. The multiple compartmental localization of TRPML3 suggests that TRPML3 is dynamically expressed in different compartments and plays a role in membrane trafficking. Indeed, TRPML3 is accumulated in the plasma membrane upon inhibition of endocytosis and is recruited to autophagosomes upon induction of autophagy, thereby regulating endocytosis and autophagy [83][129]. Specifically for autophagy, TRPML3 overexpression increases while its knock-down or expression of the channel-dead dominant negative TRPML3 reduces autophagy [83][129]. Mechanistically, emerging evidence suggests that palmitoylation at its C-terminal region is required for TRPML3′s function in autophagosome formation, potentially by controlling its trafficking to autophagic structures [84][130], where TRPML3 may promote autophagosome maturation by providing Ca2+ in the fusion process through a specific interaction with GATE16, a mammalian ATG8 homologue [85][131].
4.2. TPC Channels in Autophagy
The role of TPCs in autophagy has been conflicting. Pereira et al. [86][132] showed that in astrocytes, NAADP and TPC2 overexpression increased the levels of autophagy markers, LC3 and beclin-1, and NAADP-mediated increases in LC3II levels were reduced in cells expressing a dominant–negative TPC2 construct. In the meantime, Leucine-rich repeat kinase 2 (LRRK2), an important regulator of autophagy involved in late-onset familial Parkinson’s disease (PD) [87][133], activated the CaMKKβ)/AMPK pathway, which was followed by a persistent increase in autophagosome formation. These effects were mimicked by the lysosomal Ca2+-mobilizing messenger NAADP and reversed by an NAADP receptor antagonist or expression of dominant–negative receptor constructs, suggesting that TPC2-mediated lysosomal Ca2+ release may promote autophagy [88][134]. However, skeletal muscles from animals lacking TPC2 displayed an enhanced autophagy flux [89][135]. In addition, loss of TPCs did not appear to have gross defects in autophagy in the liver, heart, and macrophages [90][65]. There, the role of TPC2 in autophagy may be dependent on the conditions. Interestingly, Cang et al. suggested that ATP/mTOR phosphorylates and inhibits TPCs, thereby acting as a nutrient sensor to detect nutrient status in response to intracellular ATP and mTOR levels [90][65]. In contrast, in skeletal muscle, the loss of TPC2 leads to a reduced mTOR [89][135]. It seems that TPC2 and mTOR form a feedback regulatory loop in response to nutrient status.
4.3. Other Channels in Autophagy
Although most voltage gated Ca2+ channels (VGCCs) are found in the plasma membrane of excitable cells, P/Q-type VGCCs are recently reported to be expressed in lysosomes of both mice and fruit flies. Loss of VGCC leads to defects in autophagosome–lysosome fusion, indicating an important role of Ca2+ flux through this channel in autophagy [91][64].
5. Lysosomal Ca2+, Autophagy, and Cancer
An increasing number of tumorigenic pathways have been associated with an altered expression level or abnormal activation of Ca2+ regulatory membrane proteins including Ca2+ channels, transporters, or Ca2+-ATPases [92][93][94][95][96][97][136,137,138,139,140,141]. Abnormal autophagy has also been implicated in cancer development. It protects against the initiation of carcinogenesis, but also has a role enabling the survival of cells in solid tumors where nutrients are limited [98][99][100][101][102][142,143,144,145,146]. Given that lysosomal Ca2+ channels play an important role in autophagy, the role of lysosomal Ca2+ channels in cancer development has attracted great attention in recent years [24][25][103][104][105][24,25,147,148,149]. It is believed that impaired lysosomal Ca2+ signaling is a culprit in malignant tumor development [74][122]. Indeed, emerging evidence has demonstrated that lysosomal Ca2+ signaling underlies several cancer hallmarks involving proliferation, metastasis, and angiogenesis and contributes to multidrug resistance in cancer therapy [74][106][107][108][109][110][111][112][113][114][115][116][122,150,151,152,153,154,155,156,157,158,159,160]. Here we discuss the roles of the two major Ca2+ permeable channels, TRPMLs and TPCs, in cancer.
