“Theranostics,” a new concept of medical advances featuring a fusion of therapeutic and diagnostic systems, provides promising prospects in personalized medicine, especially cancer.
- theranostics
- single chain variable fragment of IgG (scFv)
- drug delivery system (DDS)
- siRNA
- PDT
1. Introduction
Theranostics is a novel term invented for drugs and mechanisms that are used for simultaneous diagnosis and treatment[1] or a simpler definition of diagnosis plus therapy[2]. The first clinical application of “theranostics” was conceptualized in 1946[3], utilizing the radioactive iodine therapy, [131I ] for patients with metastasized thyroid cancer. Thereafter, various radioligand-based therapies including radio-attached monoclonal antibody, small-molecule inhibitors of prostate-specific membrane antigen (PSMA), and α- and β-emitting radioisotopes have gained popularity in early phase clinical trials, particularly in PSMA expressed prostate cancer[4][5][6][7][8]. Cancer is among the top five major diseases that caused millions of deaths in the 20th century and yet remains a challenging disease to treat causing significant morbidity and/or mortality with over 10 million new cases annually[1]. Cancer therapy, for many decades, has relied on the conventional radiotherapy and chemotherapy, which have significant drawbacks and side effects where non-cancerous cells are also greatly affected by chemotherapeutic action [9]. Although recent medical advancements in the form of targeted treatments, early detection, and behavioral changes have improved cancer prognoses, many treatment options are still reported to be ineffective at preventing recurrences. Moreover, the invasive nature, drug resistance, and systemic toxicity side effects of these treatment options are highly disputed[10]. Previous literatures reported that as much as 70% of ovarian cancers and several types of pancreatic cancers have already metastasized even before diagnosis, thus imploring the need for an earlier and more precise method of diagnosis coupled with targeted treatment[11][12]. The state-of-the-art theranostics concept shifts from the conventional one-size-fits-all medicine approach to a more holistic personalized medicine approach. The goal of this therapy is to offer the right treatment, for the right patient, at the right time while providing the right dose with a more targeted and efficient pharmacotherapy profile. When developing these theranostic-based technologies for clinical translation, it is imperative to focus on adequate blood plasma circulation time, specific delivery to cancerous tissues only while successfully evading normal tissues and organs accumulations, lack of an immune response, and simultaneous treatment coupled with non-invasive monitoring for successful drug delivery. Moreover, the delivery system should also be preferably non-invasive, non-toxic, and biodegradable[13].
2. Polymeric Micelle-Type DDS Carrier as the “Core” of Theranostics Technology
[14]
[9]
[15]
[16]
[17]
[20]
[17]
L
[24]
b
L
[27]
[28]
L
10
[31]
18
L
18
[32]
18
in vivo
[32]
N
131
131
18
[33]
131
131
[33]
[33]
[34]
[35]
[38]
[44]
[45]
[48]
3
[53]
[53]
[53]
[54]
m
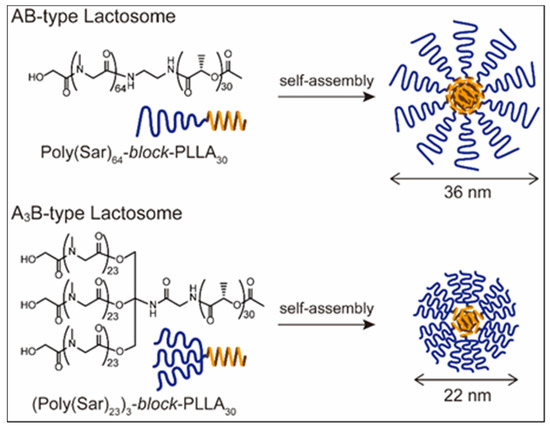
block
L
n
n
[57]
[54]
[24]
2
3
3
[60]
3
[55]
3
3
Figure 2B shows that the AB-type Lactosome, upon first administration, spread over the whole body and gradually accumulated in the tumor region via the EPR effect. However, upon second administration (after seven days) the AB-type Lactosome immediately accumulated in the liver as previously reported (
Figure 2D)
[38]
3
Figure 2C, E. There are several ABC attenuation factors regarding this: 1. By increasing the local density of the poly(sarcosine) chains on the micelle surface, which is 0.30 chain/nm
2
3
2
[55]
3
3
[56]
3
3
[55]
[56]
3
in vivo disposition was elucidated[57][58][59][60].
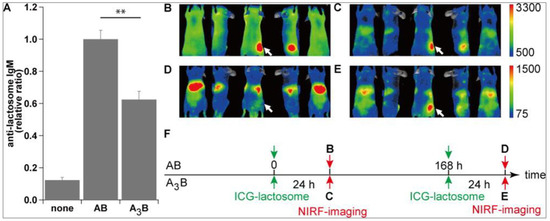
Figure 26.
3
A
n
B,D
3
C,E
3
Copyright © 2014, American Chemical Society
References
- Naveed Ahmed; Hatem Fessi; Abdelhamid Elaissari; Theranostic applications of nanoparticles in cancer. Drug Discovery Today 2012, 17, 928-934.
- Warner, S. Diagnostics plus therapy = theranostics. Scientist 2004, 38-39.
- S. M. Seidlin; L. D. Marinelli; Eleanor Oshry; Radioactive Iodine Therapy. Journal of the American Medical Association 1946, 132, 838-847.
- N Deb; M Goris; K Trisler; S Fowler; J Saal; S Ning; M Becker; C Marquez; S Knox; Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clinical Cancer Research 1996, 2, 1289-1297.
- K. P. Maresca; S. M. Hillier; F. J. Femia; D. Keith; C. Barone; J. L. Joyal; C. N. Zimmerman; A. P. Kozikowski; J. A. Barrett; W. C. Eckelman; et al.J. W. Babich A Series of Halogenated Heterodimeric Inhibitors of Prostate Specific Membrane Antigen (PSMA) as Radiolabeled Probes for Targeting Prostate Cancer. Journal of Medicinal Chemistry 2009, 52, 347-357.
- Hillier, S.; Merkin, R.; Maresca, K.; Zimmerman, C.; Barrett, J.; Tesson, M.; Eckelman, W.; Mairs, R.; Joyal, J.; Babich, J.; et al. [131I]MIP-1375, a small molecule prostate-specific membrane antigen (PSMA) inhibitor for targeted therapy of prostate cancer (PCa). The Journal of Nuclear Medicine 2011, 52, 361.
- Michael S Hofman; John Violet; Rodney J Hicks; Justin Ferdinandus; Sue Ping Thang; Tim Akhurst; Amir Iravani; Grace Kong; Aravind Ravi Kumar; Declan G Murphy; et al.Peter EuPrice JacksonMark ScalzoScott G WilliamsShahneen Sandhu [ 177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. The Lancet Oncology 2018, 19, 825-833.
- Clemens Kratochwil; Frank Bruchertseifer; Frederik L Giesel; Mirjam Weis; Frederik A Verburg; Felix Mottaghy; Klaus Kopka; Christos Apostolidis; Uwe Haberkorn; Alfred Morgenstern; et al. 225Ac-PSMA-617 for PSMA-Targeted -Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. Journal of Nuclear Medicine 2016, 57, 1941-1944.
- Saumya Shrivastava; Saloni Jain; Deepak Kumar; Shankar Lal Soni; Mukesh Sharma; A Review on Theranostics: An Approach to Targeted Diagnosis and Therapy. Asian Journal of Pharmaceutical Research and Development 2019, 7, 63-69.
- Rebecca L. Siegel; Kimberly D. Miller; Ahmedin Jemal; Cancer statistics, 2016. CA: A Cancer Journal for Clinicians 2016, 66, 7-30.
- Ahu Yuan; Jinhui Wu; Xiaolei Tang; Lili Zhao; Feng Xu; Yiqiao Hu; Application of Near-Infrared Dyes for Tumor Imaging, Photothermal, and Photodynamic Therapies. Journal of Pharmaceutical Sciences 2013, 102, 6-28.
- Jyothi U. Menon; Parth Jadeja; Pranjali Tambe; Khanh Vu; Baohong Yuan; Kytai T. Nguyen; Nanomaterials for Photo-Based Diagnostic and Therapeutic Applications. Theranostics 2013, 3, 152-166.
- Alexandra Sneider; Derek VanDyke; Shailee Paliwal; Prakash Rai; Remotely Triggered Nano-Theranostics For Cancer Applications. Nanotheranostics 2017, 1, 1-22.
- Madaswamy S. Muthu; David Tai Leong; Lin Mei; Si-Shen Feng; Nanotheranostics ˗ Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660-677.
- Hiroshi Maeda; Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjugate Chemistry 2010, 21, 797-802.
- Muthu Madaswamy Sona; Matte Kasi Viswanadh; Rahul Pratap Singh; Poornima Agrawal; Abhishesh Kumar Mehata; Datta Maroti Pawde; Narendra; Roshan Sonkar; Madaswamy Sona Muthu; Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics 2018, 2, 70-86.
- Katrin Knop; Richard Hoogenboom; Dagmar Fischer; Ulrich S. Schubert; Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angewandte Chemie International Edition 2010, 49, 6288-6308.
- Ary Wolfgang Richter; Eva Åkerblom; Polyethylene Glycol Reactive Antibodies in Man: Titer Distribution in Allergic Patients Treated with Monomethoxy Polyethylene Glycol Modified Allergens or Placebo, and in Healthy Blood Donors. International Archives of Allergy and Immunology 1984, 74, 36-39.
- Jonathan K. Armstrong; Georg Hempel; Susanne Koling; Linda S. Chan; Timothy Fisher; Herbert J. Meiselman ScD; George Garratty; Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 2007, 110, 103-111.
- Nancy J Ganson; Susan J Kelly; Edna Scarlett; John S Sundy; Michael S Hershfield; Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Research & Therapy 2006, 8, R12-R12.
- So-Hee Lee; Sun Hyuk Hwang; Jin Soo Park; Hae-Sim Park; Yoo Seob Shin; Anaphylaxis to Polyethylene Glycol (Colyte®) in a Patient with Diverticulitis. Journal of Korean Medical Science 2016, 31, 1662-1663.
- A. Chanan-Khan; J. Szebeni; S. Savay; L. Liebes; N. M. Rafique; C. R. Alving; F. M. Muggia; Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil®): possible role in hypersensitivity reactions. Annals of Oncology 2003, 14, 1430-1437.
- Janos Szebeni; Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology 2005, 216, 106-121.
- Akira Makino; Ryo Yamahara; Eiichi Ozeki; Shunsaku Kimura; Preparation of Novel Polymer Assemblies, “Lactosome”, Composed of Poly(L-lactic acid) and Poly(sarcosine). Chemistry Letters 2007, 36, 1220-1221.
- Akira Makino; Shinae Kizaka-Kondoh; Ryo Yamahara; Isao Hara; Tatsuya Kanzaki; Eiichi Ozeki; Masahiro Hiraoka; Shunsaku Kimura; Near-infrared fluorescence tumor imaging using nanocarrier composed of poly(l-lactic acid)-block-poly(sarcosine) amphiphilic polydepsipeptide. Biomaterials 2009, 30, 5156-5160.
- Akira Makino; Eri Hara; Isao Hara; Ryo Yamahara; Kensuke Kurihara; Eiichi Ozeki; Fumihiko Yamamoto; Shunsaku Kimura; Control of in vivo blood clearance time of polymeric micelle by stereochemistry of amphiphilic polydepsipeptides. Journal of Controlled Release 2012, 161, 821-825.
- Benjamin Weber; Alexander Birke; Karl Fischer; Manfred Schmidt; Matthias Barz; Solution Properties of Polysarcosine: From Absolute and Relative Molar Mass Determinations to Complement Activation. Macromolecules 2018, 51, 2653-2661.
- Akira Makino; Shunsaku Kimura; Solid Tumor-Targeting Theranostic Polymer Nanoparticle in Nuclear Medicinal Fields. The Scientific World Journal 2014, 2014, 1-12.
- Bhuvanesh Gupta; Nilesh Revagade; Jöns Hilborn; Poly(lactic acid) fiber: An overview. Progress in Polymer Science 2007, 32, 455-482.
- Joseph Jagur-Grodzinski; Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polymers for Advanced Technologies 2006, 17, 395-418.
- Tomoko Fujiwara; Yoshiharu Kimura; Macromolecular Organization of Poly(L-lactide)-block-Polyoxyethylene into Bio-Inspired Nano-Architectures. Macromolecular Bioscience 2002, 2, 11-23.
- Fumihiko Yamamoto; Ryo Yamahara; Akira Makino; Kensuke Kurihara; Hideo Tsukada; Eri Hara; Isao Hara; Shinae Kizaka-Kondoh; Yasuhito Ohkubo; Eiichi Ozeki; et al.Shunsaku Kimura Radiosynthesis and initial evaluation of 18F labeled nanocarrier composed of poly(L-lactic acid)-block-poly(sarcosine) amphiphilic polydepsipeptide. Nuclear Medicine and Biology 2013, 40, 387-394.
- Eri Hara; Akira Makino; Kensuke Kurihara; Motoki Ueda; Isao Hara; Takashi Kawabe; Fumihiko Yamamoto; Eiichi Ozeki; Kaori Togashi; Shunsaku Kimura; et al. Radionuclide therapy using nanoparticle of 131I-Lactosome in combination with percutaneous ethanol injection therapy. Journal of Nanoparticle Research 2013, 15, 1-10.
- Toru Funayama; Masataka Sakane; Tetsuya Abe; Isao Hara; Eiichi Ozeki; Naoyuki Ochiai; Intraoperative Near-infrared Fluorescence Imaging with Novel Indocyanine Green-Loaded Nanocarrier for Spinal Metastasis: A Preliminary Animal Study. The Open Biomedical Engineering Journal 2012, 6, 80-84.
- Akiya Akahoshi; Eiji Matsuura; Eiichi Ozeki; Hayato Matsui; Kazunori Watanabe; Takashi Ohtsuki; Enhanced cellular uptake of lactosomes using cell-penetrating peptides. Science and Technology of Advanced Materials 2016, 17, 245-252.
- Melissa Siaw Han Lim; Yuki Nishiyama; Takashi Ohtsuki; Kazunori Watanabe; Hirotsugu Kobuchi; Kazuko Kobayashi; Eiji Matsuura; Lactosome-Conjugated siRNA Nanoparticles for Photo-Enhanced Gene Silencing in Cancer Cells. Journal of Pharmaceutical Sciences 2021, 110, 1788-1798.
- Melissa Lim; Takashi Ohtsuki; Fumiaki Takenaka; Kazuko Kobayashi; Masaru Akehi; Hirotaka Uji; Hirotsugu Kobuchi; Takanori Sasaki; Eiichi Ozeki; Eiji Matsuura; et al. A Novel 89Zr-labeled DDS Device Utilizing Human IgG Variant (scFv): “Lactosome” Nanoparticle-Based Theranostics for PET Imaging and Targeted Therapy. Life 2021, 11, 158.
- Eri Hara; Akira Makino; Kensuke Kurihara; Fumihiko Yamamoto; Eiichi Ozeki; Shunsaku Kimura; Pharmacokinetic change of nanoparticulate formulation “Lactosome” on multiple administrations. International Immunopharmacology 2012, 14, 261-266.
- Tatsuhiro Ishida; Hiroshi Kiwada; Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. International Journal of Pharmaceutics 2008, 354, 56-62.
- Amr S. Abu Lila; Hiroshi Kiwada; Tatsuhiro Ishida; The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. Journal of Controlled Release 2013, 172, 38-47.
- Hiroyuki Koide; Tomohiro Asai; Kentaro Hatanaka; Shuji Akai; Takayuki Ishii; Eriya Kenjo; Tatsuhiro Ishida; Hiroshi Kiwada; Hideo Tsukada; Naoto Oku; et al. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. International Journal of Pharmaceutics 2010, 392, 218-223.
- Tetyana V. Obukhanych; Michel C. Nussenzweig; T-independent type II immune responses generate memory B cells. Journal of Experimental Medicine 2006, 203, 305-310.
- D.E. Mosier; B. Subbarao; Thymus-independent antigens: complexity of B-lymphocyte activation revealed. Immunology Today 1982, 3, 217-222.
- Cheol Joo Kim; Eri Hara; Akira Shimizu; Manabu Sugai; Shunsaku Kimura; Activation of B1a Cells in Peritoneal Cavity by T Cell-Independent Antigen Expressed on Polymeric Micelle. Journal of Pharmaceutical Sciences 2015, 104, 1839-1847.
- Taro Shimizu; Yu Mima; Yosuke Hashimoto; Masami Ukawa; Hidenori Ando; Hiroshi Kiwada; Tatsuhiro Ishida; Anti-PEG IgM and complement system are required for the association of second doses of PEGylated liposomes with splenic marginal zone B cells. Immunobiology 2015, 220, 1151-1160.
- Taro Shimizu; Tatsuhiro Ishida; Hiroshi Kiwada; Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology 2013, 218, 725-732.
- Amr Selim Abu Lila; Masako Ichihara; Taro Shimizu; Tatsuhiro Ishida; Hiroshi Kiwada; Ex-Vivo/in-Vitro Anti-polyethylene Glycol (PEG) Immunoglobulin M Production from Murine Splenic B Cells Stimulated by PEGylated Liposome. Biological and Pharmaceutical Bulletin 2013, 36, 1842-1848.
- Cheol Joo Kim; Eri Hara; Akira Shimizu; Manabu Sugai; Shunsaku Kimura; Activation of B1a Cells in Peritoneal Cavity by T Cell-Independent Antigen Expressed on Polymeric Micelle. Journal of Pharmaceutical Sciences 2015, 104, 1839-1847.
- James J. Mond; T cell independent antigens. Current Opinion in Immunology 1995, 7, 349-354.
- James J. Mond; Andrew Lees; Clifford M. Snapper; T Cell-Independent Antigens Type 2. Annual Review of Immunology 1995, 13, 655-692.
- Xinyu Wang; Tatsuhiro Ishida; Hiroshi Kiwada; Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. Journal of Controlled Release 2007, 119, 236-244.
- Kazuaki Taguchi; Yukino Urata; Makoto Anraku; Hiroshi Watanabe; Daisuke Kadowaki; Hiromi Sakai; Hirohisa Horinouchi; Koichi Kobayashi; Eishun Tsuchida; Toru Maruyama; et al.Masaki Otagiri Hemoglobin Vesicles, Polyethylene Glycol (PEG)ylated Liposomes Developed as a Red Blood Cell Substitute, Do Not Induce the Accelerated Blood Clearance Phenomenon in Mice. Drug Metabolism and Disposition 2009, 37, 2197-2203.
- Eri Hara; Akira Makino; Kensuke Kurihara; Manabu Sugai; Akira Shimizu; Isao Hara; Eiichi Ozeki; Shunsaku Kimura; Evasion from accelerated blood clearance of nanocarrier named as “Lactosome” induced by excessive administration of Lactosome. Biochimica et Biophysica Acta (BBA) - General Subjects 2013, 1830, 4046-4052.
- Eri Hara; Motoki Ueda; Cheol Joo Kim; Akira Makino; Isao Hara; Eiichi Ozeki; Shunsaku Kimura; Suppressive immune response of poly-(sarcosine) chains in peptide-nanosheets in contrast to polymeric micelles. Journal of Peptide Science 2014, 20, 570-577.
- Eri Hara; Motoki Ueda; Akira Makino; Isao Hara; Eiichi Ozeki; Shunsaku Kimura; Factors Influencing in Vivo Disposition of Polymeric Micelles on Multiple Administrations. ACS Medicinal Chemistry Letters 2014, 5, 873-877.
- Kensuke Kurihara; Motoki Ueda; Isao Hara; Eiichi Ozeki; Kaori Togashi; Shunsaku Kimura; Control of in vivo disposition and immunogenicity of polymeric micelles by adjusting poly(sarcosine) chain lengths on surface. Journal of Nanoparticle Research 2017, 19, 242.
- Motoki Ueda; Akira Makino; Tomoya Imai; Junji Sugiyama; Shunsaku Kimura; Rational design of peptide nanotubes for varying diameters and lengths. Journal of Peptide Science 2010, 17, 94-99.
- Akihiro Nomura; Kenji Okayasu; Kohji Ohno; Takeshi Fukuda; Yoshinobu Tsujii; Lubrication Mechanism of Concentrated Polymer Brushes in Solvents: Effect of Solvent Quality and Thereby Swelling State. Macromolecules 2011, 44, 5013-5019.
- Yoshinobu Tsujii; Kohji Ohno; Shinpei Yamamoto; Atsushi Goto; Takeshi Fukuda; Structure and Properties of High-Density Polymer Brushes Prepared by Surface-Initiated Living Radical Polymerization. Advances in Polymer Science 2006, 197, 1-45.
- Akira Makino; Eri Hara; Isao Hara; Eiichi Ozeki; Shunsaku Kimura; Size Control of Core–Shell-type Polymeric Micelle with a Nanometer Precision. Langmuir 2014, 30, 669-674.