Bladder cancer (BC) is the tenth most common cancer worldwide with a high recurrence rate, morbidity and mortality. Therefore, chemoprevention and improved treatment of BC are of paramount importance. Epidemiological studies suggest that adequate vitamin A intake may be associated with reduced BC risk. In addition, retinoids, natural and synthetic derivatives of vitamin A, are intensively studied in cancer research due to their antioxidant properties and their ability to regulate cell growth, differentiation, and apoptosis. Findings from in vivo and in vitro models of BC show great potential for the use of retinoids in the chemoprevention and treatment of BC. However, translation to the clinical practice is limited.
- bladder cancer
- vitamin A
- retinoids
1. Vitamin A and Bladder Cancer
Vitamin A is a generic term for a group of lipophilic isoprenoids consisting of a cyclic group and a linear chain with a hydrophilic polar group that includes the major biologically active forms retinol, retinal, and retinoic acid (RA) [1]. Since vitamin A cannot be synthesized in the human body, it must be obtained from the diet [2]. The importance of vitamin A for human health was already known to the ancient Egyptians around 1500–1800 B.C., although they did not know vitamin A as such. They recommended compressed animal livers for the treatment of night blindness or nyctalopia. Today we know that the liver is the richest source of vitamin A and that night blindness is caused by vitamin A deficiency (VAD) [3][4][5]. Rhodopsin with its covalently bound cofactor retinal is a major light-sensitive receptor protein involved in visual phototransduction and essential for normal vision. But the importance of vitamin A goes beyond visual health. Vitamin A is a regulator of cell growth and differentiation, embryogenesis, reproduction, epithelial cell integrity, and immune function [1][6][7]. In addition, it has antioxidant properties [8] and plays a role in protecting against oxidative stress damage and inflammation [1][9]. Recent data also indicate that vitamin A regulates the interactions between eukaryotic host cells and symbiotic microbes, as well as the complexity of the microbiome. On the other hand, the microbiome regulates vitamin A metabolism in the host [10][11].
Vitamin A belongs to the retinoids, a group of over 4000 molecules, which are natural and synthetic compounds that are structurally similar or share functional similarities [2][12][13]. Retinoids are classified into four generations based on the time of introduction and structural features: (i) first generation: retinol, retinaldehyde, all-trans RA (ATRA), tretinoin, isotretinoin; (ii) second generation: etretinate, acitretin; (iii) third generation: adapalene, tazarotene, bexarotene; (iv) fourth generation: seletinoid G [14]. The current use of retinoids in medicine is broad, especially in the field of skin health. For example, they are used for the treatment of various inflammatory and keratinization skin diseases (e.g., psoriasis, pityriasis rubra pilaris, lichen planus), as well as basal cell carcinoma [14]. Moreover, retinoids have been used successfully for the treatment of several other cancers, especially acute promyelocytic leukaemia in adults and neuroblastoma in children [15][16].
Bladder cancer (BC), which usually arises from the urothelial cells, is one of the ten most common cancers worldwide. As it has a high recurrence rate of 50–70% and represents a huge social and economic burden [17][18][19], new prevention and treatment strategies are needed. Retinoids are among the best-studied chemopreventive agents for various diseases and are used in clinical practice for chemoprevention and treatment of several cancers [15][20]. Meta-analyses of epidemiological studies indicate that high dietary vitamin A intake reduces the risk of BC [21][22]. Several preclinical studies have shown great potential of retinoids for chemoprevention and treatment of BC, however, translation into clinical use remains limited due to application challenges. Nevertheless, novel synthetic retinoids and retinoid delivery systems have been developed, which, together with the discovery of novel therapeutic targets in the retinoid pathway, offer new opportunities for successful translation of retinoid application into the clinical setting.
2. The Role of Dietary Vitamin A in Bladder Cancer: The Epidemiologic Evidence
Vitamin A and retinoids are among the best-studied micronutrients and have great potential for prevention and cancer treatment due to their differentiating, antiproliferative, pro-apoptotic, and antioxidant effects combined with selectivity, high receptor binding affinity, and ability to directly modulate gene expression programs [15][23].
An association between VAD and the incidence of cancer was first demonstrated around 1920 in animal studies showing that VAD increased the incidence of spontaneous and carcinogen-induced tumours [24][25][26][27]. In 1979, a retrospective study of human dietary habits and BC showed an increased risk in people with low vitamin A intake [28], implicating vitamin A as a potential agent for BC prevention. Despite the fact that vitamin A is present in a wide variety of foods, many people do not consume this nutrient adequately due to malnutrition or selective diets, leading to VAD. Therefore, the impact of vitamin A intake on BC risk has important public health implications [21][29][30].
Typically, VAD develops in environments of ecological, social and economic deprivation. Recent analysis showed a decline in VAD prevalence primarily due to decrease in East and Southeast Asia, Oceania, Latin America and the Caribbean, while it remains high in South Asia and sub-Saharan Africa [31]. Moreover, we have to point out that Western diets containing mainly processed foods can lead to subclinical VAD, which often goes unnoticed but may be implicated in the development of some cancers [32].
The highest rates of BC are observed in developed countries in Europe, Northern America, and Western Asia, but also in Syrian, Israeli, Egyptian and Turkish men. Approximately threefold lower rates are seen in Southeast Asia (except Japan) and in Latin America and Northern Africa in both sexes, and the lowest in Sub-Saharan Africa and some Middle Eastern and Central Asian countries [17][19][33][34].
Looking at the global distribution of BC incidence and VAD, the association between the two is not immediately apparent. Nevertheless, numerous population-based epidemiological studies investigated the relationship between dietary vitamin A and BC risk, including several meta-analyses [21][22][35][36]. While older studies concluded that dietary retinol and β-carotene play a minimal role in BC [35], more recent studies show a preventive effect of vitamin A on BC. A meta-analysis of 25 studies investigating the quantitative effects of vitamin A on BC revealed that high vitamin A intake and high blood retinol levels were associated with a reduced risk of BC [21]. The most recent meta-analysis of 22 studies conducted in Northern America, Europe, or Japan (19 of which were included in the previous analysis by Tang et al. [21]) indicated that the risk of BC decreased by 76% for every 1 µmol/L increase in circulating concentrations of α-carotene, and by 27% for every 1 µmol/L increase in circulating concentrations of β-carotene. When comparing high and low total dietary carotenoid intake, high intake was associated with a 15% reduced risk of BC in men [22].
On the other hand, very high intakes of preformed vitamin A present in animal foods and pharmaceutical supplements can cause acute or chronic toxicity, while very high doses of provitamin A (carotenoids) from plants do not. Acute hypervitaminosis A is a consequence of the ingestion (usually accidental) of more than 300,000 IU of vitamin A as a single dose or several repeated doses over a few days, whereas chronic hypervitaminosis A is a result of continued ingestion of more than 100,000 IU daily for months or years [37]. In addition, a single dose of more than 25,000 IU of vitamin A may be teratogenic if consumed between the 15th and 60th day after conception [38].
Although the evidence for the correlation between BC aetiology and diet are not yet conclusive, diet is considered one of the modifiable risk factors for BC prevention [39][40]. There is still a large gap to be filled in understanding the molecular mechanisms by which vitamin A affects urothelium and urothelial carcinogenesis. To address this issue, various in vivo and in vitro models mimicking human BC have been widely used.
3. Experimental Models of Bladder Cancer Play a Key Role in Understanding the Chemopreventive and Therapeutic Effects of Vitamin A and Retinoids
Several retinoids, such as ATRA, 13-cis-RA, and N-(4-hydroxyphenyl)-retinamide (4-HPR, or fenretinide), showed promising chemopreventive effects on BC both in vitro and in vivo (
and
, respectively). In vitro studies suggest that retinoids exert their chemopreventive effects on BC through cytostatic, pro-apoptotic, growth inhibitory, cell cycle distribution, and gene expression modulating/regulating functions [41][42][43][44][45][46]. The study by Boström et al. suggested that retinoids may downregulate the expression of matrix metalloproteinases (MMPs), which play an important role in the process of degradation of extracellular matrix essential for tumour growth and invasion [47]. The mechanism of retinoid BC chemoprevention may also include reversion of epithelial-mesenchymal transition, a key process in cancer cell invasion and migration. Wang et al. showed that the synthetic retinoid 4-HPR increased the expression of E-cadherin in invasive BC cell lines and induced the translocation of β-catenin from the nucleus to the cytoplasm, resulting in an altered BC cell morphology that resembles epithelial rather than invasive cancer cells, presumably leading to reduced cell infiltration [48].
In vitro studies investigating the effects of retinoids in human BC cell lines.
| Retinoids | In Vitro Model–Cell Line | Effects | Reference |
|---|
| ATRA 1 | HT-1376 | BBN (rat) | Inhibition of cell growth by inhibition of transcription factor AP-1 activity requiring RARα or RARβ mediated by the orphan receptor chicken ovalbumin upstream promoter-transcription factor (COUP-TF). | [85][49] | ||||
| Increased incidence and size of hyperplasia, papilloma and carcinoma. | [ | 90 | ][53] | RT112 | Inhibition of epidermal growth factor (EGF)-induced cell growth. | [72][42] | ||
| Etretinate 2 | BBN (rat) | Inhibition of urothelial papillary or nodular hyperplasia in a dose-dependent manner. | [92][54] | T24 | Induction of apoptosis. | |||
| No effect on BC. | Redistribution of apoptosis regulators Bax and Bcl-2, correlating with keratin 18 network reorganization. |
[93][55][75][45] | ||||||
| Induction of dose- and time-dependent cell proliferation. Downexpression of cellular retinol-binding protein-II (CRABP-II). Direct inhibition of peroxisome proliferator-activated receptor PPARβ/δ potentiating cell proliferation. |
[86][50] | |||||||
| RA 2 | EJ | |||||||
| Retinyl acetate | BBN (mouse) | Reduction of urothelial atypia and apoptosis in early BC. | [89][56] | Inhibition of cell growth and decreased expression of mutant p53. | [87 | |||
| FANFT (mouse) | Inhibition of squamous and urothelial carcinomas. | [94][57] | ][51] | |||||
| 4-HPR 3 | T24 | Increased expression of E-cadherin and translocation of β-catenin from the nucleus to the cytoplasm. | [78] | |||||
| 4-HPR 3 | [ | BBN (mouse) | 48 | No reduction in tumour incidence. | ] | |||
| [ | 95 | ] | [58] | Preventive effect. | [ | 114][84] | ATRA 1 9-cis-RA 4 13-cis-RA 5 |
RT4 T24 |
| Phase ND | Inhibition of matrix metalloproteinases (MMPs). | |||||||
| MNU (rat) Prospective randomized, placebo controlled, double-blinded | [ | 77 | ][47] | |||||
| Inhibition of tumour growth when combined with the chemotherapeutic agent ADM. | [ | 91][59] | ATRA 1 Bexarotene 6 4-HPR 3 9-cis-RA 4 |
RT4 T24 UM-UC-2/3/6/9/10/11/13/14 |
Resistance to ATRA and 9-cis-RA growth inhibition and apoptosis induction in most of the examined cell lines, which did not express RARβ. 4-HPR was the most potent growth inhibitor and apoptosis inducer. |
[88][52] | ||
| ATRA 1 CD437 7 4-HPR 3 |
RT4 T24 UM-UC-2/3/6/10/13/14 |
Stronger effects on growth inhibition and apoptosis induction by synthetic retinoids (4-HPR and CD437) compared to natural (ATRA). Induction of expression of different nuclear retinoid receptors (RARα, RARβ, RARγ) by different retinoids. |
[74][44] |
In vivo studies investigating the effects of retinoids in carcinogen-based animal models of BC. In all experiments listed here, animals were fed with retinoid-supplemented diets.
| Retinoid | In Vivo Model–Carcinogen (Species) | Effects | Reference |
|---|
| Bexarotene 1 | ||||||||
| 37 (59.3) | ||||||||
| 42 (59.6) | ||||||||
| Well-tolerated side effects. | Cardiac toxicity in 3 patients. | Similar first recurrence time but increased interval length for subsequent tumour recurrences. |
[115][85] | |||||
| 13-cis-RA 4 | BBN (rat) | Inhibition of urothelial carcinomas and other proliferative lesions of the bladder. Reduction in the incidence of hyperplasia, atypia, and urothelial carcinomas by simultaneous or delayed retinoid administration. |
[96 | |||||
| Recurring non-invasive bladder tumours | ] | [ | Phase ND randomized, placebo controlled60] [97][61] |
|||||
| 47 | 49 | Patient dropout due to side effects (17 patients). | No effect on outcome. | [ | BBN (mouse) | Reduction in the incidence of invasive urothelial carcinoma in a dose-dependent manner. | [98,99][62][63] | |
| MNU (rat) | Inhibition of urothelial and squamous carcinomas and proliferative epithelial lesions by simultaneous or delayed retinoid administration. | [100,101][64][65] | ||||||
| ATRA 5 13-cis-RA 4 |
MNU (rat) | Reduction in number and size of tumours. | [102][66] | |||||
| ER 6 2-HER 7 13-cis-RA 4 |
BBN (rat, mouse) | Reduction in incidence, number, and severity of low-grade papillary urothelial carcinomas. ER and 2-HER were less toxic to rats than 13-cis-RA. |
[103][67] | |||||
| FANFT (rat) | No inhibition of incidence or severity of BC. | [104,105][68][69] |
Animal studies have examined chemopreventive and therapeutic effects of retinoids in carcinogen-based in vivo models of BC (
). In these models, tumours develop after animals are treated with carcinogens that mimic environmental exposures known to be a major cause of BC. Carcinogen-based BC models recapitulate the high mutational burden and complexity of human BC [70][71]. Among the carcinogens, the most prevalently used is N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN), a compound closely related to some of the carcinogens found in tobacco smoke, showing remarkable specificity for the urinary bladder [72][73]. Animals orally administered BBN (
a) develop bladder tumours recapitulating the histology of human BC and its morphological, biological, and molecular features [74]. To a lesser extent, the chemopreventive effects of retinoids in BC have also been investigated in N-methyl-N-nitrosourea (MNU) and N-4-(5-nitro-2-furyl)-2-thiazolylformamide (FANFT) in vivo models of BC (
). MNU is a direct-acting carcinogen that is locally instilled into the bladder, and MNU-induced BC in animals displays an immunophenotype similar to human urothelial carcinoma [75]. FANFT is a heterocyclic nitro compound and an indirect chemical carcinogen that stimulates the bladder mucosa to develop carcinoma when animals are fed with FANFT [71].
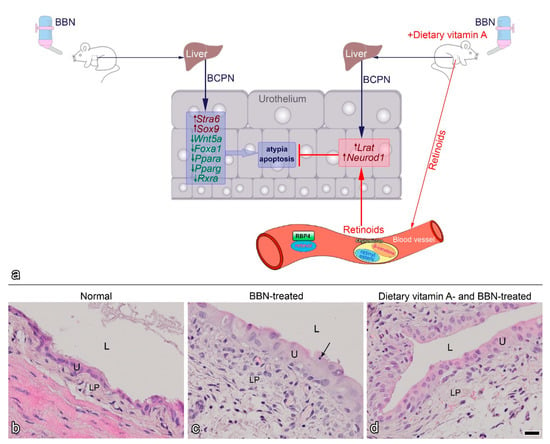
Proposed model of BBN-induced early bladder carcinogenesis and the effects of dietary vitamin A (modified from [56]) (
). BBN (N-butyl-N-(4-hydroxybutyl)-nitrosamine) administered orally to experimental mice via drinking water is metabolized in the liver to BCPN (N-butyl-N-(3-carboxypropyl)-nitrosamine), which reaches urothelium through urine and can affect urothelial cells. BCPN binds to cellular macromolecules, including DNA, and initiates the early carcinogenic process in urothelial cells, manifested by altered RA signalling. Expression of the RA receptor
and the transcription factor
is upregulated, while the expression of the RA pathway regulator
and the transcription factors
,
,
and
is downregulated. Histologically, early urothelial carcinogenesis reveals as urothelial atypia and increased apoptosis. When experimental mice received BBN and dietary vitamin A in the form of a vitamin A-rich diet, retinoids reach the urothelium via the blood circulation. RA signalling is altered in urothelial cells in a different way, compared to BBN-treatment alone. In this case, the expression of
and the transcription factor
are upregulated, while the expression of other genes is not significantly altered. Retinoids from the blood reduce BBN-induced atypia and apoptosis. Representative histological section of an early mouse bladder carcinogenesis model: (
) normal bladder mucosa. (
) two weeks of BBN treatment causes early carcinogenesis with characteristic atypia and apoptotic bodies (black arrow). (
) three weeks of dietary vitamin A together with 2 weeks of BBN treatment decrease urothelial changes. L, lumen of bladder; LP, lamina propria; U, urothelium. Scale bar: 20 µm (
–
).
The study of early bladder carcinogenesis using the BBN model showed that dietary vitamin A (supplemented as retinyl acetate) decreased BBN-induced urothelial atypia and apoptosis [56] (
). Moreover, during early bladder carcinogenesis RA signalling was altered as the expression of several genes was up- or downregulated, while a vitamin A-rich diet prevented this altered expression. In fact, dietary vitamin A together with BBN treatment resulted in upregulation of
and the transcription factor
(
a). In addition, LRAT was observed to be translocated from the cytoplasm to the nuclei of urothelial cells in BBN-treated animals [56]. These results suggest that dietary vitamin A indeed alters cancer-related dysregulation of retinoid signalling and gene expression at early stages of cancer transformation.
It is important to emphasize that anti-cancer activity varies between different retinoid derivatives. Synthetic retinoids (4-HPR and CD437–also known as Ro 472077) have been shown to have stronger effects on growth inhibition and apoptosis than naturally occurring retinoids, e.g., ATRA [44]. Different retinoids induced the expression of different nuclear retinoid receptors (
,
,
) and differentially altered the expression of apoptosis-associated genes (
,
,
,
) [44][52]. Moreover, it was shown that ATRA treatment was not always effective due to some resistance mechanisms and that ATRA could even induce a dose- and time-dependent cell proliferation [50]. A similar effect was also shown for bexarotene (also known as LGD1069 or Ro 26-445, brand name Targretin), which increased the incidence and size of tumours that developed in the BBN model [53]. On the other hand, the combination of retinoids, e.g., 4-HPR, with the chemotherapeutic agents, such as adriamycin (ADM), increased the antitumour effects of the chemotherapeutic agents compared to the antitumour effects when both chemicals were used separately [59].
Another important effect of retinoids demonstrated in in vivo models is the discovery that delayed administration of retinoids also inhibits urinary bladder carcinogenesis [61][65]. For example, delaying the administration of a 13-cis-RA supplement for several weeks after the last administration of BBN to rats did not result in a loss of the chemopreventive effect of 13-cis-RA [61]. This is important for the clinical settings because the onset of retinoid administration in a clinical situation would also likely be delayed with respect to the earliest preneoplastic changes in BC patients.
Unfortunately, the preclinical studies have shown limited predictive potential for the clinical trials. All animal studies with retinoids utilized carcinogen-based BC models, whereas retinoid effects have not been studied in engraftment models, in which cells or tissues are grown in recipient hosts, or in genetically engineered mouse models based on activation or inactivation of gene function in the bladder. Therefore, we believe that combining different in vivo models in chemopreventive studies with retinoids could be the way to improve the predictive potential and translate preclinical experiments into clinical trials with positive outcome. We must also point out that interspecies variations must be considered for a correct interpretation of the results. For example, Chopra et al. highlighted the differences in the expression and distribution of PPAR and RXR isoforms between rat and human urothelium, which may underlie a different response to PPAR agonists [76]. This interspecies gap can be overcome by ex vivo studies on human biopsy specimens, which are extremely under-researched. Moreover, carefully designed clinical trials utilizing promising retinoids and retinoid/chemotherapeutic combinations are of utmost importance.
4. Clinical Trials of Retinoids for Chemoprevention and Treatment of Bladder Cancer and Limitations of Their Use
Retinoids have been successfully used in several clinical applications, such as the treatment of acute promyelocytic leukaemia with orally administered ATRA [77] and high-risk neuroblastoma with 13-cis-RA [78]. Clinical trials of BC chemoprevention with retinoids (
) have often failed, have not shown efficacy, and have not produced results comparable to in vivo and in vitro studies. It is important to stress out that there have been only a handful of clinical trials conducted, some with small group sizes and some without a placebo group that consequently may not have resulted in statistical significance (
).
Clinical trials investigating the chemopreventive effects of retinoids in BC.
| Retinoid | BC Stage | Phase Study Type | No. of Retinod-Treated Patients (Mean Age) | No. of Control Patients (Mean Age) |
Outcome | Reference |
|---|
| 4-HPR 1 | Ta, T1 | Phase IIa | 12 (68) | 12 (65) | Well-tolerated side effects. Indication of reduced proliferation, delayed development of DNA aneuploidy or its reversal to diploidy. |
[109][79] |
| Phase IIb randomized |
49 (63.8) | 50 (61.6) | Well-tolerated side effects. No effect on DNA content distribution and morphology of urothelial cells. No effect on recurrence-free survival. |
[110][80] | ||
| Twenty-year follow-up of randomized [110][80] | 33 | 29 | No effect on outcome. Inverse association between baseline VEGF levels and BC survival. |
[111][81] | ||
| Phase IIb randomized |
24 (60.1) | 19 (61) | Lower IGF-I levels. | [112][82] | ||
| Tis, Ta, T1 | Phase III randomized, placebo controlled |
70 (64.5) | 67 (64.5) | Well-tolerated side effects. No effect on time-to-recurrence. Subgroup analysis indicated that high-risk patients co-treated with BCG had a lower risk of recurrence. |
[113][83] | |
| Etretinate 2 | Ta, T1 | Phase ND randomized, placebo controlled, double-blinded |
15 (68.8) | 15 (64.1) | Well-tolerated at final maintenance dose. Disturbing side effects at high doses. | |
| 116 | ||||||
| ] | [ | 86 | ] | |||
| 13-cis-RA 3 | Ta, T1 | Phase I/II | 14 | / | Toxicity and lack of positive results led to termination of the study. | [117][87] |
| COMBINED TREATMENT | ||||||
| ATRA 4 + ketonazole |
Ta, T1 | Phase ND | 16 | 25 | Well-tolerated side effects. Improved survival time and decreased recurrence rate. |
[118][88] |
| 13-cis-RA 3 + entinostat |
Epithelial tumours, including urothelial carcinoma | Phase I | 18 (5 with BC) |
/ | Well tolerated. No objective responses were observed. |
[119][89] |
A phase III multicentre randomized study in patients with Ta tumours treated with BCG found no benefit from the synthetic retinoid 4-HPR [83][90]. Nevertheless, a subgroup analysis showed that high-risk patients co-treated with 4-HPR and BCG had a lower risk of recurrence compared to the placebo group. Additionally, 4-HPR was shown to decrease plasma levels of insulin-like growth factor I (IGF) in patients with superficial BC [82]. Given the increasingly recognized importance of circulating IGFs in the pathogenesis of various solid tumours, these findings strengthen the rationale for further investigation of 4-HPR as a chemopreventive agent for BC.
The pharmacological use of retinoids encounters several limitations, such as the low concentrations of retinoids at the tumour site, short half-life, poor water solubility, susceptibility to light, heat, and oxidants, and rapid degradation during digestion resulting in low bioavailability and bioaccessibility [20][50]. One of the ways to increase retinoid plasma levels is to combine retinoid treatment with agents that inhibit retinoid degradation, which was tested in a BC clinical trial. The study of Ta and T1 BC patients showed that treatment with a combination of ATRA and ketoconazole (a potent inhibitor of RA-catabolizing cytochrome P450s) significantly improved patient survival and reduced the recurrence rate compared with the control group [88].
One of the limitations to the successful use of retinoids for chemoprevention and treatment is also retinoid resistance. Many potential mechanisms have been proposed for retinoid resistance, including reduced retinoid uptake, increased ATRA catabolism by P450s (CYP26), active drug efflux by membrane transporters, downregulated expression of various RAR genes (promoter methylation), altered expression of co-activators or downstream target genes, and changes in the activities of other signalling pathways [15]. Lu et al. demonstrated a positive correlation between the expression of octamer-binding transcription factor (Oct4) and tumour recurrence in BC. Furthermore, inhibition of Oct4 by ATRA synergistically increased sensitivity to the chemotherapeutic agent cisplatin in preclinical BC studies [91]. Therefore, inhibition of Oct4 could be a therapeutic strategy to overcome drug resistance and reduce the recurrence rate. Combining retinoids with epigenetic drugs also shows great potential to restore tumour response to retinoids. For example, histone acetylation regulates gene transcription so it was proposed that it could restore tumour sensitivity to retinoids. Indeed, the combination of 13-cis-RA and the histone deacetylase (HDAC) inhibitor entinostat was shown to induce histone acetylation in patients with solid tumours including urothelial carcinoma. Although no tumour responses were observed, further evaluation of this combination is warranted [89].
Finally, the use of retinoids in the clinical practice is also limited because long-term administration of natural retinoids is associated with toxicity manifested by hepatic and lipid changes, dry skin, teratogenicity, and bone and connective tissue damage [92]. Treatment with the synthetic retinoid etretinate has been shown to significantly reduce the annual transurethral resection rate in patients with superficial papillary bladder tumours. However, significant cardiac toxicity occurred in the etretinate group [84][85]. To reduce the toxicity of retinoids, novel synthetic retinoids are being developed. For example, the newly developed synthetic retinoid WYC-209 inhibited the growth of tumour repopulating cells of several cancer cell lines (human melanoma, lung cancer, ovarian cancer, and breast cancer) and inhibited lung metastasis in vivo, with low in vivo toxicity [93].
5. Novel Retinoid Delivery Systems
One approach to avoid retinoid degradation, increase bioavailability and bioaccessibility, and reduce toxicity is to encapsulate retinoids in various drug delivery systems such as nanoparticles, micelles, liposomes, or bind them to nanoparticles, proteins or polymers [20]. For example, conjugation of RA to nanoparticles such as RA-poly(ethylene glycol)-thiol gold nanoparticle conjugates showed superior activity against the cervical carcinoma cell line compared to free RA, which is attributed to increased rates of drug transport through nanoparticle uptake compared to passive diffusion of free drug [94]. It has been demonstrated that a nanoformulation of 4-HPR complexed with a solubilizing excipient 2-hydroxypropyl-beta-cyclodextrin (nanofenretinide) was shown to increase the bioavailability and therapeutic efficacy of 4-HPR in vitro and in vivo in the absence of macroscopic toxic effects [95]. Next, a 20% soy oil-in-water emulsion of 4-HPR was developed and a phase I study in patients with malignant solid tumours demonstrated a manageable safety profile and achieved higher plasma steady-state concentrations of the active metabolite compared to previous formulations [96].
Novel retinoid-based formulations also show great potential against CSCs. For example, a nano-micellar formulation of 4-HPR based on its encapsulation in the lipid matrix displayed pronounced antitumour activity against lung, colon, and melanoma CSCs both in vitro and in vivo, in the absence of systemic toxicity, suggesting its potential usefulness for the treatment of solid tumours of various origins [97]. Moreover, ATRA and the chemotherapeutic agent doxorubicin were simultaneously encapsulated in the same nanoparticle, which improved the suppression of breast tumour growth while synergistically reducing the incidence of CSCs in preclinical settings [98].
The anticancer efficiency of encapsulated retinoids can be further enhanced when combined with immunotherapy. For example, the lipid-coated biodegradable hollow mesoporous silica nanoparticles with co-encapsulation of ATRA, doxorubicin and interleukin-2 (IL-2) showed great potential for developing a viable strategy to remodel the tumour immune microenvironment and achieve enhanced antitumour effect [99].
Finally, nanoencapsulation may enhance the effect of dietary vitamin A supplementation. Novel carotenoid delivery systems have gained much attention in the food industry due to their enhanced absorption and bioavailability. After oral ingestion, nanocarriers can easily penetrate the mucus barriers, resulting in better cellular uptake [100][101][102]. Currently, polymeric nanocapsules are the most widely used due to their high encapsulation efficiency, stability during storage and controlled release of the encapsulated carotenoid [103].
6. Novel Retinoid Pathway Therapeutic Targets
Several recent studies have suggested new therapeutic targets related to components of the retinoid pathway. For example, RORC, CRBP1, ALDH1A1 and TUBB3 (discussed in Chapter 3) have been proposed as potential therapeutic targets for BC. Preclinical in vitro experiments showed that increased expression of RORC suppressed cell proliferation and glucose metabolism and induced apoptosis in BC cells. Moreover, activation of RORC in BC cells increased cisplatin-induced apoptosis. These findings established RORC and RORC-mediated signalling as potential therapeutic targets for BC [104]. Increased expression of CRBP1 in transfected BC cell lines reduced cell growth and migration activity [105]. Moreover, in other cancers with decreased expression of CRBP1 (similar to BC), it was reported that forced overexpression of CRBP1 resulted in increased susceptibility to retinoids [106][107]. Namekawa et al. showed that ALDH1A1 and its putative downstream target TUBB3 could be exploited for therapeutic options in advanced disease [108]. Inhibition of ALDH1A1 by ALDH inhibitors and silenced ALDH1A1 expression by shRNA lentiviral transfer suppressed proliferation and spheroid formation of cancer cells from long-term BC patients. In addition, knockdown of TUBB3 also suppressed proliferation of these cells [108]. Taken together, these results suggest that RORC, CRBP1, ALDH1A1 and TUBB3 may be promising candidates for gene therapy or novel targets for improved adjuvant retinoid therapy of human BC.
Recently, a negative correlation between miR-29b, which functions as an oncogenic microRNA, and RARβ expression was demonstrated in a preclinical study of urothelial carcinoma [109]. The study showed that inhibition of miR-29b suppressed cell proliferation, growth, migration, invasion, and tumour growth via RARβ. Moreover, inhibitor of growth protein 4 (ING4) was identified as a tumour suppressor that directly interacts with RARβ. Silencing of ING4 reversed the RARβ-mediated suppression of cell migration and invasion. Thus, restoring RARβ and ING4 by inhibiting miR-29b may serve as a potential therapeutic target in BC [109].
References
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258.
- Bushue, N.; Wan, Y.J. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 1285–1298.
- Al Binali, H.A. Night blindness and ancient remedy. Heart Views 2014, 15, 136–139.
- Wolf, G. A history of vitamin A and retinoids. FASEB J. 1996, 10, 1102–1107.
- Maumenee, A.E. The history of vitamin A and its ophthalmic implications. A personal viewpoint. Arch. Ophthalmol. 1993, 111, 547–550.
- Clagett-Dame, M.; Knutson, D. Vitamin A in reproduction and development. Nutrients 2011, 3, 385–428.
- Zhu, G. Vitamin A and its Derivatives-Retinoic Acid and Retinoid Pharmacology. Am. J. Biomed. Sci. Res. 2019, 3, 162–177.
- Dao, D.Q.; Ngo, T.C.; Thong, N.M.; Nam, P.C. Is Vitamin A an Antioxidant or a Pro-oxidant? J. Phys. Chem. B 2017, 121, 9348–9357.
- Siddikuzzaman; Grace, V.M. Antioxidant potential of all-trans retinoic acid (ATRA) and enhanced activity of liposome encapsulated ATRA against inflammation and tumor-directed angiogenesis. Immunopharmacol. Immunotoxicol. 2013, 35, 164–173.
- Iyer, N.; Vaishnava, S. Vitamin A at the interface of host-commensal-pathogen interactions. PLoS Pathog. 2019, 15, e1007750.
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192.
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700.
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy. Dermatol. Alergol. 2019, 36, 392–397.
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235.
- Dobrotkova, V.; Chlapek, P.; Mazanek, P.; Sterba, J.; Veselska, R. Traffic lights for retinoids in oncology: Molecular markers of retinoid resistance and sensitivity and their use in the management of cancer differentiation therapy. BMC Cancer 2018, 18, 1059.
- Chlapek, P.; Slavikova, V.; Mazanek, P.; Sterba, J.; Veselska, R. Why Differentiation Therapy Sometimes Fails: Molecular Mechanisms of Resistance to Retinoids. Int. J. Mol. Sci. 2018, 19, 132.
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904.
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Ferreira, R.; Napoli, J.; Enver, T.; Bernardino, L.; Ferreira, L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat. Commun. 2020, 11, 4265.
- Tang, J.E.; Wang, R.J.; Zhong, H.; Yu, B.; Chen, Y. Vitamin A and risk of bladder cancer: A meta-analysis of epidemiological studies. World J. Surg. Oncol. 2014, 12, 130.
- Wu, S.; Liu, Y.; Michalek, J.E.; Mesa, R.A.; Parma, D.L.; Rodriguez, R.; Mansour, A.M.; Svatek, R.; Tucker, T.C.; Ramirez, A.G. Carotenoid Intake and Circulating Carotenoids Are Inversely Associated with the Risk of Bladder Cancer: A Dose-Response Meta-analysis. Adv. Nutr. 2020, 11, 630–643.
- Uray, I.P.; Dmitrovsky, E.; Brown, P.H. Retinoids and rexinoids in cancer prevention: From laboratory to clinic. Semin. Oncol. 2016, 43, 49–64.
- Mori, S. The changes in the paraocular glands which follow the administration of diets low in fat-soluble vitamin A with notes of the effects of the same diets on the salivary glands and the mucosa of the larynx and brachea. John Hopkins Hospital. Bull. 1922, 33, 357–359.
- Wolbach, S.B.; Howe, P.R. Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med. 1925, 42, 753–777.
- Wolbach, S.B.; Howe, P.R. Vitamin A Deficiency in the Guineapig. Arch. Path. Lab. Med. 1928, 5, 239–253.
- Fujimaki, Y. Formation of gastric carcinoma in albino rats fed on deficient diets. J. Cancer Res. 1926, 10, 469–477.
- Mettlin, C.; Graham, S. Dietary risk factors in human bladder cancer. Am. J. Epidemiol. 1979, 110, 255–263.
- Martini, S.; Rizzello, A.; Corsini, I.; Romanin, B.; Fiorentino, M.; Grandi, S.; Bergamaschi, R. Vitamin A Deficiency Due to Selective Eating as a Cause of Blindness in a High-Income Setting. Pediatrics 2018, 141, S439–S444.
- Gröber, U. Vitamin A (Retinol): Stiefkind der Ernährungsmedizin. Erfahrungsheilkunde 2020, 69, 334–339.
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536.
- Surman, S.L.; Penkert, R.R.; Sealy, R.E.; Jones, B.G.; Marion, T.N.; Vogel, P.; Hurwitz, J.L. Consequences of Vitamin A Deficiency: Immunoglobulin Dysregulation, Squamous Cell Metaplasia, Infectious Disease, and Death. Int. J. Mol. Sci. 2020, 21, 5570.
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108.
- He, H.; Xie, H.; Chen, Y.; Li, C.; Han, D.; Xu, F.; Lyu, J. Global, regional, and national burdens of bladder cancer in 2017: Estimates from the 2017 global burden of disease study. BMC Public Health 2020, 20, 1693.
- Steinmaus, C.M.; Nuñez, S.; Smith, A.H. Diet and bladder cancer: A meta-analysis of six dietary variables. Am. J. Epidemiol. 2000, 151, 693–702.
- Luo, X.; Lu, H.; Li, Y.; Wang, S. Carrot intake and incidence of urothelial cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 77957–77962.
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201.
- WHO. Guidelines Approved by the Guidelines Review Committee. In Guideline: Vitamin A Supplementation in Pregnant Women; WHO: Geneva, Switzerland, 2011.
- Piyathilake, C. Dietary factors associated with bladder cancer. Investig. Clin. Urol. 2016, 57, S14–S25.
- Al-Zalabani, A.H.; Stewart, K.F.; Wesselius, A.; Schols, A.M.; Zeegers, M.P. Modifiable risk factors for the prevention of bladder cancer: A systematic review of meta-analyses. Eur. J. Epidemiol. 2016, 31, 811–851.
- Clifford, J.L.; Sabichi, A.L.; Zou, C.; Yang, X.; Steele, V.E.; Kelloff, G.J.; Lotan, R.; Lippman, S.M. Effects of novel phenylretinamides on cell growth and apoptosis in bladder cancer. Cancer Epidemiol. Biomark. Prev. 2001, 10, 391–395.
- Nutting, C.; Chowaniec, J. Evaluation of the actions and interactions of retinoic acid and epidermal growth factor on transformed urothelial cells in culture: Implications for the use of retinoid therapy in the treatment of bladder cancer patients. Clin. Oncol. 1992, 4, 51–55.
- Laaksovirta, S.; Rajala, P.; Nurmi, M.; Tammela, T.; Laato, M. The cytostatic effect of 9-cis-retinoic acid, tretinoin, and isotretinoin on three different human bladder cancer cell lines in vitro. Urol. Res. 1999, 27, 17–22.
- Zou, C.; Zhou, J.; Qian, L.; Feugang, J.M.; Liu, J.; Wang, X.; Wu, S.; Ding, H.; Zou, C.; Liebert, M. Comparing the effect of ATRA, 4-HPR, and CD437 in bladder cancer cells. Front. Biosci. 2006, 11, 2007–2016.
- Chien, C.-L.; Chen, T.-W.; Lin, Y.-S.; Lu, K.-S. The apoptotic process of human bladder carcinoma T24 cells induced by retinoid. J. Biomed. Sci. 2004, 11, 631–640.
- Southgate, J.; Hutton, K.; Thomas, D.; Trejdosiewicz, L.K. Normal human urothelial cells in vitro: Proliferation and induction of stratification. Lab. Investig. 1994, 71, 583.
- Boström, P.J.; Ravanti, L.; Reunanen, N.; Aaltonen, V.; Söderström, K.O.; Kähäri, V.M.; Laato, M. Expression of collagenase-3 (matrix metalloproteinase-13) in transitional-cell carcinoma of the urinary bladder. Int. J. Cancer 2000, 88, 417–423.
- Wang, E.; Li, J.; Yang, G.; Zhong, S.; Liu, T. Impact of 4HPR on the expression of E-Cad in human bladder transitional epithelial cancer cells T24. Acta Acad. Med. Wuhan 2012, 32, 237–241.
- Lin, F.; Kolluri, S.K.; Chen, G.-q.; Zhang, X.-k. Regulation of retinoic acid-induced inhibition of AP-1 activity by orphan receptor chicken ovalbumin upstream promoter-transcription factor. J. Biol. Chem. 2002, 277, 21414–21422.
- Costantini, L.; Molinari, R.; Farinon, B.; Lelli, V.; Timperio, A.M.; Merendino, N. Docosahexaenoic Acid Reverted the All-trans Retinoic Acid-Induced Cellular Proliferation of T24 Bladder Cancer Cell Line. J. Clin. Med. 2020, 9, 2494.
- Zhiping, W.; Zhihua, Z.; Yinmei, L.; Yirong, C.; Qinxi, L.; Dashan, Q.; Guodong, L.; Liufang, W. Effect of retinoic acid and its complexes with transition metals on human bladder cancer cell line EJ in vitro. Urol. Res. 2000, 28, 191–195.
- Zou, C.; Liebert, M.; Zou, C.; Grossman, H.B.; Lotan, R. Identification of effective retinoids for inhibiting growth and inducing apoptosis in bladder cancer cells. J. Urol. 2001, 165, 986–992.
- Lubet, R.A.; Clapper, M.L.; McCormick, D.L.; Pereira, M.A.; Chang, W.; Steele, V.E.; Fischer, S.M.; Juliana, M.M.; Grubbs, C.J. Chemopreventive efficacy of Targretin in rodent models of urinary bladder, colon/intestine, head and neck and mammary cancers. Oncol. Rep. 2012, 27, 1400–1406.
- Murasaki, G.i.; Miyata, Y.; Babaya, K.; Arai, M.; Fukushima, S.; Ito, N. Inhibitory effect of an aromatic retinoic acid analog on urinary bladder carcinogenesis in rats treated with N-butyl-N-(4-hydroxybutyl) nitrosamine. GANN 1980, 71, 333–340.
- Fujita, J.; Tokuda, H.; Ito, Y.; Yoshida, O. Therapeutic effect of a retinoid (Ro 10-9359) on rats with bladder tumours induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine upon administration alone or in combination with mitomycin C. Urol. Res. 1983, 11, 227–230.
- Zupančič, D.; Korać-Prlić, J.; Kreft, M.E.; Franković, L.; Vilović, K.; Jeruc, J.; Romih, R.; Terzić, J. Vitamin A Rich Diet Diminishes Early Urothelial Carcinogenesis by Altering Retinoic Acid Signaling. Cancers 2020, 12, 1712.
- Dawson, W.; Miller, W.; Liles, W. Retinyl acetate prophylaxis in cancer of the urinary bladder. Investig. Urol. 1979, 16, 376–377.
- Hemstreet III, G.P.; Rao, J.Y.; Hurst, R.E.; Bonner, R.B.; Jones, P.L.; Vaidya, A.M.; Fradet, Y.; Moon, R.C.; Kelloff, G.J. Intermediate endpoint biomarkers for chemoprevention. J. Cell Biochem. 1992, 50, 93–110.
- Qian, L.; Ding, H.; Zhou, J.; Wang, X.; Shao, P.; Wu, S.; Yang, J.; Feugang, J.M.; Zou, C. Intravesical N-(4-hydroxyphenyl) retinamide and adriamycin induces apoptosis in bladder cancer. Front. Biosci. 2006, 11, 2045–2051.
- Grubbs, C.J.; Moon, R.C.; Squire, R.A.; Farrow, G.M.; Stinson, S.F.; Goodman, D.G.; Brown, C.C.; Sporn, M.B. 13-cis-Retinoic acid: Inhibition of bladder carcinogenesis induced in rats by N-butyl-N-(4-hydroxybutyl) nitrosamine. Science 1977, 198, 743–744.
- Becci, P.J.; Thompson, H.J.; Grubbs, C.J.; Brown, C.C.; Moon, R.C. Effect of delay in administration of 13-cis-retinoic acid on the inhibition of urinary bladder carcinogenesis in the rat. Cancer Res. 1979, 39, 3141–3144.
- Becci, P.J.; Thompson, H.J.; Grubbs, C.J.; Squire, R.A.; Brown, C.C.; Sporn, M.B.; Moon, R.C. Inhibitory effect of 13-cis-retinoic acid on urinary bladder carcinogenesis induced in C57BL/6 mice by N-butyl-N-(4-hydroxybutyl) nitrosamine. Cancer Res. 1978, 38, 4463–4466.
- Becci, P.J.; Thompson, H.J.; Strum, J.M.; Brown, C.C.; Sporn, M.B.; Moon, R.C. N-butyl-N-(4-hydroxybutyl) nitrosamine-induced urinary bladder cancer in C57BL/6× DBA/2 F1 mice as a useful model for study of chemoprevention of cancer with retinoids. Cancer Res. 1981, 41, 927–932.
- Sporn, M.B.; Squire, R.A.; Brown, C.C.; Smith, J.M.; Wenk, M.L.; Springer, S. 13-cis-retinoic acid: Inhibition of bladder carcinogenesis in the rat. Science 1977, 195, 487–489.
- Squire, R.A.; Sporn, M.B.; Brown, C.C.; Smith, J.M.; Wenk, M.L.; Springer, S. Histopathological evaluation of the inhibition of rat bladder carcinogenesis by 13-cis-retinoic acid. Cancer Res. 1977, 37, 2930–2936.
- Tannenbaum, M.; Tannenbaum, S.; Richelo, B.; Trown, P. Effects of 13-cis and all-trans-retinoic acid on the development of bladder cancer in rats: An ultrastructural study. Scan. Electron. Microsc. 1979, 3, 673–678.
- Thompson, H.J.; Becci, P.J.; Grubbs, C.J.; Shealy, Y.F.; Stanek, E.J.; Brown, C.C.; Sporn, M.B.; Moon, R.C. Inhibition of urinary bladder cancer by N-(ethyl)-all-trans-retinamide and N-(2-hydroxyethyl)-all-trans-retinamide in rats and mice. Cancer Res. 1981, 41, 933–936.
- Croft, W.A.; Croft, M.A.; Paulus, K.P.; Williams, J.H.; Wang, C.Y.; Lower, G.M., Jr. Synthetic retinamides: Effect on urinary bladder carcinogenesis by FANFT in Fischer rats. Carcinogenesis 1981, 2, 515–517.
- Croft, W.A.; Croft, M.A.; Paulus, K.P.; Williams, J.H.; Wang, C.Y.; Lower, G.M., Jr. 13-cis-retinoic acid: Effect on urinary bladder carcinogenesis by N-[4-(5-nitro-2-furyl)-2-thiazolyl]-formamide in Fischer rats. Cancer Lett. 1981, 12, 355–360.
- Kobayashi, T.; Owczarek, T.B.; McKiernan, J.M.; Abate-Shen, C. Modelling bladder cancer in mice: Opportunities and challenges. Nar. Rev. Cancer 2015, 15, 42–54.
- John, B.A.; Said, N. Insights from animal models of bladder cancer: Recent advances, challenges, and opportunities. Oncotarget 2017, 8, 57766.
- He, Z.; Kosinska, W.; Zhao, Z.-L.; Wu, X.-R.; Guttenplan, J.B. Tissue-specific mutagenesis by N-butyl-N-(4-hydroxybutyl)nitrosamine as the basis for urothelial carcinogenesis. Mutat. Res. 2012, 742, 92–95.
- Nagao, M.; Suzuki, E.; Yasuo, K.; Yahagi, T.; Seino, Y. Mutagenicity of N-butyl-N-(4-hydroxybutyl)nitrosamine, a bladder carcinogen, and related compounds. Cancer Res. 1977, 37, 399–407.
- Ariel, I.; Ayesh, S.; Gofrit, O.; Ayesh, B.; Abdul-Ghani, R.; Pizov, G.; Smith, Y.; Sidi, A.A.; Birman, T.; Schneider, T.; et al. Gene expression in the bladder carcinoma rat model. Mol. Carcinog. 2004, 41, 69–76.
- Kates, M.; Nirschl, T.; Sopko, N.A.; Matsui, H.; Kochel, C.M.; Reis, L.O.; Netto, G.J.; Hoque, M.O.; Hahn, N.M.; McConkey, D.J. Intravesical BCG induces CD4+ T-cell expansion in an immune competent model of bladder cancer. Cancer Immunol. Res. 2017, 5, 594–603.
- Chopra, B.; Hinley, J.; Oleksiewicz, M.B.; Southgate, J. Trans-species comparison of PPAR and RXR expression by rat and human urothelial tissues. Toxicol. Pathol. 2008, 36, 485–495.
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.-J. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643.
- Villablanca, J.G.; Khan, A.A.; Avramis, V.I.; Seeger, R.C.; Matthay, K.K.; Ramsay, N.; Reynolds, C.P. Phase I trial of 13-cis-retinoic acid in children with neuroblastoma following bone marrow transplantation. J. Clin. Oncol. 1995, 13, 894–901.
- Decensi, A.; Bruno, S.; Costantini, M.; Torrisi, R.; Curotto, A.; Gatteschi, B.; Nicolò, G.; Polizzi, A.; Perloff, M.; Malone, W.F.; et al. Phase IIa study of fenretinide in superficial bladder cancer, using DNA flow cytometry as an intermediate end point. J. Natl. Cancer Inst. 1994, 86, 138–140.
- Decensi, A.; Torrisi, R.; Bruno, S.; Costantini, M.; Curotto, A.; Nicolò, G.; Malcangi, B.; Baglietto, L.; Bruttini, G.P.; Gatteschi, B.; et al. Randomized trial of fenretinide in superficial bladder cancer using DNA flow cytometry as an intermediate end point. Cancer Epidemiol. Biomarkers Prev. 2000, 9, 1071–1078.
- Puntoni, M.; Petrera, M.; Campora, S.; Garrone, E.; Defferrari, C.; Torrisi, R.; Johansson, H.; Bruno, S.; Curotto, A.; DeCensi, A. Prognostic Significance of VEGF after Twenty-Year Follow-up in a Randomized Trial of Fenretinide in Non–Muscle-Invasive Bladder Cancer. Cancer Prev. Res. 2016, 9, 437–444.
- Torrisi, R.; Mezzetti, M.; Johansson, H.; Barreca, A.; Pigatto, F.; Robertson, C.; Decensi, A. Time course of fenretinide-induced modulation of circulating insulin-like growth factor (IGF)-i, IGF-II and IGFBP-3 in a bladder cancer chemoprevention trial. Int. J. Cancer. 2000, 87, 601–605.
- Sabichi, A.L.; Lerner, S.P.; Atkinson, E.N.; Grossman, H.B.; Caraway, N.P.; Dinney, C.P.; Penson, D.F.; Matin, S.; Kamat, A.; Pisters, L.L. Phase III Prevention Trial of Fenretinide in Patients with Resected Non–Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2008, 14, 224–229.
- Alfthan, O.; Tarkkanen, J.; Gröhn, P.; Heinonen, E.; Pyrhönen, S. Tigason®(etretinate) in prevention of recurrence of superficial bladder tumors. Eur. Urol. 1983, 9, 6–9.
- Studer, U.E.; Jenzer, S.; Biederman, C.; Chollet, D.; Rainer, K.; von Toggenburg, H.; Vonbank, F. Adjuvant treatment with a vitamin A analogue (etretinate) after transurethral resection of superficial bladder tumors. Eur. Urol. 1995, 28, 284–290.
- Pedersen, H.; Wolf, H.; Jensen, S.K.; Lund, F.; Hansen, E.; Olsen, P.R.; Sørensen, B.L. Administration of a retinoid as prophylaxis of recurrent non-invasive bladder tumors. Scand. J. Urol. Nephrol. 1984, 18, 121–123.
- Prout, G.R., Jr.; Barton, B.A.; Kontz, W., Jr.; Loening, S.; Flangan, M.; Branen, G. 13-cis-Retinoic acid in chemopreventiion of superficial bladder cancer. J. Cell Biochem. 1992, 50, 148–152.
- Hameed, D.A.; el-Metwally, T.H. The effectiveness of retinoic acid treatment in bladder cancer: Impact on recurrence, survival and TGFalpha and VEGF as end-point biomarkers. Cancer Biol. Ther. 2008, 7, 92–100.
- Pili, R.; Salumbides, B.; Zhao, M.; Altiok, S.; Qian, D.; Zwiebel, J.; Carducci, M.A.; Rudek, M.A. Phase I study of the histone deacetylase inhibitor entinostat in combination with 13-cis retinoic acid in patients with solid tumours. Br. J. Cancer 2012, 106, 77–84.
- Sabichi, A.L.; Lerner, S.P.; Grossman, H.B.; Lippman, S.M. Retinoids in the chemoprevention of bladder cancer. Curr. Opin. Oncol 1998, 10, 479–484.
- Lu, C.S.; Shieh, G.S.; Wang, C.T.; Su, B.H.; Su, Y.C.; Chen, Y.C.; Su, W.C.; Wu, P.; Yang, W.H.; Shiau, A.L.; et al. Chemotherapeutics-induced Oct4 expression contributes to drug resistance and tumor recurrence in bladder cancer. Oncotarget 2016, 8, 30844–30858.
- Ozgun, G.; Senturk, S.; Erkek-Ozhan, S. Retinoic acid signaling and bladder cancer: Epigenetic deregulation, therapy and beyond. Int. J. Cancer 2020.
- Chen, J.; Cao, X.; An, Q.; Zhang, Y.; Li, K.; Yao, W.; Shi, F.; Pan, Y.; Jia, Q.; Zhou, W.; et al. Inhibition of cancer stem cell like cells by a synthetic retinoid. Nat. Commun. 2018, 9, 1406.
- Ye, L.; Song, Q. Promising potency of retinoic acid-poly(ethylene glycol)-thiol gold nanoparticle conjugates for cervical cancer treatment. Int. J. Clin. Exp. Med. 2015, 8, 10501–10507.
- Orienti, I.; Francescangeli, F.; De Angelis, M.L.; Fecchi, K.; Bongiorno-Borbone, L.; Signore, M.; Peschiaroli, A.; Boe, A.; Bruselles, A.; Costantino, A.; et al. A new bioavailable fenretinide formulation with antiproliferative, antimetabolic, and cytotoxic effects on solid tumors. Cell Death Dis. 2019, 10, 529.
- Thomas, J.S.; El-Khoueiry, A.B.; Maurer, B.J.; Groshen, S.; Pinski, J.K.; Cobos, E.; Gandara, D.R.; Lenz, H.J.; Kang, M.H.; Reynolds, C.P.; et al. A phase I study of intravenous fenretinide (4-HPR) for patients with malignant solid tumors. Cancer Chemother. Pharmacol. 2021, 87, 525–532.
- Orienti, I.; Salvati, V.; Sette, G.; Zucchetti, M.; Bongiorno-Borbone, L.; Peschiaroli, A.; Zolla, L.; Francescangeli, F.; Ferrari, M.; Matteo, C.; et al. A novel oral micellar fenretinide formulation with enhanced bioavailability and antitumour activity against multiple tumours from cancer stem cells. J. Exp. Clin. Cancer Res. 2019, 38, 373.
- Sun, R.; Liu, Y.; Li, S.Y.; Shen, S.; Du, X.J.; Xu, C.F.; Cao, Z.T.; Bao, Y.; Zhu, Y.H.; Li, Y.P.; et al. Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials 2015, 37, 405–414.
- Kong, M.; Tang, J.; Qiao, Q.; Wu, T.; Qi, Y.; Tan, S.; Gao, X.; Zhang, Z. Biodegradable Hollow Mesoporous Silica Nanoparticles for Regulating Tumor Microenvironment and Enhancing Antitumor Efficiency. Theranostics 2017, 7, 3276–3292.
- Du, Y.; Bao, C.; Huang, J.; Jiang, P.; Jiao, L.; Ren, F.; Li, Y. Improved stability, epithelial permeability and cellular antioxidant activity of β-carotene via encapsulation by self-assembled α-lactalbumin micelles. Food Chem. 2019, 271, 707–714.
- Hidalgo, T.; Giménez-Marqués, M.; Bellido, E.; Avila, J.; Asensio, M.C.; Salles, F.; Lozano, M.V.; Guillevic, M.; Simón-Vázquez, R.; González-Fernández, A.; et al. Chitosan-coated mesoporous MIL-100(Fe) nanoparticles as improved bio-compatible oral nanocarriers. Sci. Rep. 2017, 7, 43099.
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al. The colorful world of carotenoids: A profound insight on therapeutics and recent trends in nano delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 1–40.
- Pereira, A.R.; Mendes, T.F.; Ministro, A.; Teixeira, M.; Filipe, M.; Santos, J.M.; Barcia, R.N.; Goyri-O’Neill, J.; Pinto, F.; Cruz, P.E.; et al. Therapeutic angiogenesis induced by human umbilical cord tissue-derived mesenchymal stromal cells in a murine model of hindlimb ischemia. Stem Cell Res. Ther. 2016, 7, 145.
- Cao, D.; Qi, Z.; Pang, Y.; Li, H.; Xie, H.; Wu, J.; Huang, Y.; Zhu, Y.; Shen, Y.; Zhu, Y. Retinoic Acid–Related Orphan Receptor C Regulates Proliferation, Glycolysis, and Chemoresistance via the PD-L1/ITGB6/STAT3 Signaling Axis in Bladder Cancer. Cancer Res. 2019, 79, 2604–2618.
- Toki, K.; Enokida, H.; Kawakami, K.; Chiyomaru, T.; Tatarano, S.; Yoshino, H.; Uchida, Y.; Kawahara, K.; Nishiyama, K.; Seki, N.; et al. CpG hypermethylation of cellular retinol-binding protein 1 contributes to cell proliferation and migration in bladder cancer. Int. J. Oncol. 2010, 37, 1379–1388.
- Doldo, E.; Costanza, G.; Ferlosio, A.; Passeri, D.; Bernardini, S.; Scioli, M.G.; Mazzaglia, D.; Agostinelli, S.; Del Bufalo, D.; Czernobilsky, B.; et al. CRBP-1 expression in ovarian cancer: A potential therapeutic target. Anticancer Res. 2014, 34, 3303–3312.
- Yokoi, K.; Yamashita, K.; Ishii, S.; Tanaka, T.; Nishizawa, N.; Tsutsui, A.; Miura, H.; Katoh, H.; Yamanashi, T.; Naito, M. Comprehensive molecular exploration identified promoter DNA methylation of the CRBP1 gene as a determinant of radiation sensitivity in rectal cancer. Br. J. Cancer 2017, 116, 1046–1056.
- Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Suzuki, T.; Okamoto, K.; Ichikawa, T.; Yano, A.; Kawakami, S.; Inoue, S. ALDH1A1 in patient-derived bladder cancer spheroids activates retinoic acid signaling leading to TUBB3 overexpression and tumor progression. Int. J. Cancer 2020, 146, 1099–1113.
- Liu, H.J.; Lam, H.C.; Baglini, C.V.; Nijmeh, J.; Cottrill, A.A.; Chan, S.Y.; Henske, E.P. Rapamycin-upregulated miR-29b promotes mTORC1-hyperactive cell growth in TSC2-deficient cells by downregulating tumor suppressor retinoic acid receptor β (RARβ). Oncogene 2019, 38, 7367–7383.
 Encyclopedia
Encyclopedia
