This rentryview highlights the following main aspects: the relationship between probiotics/gut microbes with the pathogenesis of MetS, the particular positive roles of Akkermansia muciniphila supplementation in the onset of MetS, and the interaction between dietary polyphenols (prebiotics) with gut microbiota.
- prebiotics
- probiotics
- metabolic syndrome
- type 2 diabetes mellitus
- Akkermansia muciniphila
- gut bacterium
1. Introduction
The only Gram-negative emblematic Verrucomicrobia that is widespread in human intestinal mucosa is A. muciniphila [1][125]. Gene sequence analyses revealed that there are multiple gene candidates for mucin encoding and the single chromosome is contained in 2176 genes with a 55.8% GC content after the MucT type strain of A. muciniphila, as reported by Derrien (ATCC BAA-835 1/4 CIP107961T) [2][3][126,127]. This non-motile, oval-formed microorganism is purely anaerobic and with chemical organotrophic material that can withstand low levels of oxygen. A. muciniphila may create mucin-degrading enzymes and use mucin in the mucosal layer of the epithelium as a source of carbon and nitrogen. A. muciniphila splits these compounds into acetic and propionic compounds, releasing sulfate [4][5][128,129]. A. muciniphila also is 3 to 5% of the total gut microbiome population in healthy adult humans based on an analysis of its distinctive 16SrRNA signature, although this amount differs by several factors. In stable human beings, A. muciniphila has been closely linked to age. Its colonization starts at a young age and ranges between 5.0 and 8.8 log cells/g in a year equivalent to the adult stage but decreases in the elderly [6][7][130,131]. In comparison, in patients with metabolic disorders, A. muciniphila and a mucosal pathology varied, and the incidence of appendicitis and IBD was reversely associated with this [8][132]. In addition, a negative association of intestinal A. muciniphila with diabetes, obesity, and others MetS has been shown [9][133].
Dual control of A. muciniphila and metabolic disorders has demonstrated that both A. muciniphila excess and supplementation can have an effect on the host body. The anatomy of A. muciniphila can also be affected. The dissemination of A. muciniphila through early vancomycin therapy to the early intestinal colonization could help regulate the progression of autoimmune diabetes [10][134]. In an initial analysis, A. muciniphila was recognized as a potential new therapeutic agent for obese patients. Most studies have shown the advantages of using A. muciniphila in metabolic and obesity disorders for prevention and progress. T2DM is characterized by a lower A. muciniphila abundance, low inflammation, and an intestinal permeability disease [11][135]. The degree of A. muciniphila development can be used to determine the metabolic state of the body, for instance glucose homeostasis, serum lipids, and human adipocyte distribution. The reason for associating A. muciniphila with the production of obesity is not yet entirely explained. We have therefore analyzed the most recent studies into A. muciniphila’s function in obesity and learned more about its effects on the distinctive changes in the expression of pathways in metabolic homeostasis [12][136].
2. A. muciniphila and Obesity: Evidence from Mouse Models
All of the previous studies found a correlation between the caloric intake of A. muciniphila and its abundance. Prebiotic administration in high-fat diet mice eliminated the metabolic endotoxemia that characterizes the compromised MetS present among obese participants, decreased the overall fat mass, and decreased body weight [13][137]. These findings have in fact been strongly linked to an abundance of A. muciniphila [14][138]. The reduction of A. muciniphila and inflammatory markers were crucial in all circulatory parameters (i.e., glucose, insulin, leptin, and triglycerides), with 118 of the 13 genes implicated in the fatty oxidation, production, and oxidation of positive ties, whereas Bifidobacterium spp. were important. Three inflammatory features, leptin, and only two genes involved in the oxidation of fatty compounds are positively and negatively related.
Schneeberger et al. documented the inversely related levels of A. muciniphila in mice with inflammatory markers, lipid synthesis and insulin tolerance, cardiovascular risk, and adiposity markers in their plasma. After six consecutive weeks of HFD administration, the main effects were body weight gain and adiposity in mice. The results therefore suggest that an HFD particularly affects the gut bacteria and show that the abundance of A. muciniphila is decreasing steadily with sustained dietary care in mice. This bacterium also reduces, showing a causal impact, the disease development before the initiation of metabolic changes [15][139]. In comparison, the abundance of A. muciniphila increases and decreases in mice fed a diet rich in fish oil and lard, with greater regulation of the intestinal barrier system and less inflammatory tissue, which can be converted into germless receiving mice [16][55]. Other authors also found that the gut barrier dysfunction, weight, and fat gain of HFD-fed mice can be decreased at the same time with A. muciniphila [17][18][140,141]. The relation between age and A. muciniphila was finally identified in mice, as the intestinal level of the bacterium is lower in older mice. An HFD improves the adipose tissue and intestinal microbiological composition considerably compared to aging [15][139].
3. A. muciniphila and Obesity: Evidence from Human Studies
Emerging research assessed the correlation between A. muciniphila intestinal abundance and human body weight. There are proofs that these two variables have an opposite correlation [19][20][142,143]. The numbers of Bifidobacterium spp. and A. muciniphila and the number of Staphylococcus spp., Enterobacteriaceae spp., and Escherichia coli were found to decrease in overweight pregnancy. They were analyzed by quantitative real-time PCR for their gut microbiota composition. The increase in overall bacteria and Staphylococcus was linked in the whole population with increased plasma cholesterol levels, while the increased amount of Bacteroidites spp. was associated with an increased level of HDL and folic acid [21][144].
Twenty overweight or obese children and 20 average regular weight children aged 4–5 years were evaluated by Karlsson et. al. Interestingly, in obese/oversized infants, the A. muciniphila levels have decreased dramatically, whereas in the same class, the Gram-negative Enterobacteriaceae concentrations were significantly higher. Bifidobacterium levels in obese/overweight children were inversely linked to alanine aminotransferase (ALT) [22][145]. Since diabetes and overweight are linked to increased intestinal permeability and low inflammation, endotoxemia caused by LPS is considered as one of the causative agents of obesity-related metabolic disorders. An in vitro observation that confirms the epithelial barrier role of A. muciniphila may provide a working hypothesis to streamline in vivo proof that links decreased fecal A. muciniphila with diabetes and obesity rate. This could reveal a mechanism for protecting HFD obese mice from bacterial LPS endotoxemia. No relationship was currently assessed between A. muciniphila and hypothalamic food intake regulation markers. According to all data, it can be concluded that A. muciniphila influences the reaction of humans in terms of improving inflammation, insulin resistance, and glycemia on their diet of caloric constraint [23][146].
4. A. muciniphila Medicinal Role in the Treatment of Metabolic Conditions
The study of A. muciniphila has contributed to a better understanding of its possible therapeutic effects and actions in determining certain diseases and metabolic disorders. A. muciniphila, in particular, tends to be involved in the development of amine butyrate and in the propionate extracellular pool. It is also important in the development of sulfide hydrogen, which can be anti-inflammatory and a powerful antioxidant [24][25][147,148]. The involvement of A. muciniphila in metabolism enhances prebiotic intake. Both studies performed on animals with A. muciniphila revealed that it decreases body weight and fat mass rise, hepatic steatosis, inflammation, cholesterol, and atherosclerosis; it also enhances insulin sensitivity and restores intestinal barrier function by influencing various factors, such as the thickness of the mucosal membrane, close attachment proteins, antimicrobial peptides, and immunity. A. muciniphila works in particular on the immunomodulatory involvement of a special protein called Amuc 1100 [26][149].Ottman et al. recently documented the increase of glucose resistance and a decline in body weight and fat gain in mice fed an HFD relative to the untreated mice with refined recombinant Amuc 1100 protein. The critical role of A. muciniphila in intestinal health, particularly in metabolic immunomodulation, is evident in this scenario. In order to increase their therapeutic application to gastrointestinal disease, future studies will also need to accentuate the main role of this microorganism and of its proteins in metabolic regulation and immune modulation [27][150].The clinical studies focused on the obesity implying A. muciniphila and its associated biomarkers are presented in Table 12.
Table 12.
Clinical studies of obesity implying
A. muciniphila
and its associated biomarkers.
| Patients Included in the Study/Condition/Period of Study | Observations | Results | Ref. |
|---|
| 81/T2DM/3 months | A reduced-energy diet | Consumption of | A. muciniphila | according to dietary portfolio improved levels and strengthened glycemic regulation, dyslipidemia, and inflammation. | [28] | [151] | ||||
| 60/overweight + obese diabetes/45 days | 600 mg butyrate + 10 g inulin/day powder, or placebo | Insulin + butyrate supplementation may increase | A. muciniphila | , and butyrate lowers the expression of TNF-alpha mRNA, hs-CRP, MDA, and DBP. | [29] | [152] | ||||
| 28 men/obese + metabolic syndrome/35 days | 1 g resveratrol orally, 2/day or placebo | Resveratrol increases homeostasis of glucose and | A. muciniphila | abundance. | [30] | [153] | ||||
| 134 prediabetes 134 healthy controls |
Observation | There was a strong decline in the concentration of the mucin-degrading bacterium | A. muciniphila | in prediabetes. | [31] | [154] | ||||
| 49/overweight + obese/12 weeks | Calorie restriction for 6 weeks | The large amounts of | A. muciniphila | increased the distribution of fasting plasma glucose, plasma triglycerides, and body fat. | [12] | [136] | ||||
| 43/hypercholesterolemic 19 healthy controls/2 years | 27 patients with Atorvastatin treatment | Treatment with atorvastatin improved the amount of | A. muciniphila | . | [32] | [155] | ||||
| 70 female patients/T2DM 70 healthy females. |
Observation | Decreased | A. muciniphila | was linked with fasting blood/urinary glucose. | [33] | [156] | ||||
| 16 infants/obese mothers 256 infants/normal mothers as control | Observation | Prevalence of | A. muciniphila | was lower in control infants with normal mothers. | [21][34][35] | [144,157,158] | ||||
| 28/diabetes 84 healthy controls |
Metformin | Patients with diabetes who obtained metformin have a higher relative abundance of | A. muciniphila | vs. healthy controls. | [36] | [159] | ||||
| 13/morbidly obese patients/12 months | Roux-en-Y gastric bypass (RYGB) | Within the first 3 months, RYGB modified the relative abundances of 31 species, including | A. muciniphila | . This increase in abundance can be continued for 9 months. | [37] | [160] | ||||
| 53 women/obesity | Observation | There were 140 metagenomic species associated with metabolic markers, including | A. muciniphila | . | [17] | [140] | ||||
| 21/T2DM/12 months | Duodenal-jejunal by-pass surgery medical care | In the surgery control group, the amount of gut | A. muciniphila | increased. | [38][39] | [57,161] | ||||
| 32/overweight + obese insulin-resistant/3 months | Oral supplementation of 10 | 10 | A. muciniphila | bacteria, live or pasteurized | For liver dysfunction and inflammation, | A. muciniphila | decreased body weight and decreased the levels of the related blood markers, although the overall composition of the gut microbiota remained unchanged. | [40] | [59] | |
| 21/alcoholic steatohepatitis 16/healthy controls |
Observation | The concentration of fecal | A. muciniphila | vs. healthy controls (indirectly related to the incidence of hepatic disease) was diminished in ASH patients. | [41] | [162] | ||||
| 13/overweight adults/7 weeks | Interventional, fasting (1 week), followed by probiotic intake (6 weeks) Samples: feces, T1 = before fasting, T2 = during fasting, T3 = 6 weeks after probiotic intervention |
In comparison to fasting (T2), the concentration of | A. muciniphila | was higher before fasting (T1) than after probiotic action (T3) | [13] | [137] | ||||
| 3c/normal weight 3/morbid obesity 3/after gastric bypass surgery |
Interventional, gastric bypass | Reduced quantity of | A. muciniphila | in obese subjects (who obtained an elevated quantity of | A. muciniphila | after gastric bypass) vs. average weight subjects | [42] | [163] | ||
| 11/colorectal cancer 10/healthy |
Observational | Increased | A. muciniphila | level in colorectal cancer patients vs. in healthy subjects | [43] | [164] | ||||
| 71/T2DM 74/healthy controls |
Increased | A. muciniphila | abundance in T2DM patients’ feces, vs. in healthy controls patients |
[44] | [165] | |||||
| 53/obese women | No correlation of | A. muciniphila | abundance with dyslipidemia/insulin resistance | [17] | [140] | |||||
| Children (4–5-year-old) 20/normal weight 20/overweight 20/obesity |
Reduced | A. muciniphila | abundance in overweight/obese vs. in normal weight children | [22] | [145] | |||||
| Pre-diabetes/newly diagnosed T2DM: 44/normal 64/pre-diabetes 13/T2DM |
Reduced | A. muciniphila | abundance in patients with pre-diabetes/T2DM vs. subjects with regular glucose resistance | [45] | [56] | |||||
| 23/autistic children | Reduced | A. muciniphila | abundance in autistic children’ feces | [7] | [131] | |||||
| Overweight/obese adults 10/normal weight 10/overweight 10/obesity |
No correlation of | A. muciniphila | level had with the value of BMI | [46] | [166] |
Legend: BMI—body mass index; DPB—diastolic blood pressure; T2DM—type 2 diabetes mellitus.
A summary of the beneficial effects of A. muciniphila treatment on MetS conditions is schematized in Figure 12.
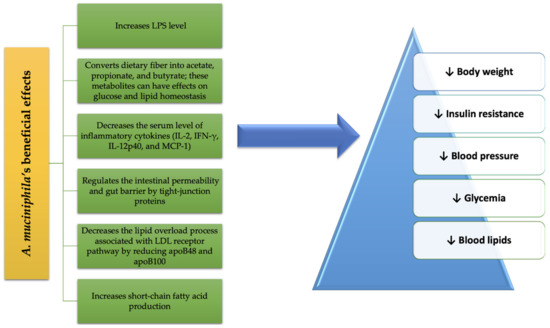
Figure 12.
A. muciniphila
’s benefits in MetS.
