The process of inorganic polymerization or the sol–gel method is an inexpensive, low-energy consuming, and stable process for obtaining high-purity ceramics, which offer versatility for the production of different kinds of devices, some of which can be used in surface-enhanced spectroscopy such as Surface Enhanced Infrared Absorption Spectroscopy (SEIRAS) and Surface Enhanced Raman Spectroscopy (SERS).
- sol–gel
- ceramics
- SEIRAS
- SERS
- spectroscopy
1. Introduction
The process of inorganic polymerization or the sol–gel method is an inexpensive, low-energy consuming, and stable process for obtaining high-purity ceramics, which offer versatility for the production of different kinds of devices, some of which can be used in surface-enhanced spectroscopy such as Surface Enhanced Infrared Absorption Spectroscopy (SEIRAS) and Surface Enhanced Raman Spectroscopy (SERS) [1,2,3]. The sol–gel process uses reactants such as alkoxides and metal salts involved in a relatively easy hydrolysis reaction [4]. The alkoxides or salts are partially hydrolyzed and then polymerized through condensation, allowing the formation of a tridimensional structure, a gel [5]. Sol–gel-derived ceramics, glasses, and composites offer distinct properties that can be taken advantage of in order to shape or fashion materials with uses ranging from thin films to matrixes, fibers, and monoliths, amongst others [6,7]. A variety of uses such as corrosion protection [8], antibacterial activity [9], bioactive behavior [10], energy storage [11], and catalytic activity [12] can be achieved. Sol–gel ceramic materials can be tailor-made in the nanoscale dimension [13]. Single component or composites [14] allow for exploitation of the properties of the constituents as well as the properties of the ultrastructure of the material [15].
The process of inorganic polymerization or the sol–gel method is an inexpensive, low-energy consuming, and stable process for obtaining high-purity ceramics, which offer versatility for the production of different kinds of devices, some of which can be used in surface-enhanced spectroscopy such as Surface Enhanced Infrared Absorption Spectroscopy (SEIRAS) and Surface Enhanced Raman Spectroscopy (SERS) [1][2][3]. The sol–gel process uses reactants such as alkoxides and metal salts involved in a relatively easy hydrolysis reaction [4]. The alkoxides or salts are partially hydrolyzed and then polymerized through condensation, allowing the formation of a tridimensional structure, a gel [5]. Sol–gel-derived ceramics, glasses, and composites offer distinct properties that can be taken advantage of in order to shape or fashion materials with uses ranging from thin films to matrixes, fibers, and monoliths, amongst others [6][7]. A variety of uses such as corrosion protection [8], antibacterial activity [9], bioactive behavior [10], energy storage [11], and catalytic activity [12] can be achieved. Sol–gel ceramic materials can be tailor-made in the nanoscale dimension [13]. Single component or composites [14] allow for exploitation of the properties of the constituents as well as the properties of the ultrastructure of the material [15].
Through various synthesis and surface modification techniques that focus on the production of nanostructures with complex shapes, it has been possible to obtain amplification surfaces with different degrees of success for spectroscopy [16]. The shape of the nanostructures can increase the number of hot spots on the amplification surface, but the function ultimately depends on the reproducibility of the nanostructures and the response, the characteristics of the materials used such as chemical and mechanical resistance, as well as the cost–benefit ratio of the materials used. The use of sol–gel ceramic precursors and the electrospinning technique make the production of fibrous ceramic enhancement substrates for infrared and Raman spectroscopy possible [17]. Such materials have an increased surface area, which provides the capability to enhance spectroscopy signals due to better contact with the analytes [18].
Sol–gel ceramics can also provide the chemical and mechanical resistance much needed for reuse of the substrates [19]. In this review, we discussed the use of the sol–gel method for the obtention of ceramics such as silica, zirconia, titania, hydroxyapatite, lithium niobate, and alumina and their role in the production of enhancement substrates. Lastly, some advances in this research path have been highlighted.
2. Electrospinning of Sol–Gel Ceramic Precursors
The production of sol–gel ceramics coupled with the electrospinning technique has been helpful in the obtention of composite materials designed for diverse purposes such as tissue engineering [102], energy devices [103], environmental solutions [104], and photocatalysis [105]. The electrospinning technique allows the production of fibers on the nanometric scale [106], providing an increase in surface area [107], which allows contact with analytes, tissues, and any chemical compound alike. The production of ceramic nanofibers with high porosity makes the surface area even greater, with successful results in most cases where applied.The production of sol–gel ceramics coupled with the electrospinning technique has been helpful in the obtention of composite materials designed for diverse purposes such as tissue engineering [20], energy devices [21], environmental solutions [22], and photocatalysis [23]. The electrospinning technique allows the production of fibers on the nanometric scale [24], providing an increase in surface area [25], which allows contact with analytes, tissues, and any chemical compound alike. The production of ceramic nanofibers with high porosity makes the surface area even greater, with successful results in most cases where applied.
Table 3 lists some cases where ceramic nanofibers obtained through the electrospinning technique were produced. Ávila-Martínez et al. [108] produced a material of ZrO1 lists some cases where ceramic nanofibers obtained through the electrospinning technique were produced. Ávila-Martínez et al. [26] produced a material of ZrO
2(
Figure 6a) obtained through the sol–gel method using zirconium butoxide as a precursor mixed on polyvinylpyrrolidone (PVP) and electrospun into fibers for the capture of Allura red dye. Average fiber diameters ranged from approximately 112 nm to 360 nm. The fibers had an estimated adsorption of 0.895 g/mg of the dye. Koo et al. [109] created yttria-stabilizaed zirconia nanofibers using metal salts such as zirconium acetate and yttrium nitrate. The fibers had average diameters between 150 and 120 nm. Pescador-Rojas et al. [110] produced a fibrous composite for heat transport applications, with titania obtained by the sol gel-method using titanium (IV) n-butoxide as a precursor and acetic acid as a catalyzer. The ZrO1a) obtained through the sol–gel method using zirconium butoxide as a precursor mixed on polyvinylpyrrolidone (PVP) and electrospun into fibers for the capture of Allura red dye. Average fiber diameters ranged from approximately 112 nm to 360 nm. The fibers had an estimated adsorption of 0.895 g/mg of the dye. Koo et al. [27] created yttria-stabilizaed zirconia nanofibers using metal salts such as zirconium acetate and yttrium nitrate. The fibers had average diameters between 150 and 120 nm. Pescador-Rojas et al. [28] produced a fibrous composite for heat transport applications, with titania obtained by the sol gel-method using titanium (IV) n-butoxide as a precursor and acetic acid as a catalyzer. The ZrO
2sol was mixed with PVP for subsequent electrospinning. The thermal diffusivity of the material was estimated as 1.52 × 10
−3cm
2s
−1.
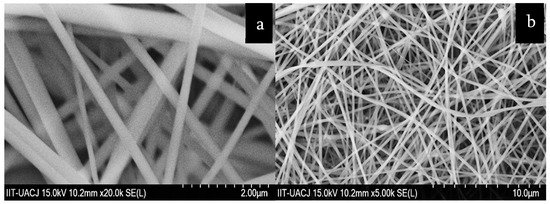
ZrO
fibers for dye capture (
) and hydroxyapatite–glass fibers for metal adsorption (
).
Ceramics used in electrospinning for nanofiber obtainment.
| Ceramic | Diameter (nm) | Use | Reference |
|---|---|---|---|
| Silica | ≈500 | Beryllium uptake | [108][26] |
| Zirconia | ≈360 | Dye sorption | [109][27] |
| Zirconia | ≤200 | Fuel cells | [110][28] |
| Titania | ≤80 | Heat transport | [111][29] |
| Titania | ≈160 | Antimicrobial activity | [112][30] |
| Hydroxyapatite | ≤330 | Bioactive behavior | [113][31] |
| Hydroxyapatite | ≈150 | Metal adsorption | [114][32] |
| Lithium niobate | ≈190 | Piezoelectric behavior | [115][33] |
| Alumina | ≤250 | Filtration device | [116][34] |
| Alumina | ≤250 | Catalyst support | [117][35] |
Lopez de Dicastillo et al. [29] produced a TiO
2 material of hollow nanofibers or nanotubes using tetrakis (dimethylamide) titanium as a precursor and electrospun with a polymer; then, the polymeric template was removed. The material was tested in concentrations of 150 to 400 µg/mL for antibacterial activity with high or total inhibition of bacteria. Garibay-Alvarado et al. [112] produced a composite of coaxial fibers, with a silica core and a hydroxyapatite sheath. Both materials were obtained by the sol–gel method using TEOS as a precursor for the silica, and triethyl phosphite and calcium nitrate for hydroxyapatite. The fibers had approximate diameters ranging from 510 to 560 nm. Roque-Ruiz et al. [113] produced a fibrous composite consisting of silica and hydroxyapatite obtained through the sol–gel method and then mixed in a single polymeric phase for use in the capture of cadmium and lead from aqueous solution. The precursors used were TEOS and triethyl phosphite/calcium nitrate for silica and hydroxyapatite, respectively. The mean diameter of the fibers was approximately 150 nm. The material achieved an amount adsorbed of Cdmaterial of hollow nanofibers or nanotubes using tetrakis (dimethylamide) titanium as a precursor and electrospun with a polymer; then, the polymeric template was removed. The material was tested in concentrations of 150 to 400 µg/mL for antibacterial activity with high or total inhibition of bacteria. Garibay-Alvarado et al. [30] produced a composite of coaxial fibers, with a silica core and a hydroxyapatite sheath. Both materials were obtained by the sol–gel method using TEOS as a precursor for the silica, and triethyl phosphite and calcium nitrate for hydroxyapatite. The fibers had approximate diameters ranging from 510 to 560 nm. Roque-Ruiz et al. [31] produced a fibrous composite consisting of silica and hydroxyapatite obtained through the sol–gel method and then mixed in a single polymeric phase for use in the capture of cadmium and lead from aqueous solution. The precursors used were TEOS and triethyl phosphite/calcium nitrate for silica and hydroxyapatite, respectively. The mean diameter of the fibers was approximately 150 nm. The material achieved an amount adsorbed of Cd
2+of 93.3 mg/g and 466.98 mg/g of Pb
2+(
Figure 6b). Garibay-Alvarado et al. [114] produced a fibrillar material of lithium niobate obtained through the sol–gel method, using lithium–niobium ethoxide as a precursor and acetic acid as a catalyzer. The resulting sol was mixed at different concentrations with PVP and then electrospun and sintered. Fibers had average diameters ranging from 330 to 760 nm. Wang et al. [115] produced a γ-alumina fibrous material for filtration. Acetic acid, formic acid, and aluminum powder were used in order to obtain aluminum formate. Aluminum formate was then mixed with polyethylene oxide and later sintered at different temperatures. It was observed that, between 700 and 900 °C, the γ phase of alumina appeared and that, at 1000 °C, the δ and α phases appeared. The material was self-standing as γ-alumina, but as δ and α phases, it became brittle. The average diameter varied between 200 to 250 nm as the sintering temperature increased. The filtration efficiency of the fibers was higher than 99.9%.1b). Garibay-Alvarado et al. [32] produced a fibrillar material of lithium niobate obtained through the sol–gel method, using lithium–niobium ethoxide as a precursor and acetic acid as a catalyzer. The resulting sol was mixed at different concentrations with PVP and then electrospun and sintered. Fibers had average diameters ranging from 330 to 760 nm. Wang et al. [33] produced a γ-alumina fibrous material for filtration. Acetic acid, formic acid, and aluminum powder were used in order to obtain aluminum formate. Aluminum formate was then mixed with polyethylene oxide and later sintered at different temperatures. It was observed that, between 700 and 900 °C, the γ phase of alumina appeared and that, at 1000 °C, the δ and α phases appeared. The material was self-standing as γ-alumina, but as δ and α phases, it became brittle. The average diameter varied between 200 to 250 nm as the sintering temperature increased. The filtration efficiency of the fibers was higher than 99.9%.
3. Sol-Gel Ceramics for SEIRAS and SERS
Different approaches have been taken to incorporate electrospun ceramic nanofibers on the SEIRAS and SERS technologies in order to resolve some of the issues of the mass adoption of such techniques, for example, the reusability, reproducibility, and stability of the enhancement substrates. Xie et al. [117] developed a material using titanium and fluorine-doped tin oxide sheets as supports. The precursor of choice for the sol–gel titania was titanium butoxide. They were able to obtain two kinds of morphologies: a nanorod array where the surface of the support is covered with rods arranged randomly, mostly pointing up and covered with silver nanoparticles, and a nanopore array, a honeycomb-like structure with more regular features and covered with silver nanoparticles. The materials were capable of detecting concentrations of 5 × 10
Different approaches have been taken to incorporate electrospun ceramic nanofibers on the SEIRAS and SERS technologies in order to resolve some of the issues of the mass adoption of such techniques, for example, the reusability, reproducibility, and stability of the enhancement substrates. Xie et al. [35] developed a material using titanium and fluorine-doped tin oxide sheets as supports. The precursor of choice for the sol–gel titania was titanium butoxide. They were able to obtain two kinds of morphologies: a nanorod array where the surface of the support is covered with rods arranged randomly, mostly pointing up and covered with silver nanoparticles, and a nanopore array, a honeycomb-like structure with more regular features and covered with silver nanoparticles. The materials were capable of detecting concentrations of 5 × 10
−12 M of 4-mercaptobenzoic acid. The use of titania’s photocatalytic activity allowed for the material to be regenerated using UV radiation. Xie and Meng [118] created a material composed of sol–gel titania, a nanotube array covered in silver nanoparticles and decorated with graphene oxide. The addition of graphene oxide was proposed as a solution for increased adsorption since it can chemically enhance the spectroscopy signals in comparison with a TiO
M of 4-mercaptobenzoic acid. The use of titania’s photocatalytic activity allowed for the material to be regenerated using UV radiation. Xie and Meng [36] created a material composed of sol–gel titania, a nanotube array covered in silver nanoparticles and decorated with graphene oxide. The addition of graphene oxide was proposed as a solution for increased adsorption since it can chemically enhance the spectroscopy signals in comparison with a TiO
2
/Ag material. The material was successful in enhancing the Raman signals of bisphenol A concentrations as low as 5 × 10
−7
M.
Roque-Ruiz et al. [119] developed a fibrous material composed of coaxial fibers with an amorphous silica core and a crystalline TiO
Roque-Ruiz et al. [37] developed a fibrous material composed of coaxial fibers with an amorphous silica core and a crystalline TiO
2
sheath, mostly anatase (
Figure 7a). Tetraethyl orthosilicate and titanium tetra-isopropoxide were used as precursors. The composite was decorated with silver nanoparticles that formed an outer cover of dendritic structures. Concentrations of 1 nM pyridine were tested and different peaks in the Raman spectrum were amplified between 3 and 13 orders of magnitude. Singh et al. [120] developed a material consisting of a silica thin film covered by TiO
2a). Tetraethyl orthosilicate and titanium tetra-isopropoxide were used as precursors. The composite was decorated with silver nanoparticles that formed an outer cover of dendritic structures. Concentrations of 1 nM pyridine were tested and different peaks in the Raman spectrum were amplified between 3 and 13 orders of magnitude. Singh et al. [38] developed a material consisting of a silica thin film covered by TiO
2
and gold nanoparticles. The titania nanoparticles were obtained using the sol–gel method and deposited on top of a silicon substrate, then covered with a thin film of gold, and annealed in order to form the gold nanoparticles. The substrate was tested for enhancement, achieving an enhancement factor of 10
7
for R6G and 10
8 for methylene blue. Prakashan et al. [121] produced a ceramic matrix of SiO
for methylene blue. Prakashan et al. [39] produced a ceramic matrix of SiO
2
–TiO
2
–ZrO
2 with gold–silver nanoparticles specifically for the detection of vitamin A. The matrix was obtained using a non-hydrolytic version of the sol–gel method, with tetraethylorthosilicate, titanium (IV) isopropoxide, and zirconium (IV) propoxide as precursors, and then, the nanoparticles were mixed into the matrix using sonication. Although the substrate was not tested for enhancement of Raman or infrared signals, it was tested for amplification of UV/Vis spectroscopy utilizing surface plasmon resonance sensing, achieving a detection of concentrations as low as 10 µM of vitamin A. Prakashan et al. [122] also tested a similar SiO
with gold–silver nanoparticles specifically for the detection of vitamin A. The matrix was obtained using a non-hydrolytic version of the sol–gel method, with tetraethylorthosilicate, titanium (IV) isopropoxide, and zirconium (IV) propoxide as precursors, and then, the nanoparticles were mixed into the matrix using sonication. Although the substrate was not tested for enhancement of Raman or infrared signals, it was tested for amplification of UV/Vis spectroscopy utilizing surface plasmon resonance sensing, achieving a detection of concentrations as low as 10 µM of vitamin A. Prakashan et al. [40] also tested a similar SiO
2
–TiO
2
–ZrO
2
ceramic matrix, but instead of Au and Ag nanoparticles, the gold was substituted for copper, in this case, for the detection of mercury. The spectroscopic technique used was also the sensing of SPR. The material had high selectivity for such metals, achieving detection of concentrations as low as 0.01 µM of Hg. The material could possibly be used for enhancement of infrared and Raman spectroscopy.
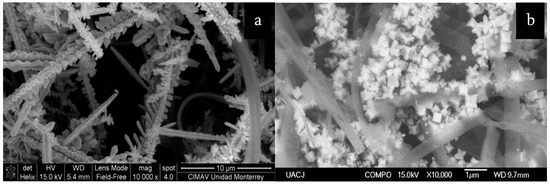
SiO
–TiO
–Ag fibrous composite with SERS activity (
) and hydroxyapatite–SiO
–Ag fibers with SERS and SEIRAS activity (
).
Hareesh et al. [123] synthesized a TiO
Hareesh et al. [41] synthesized a TiO
2
–ZrO
2
thin film with embedded silver nanoparticles for the detection of R6G through SERS. The ceramic film was obtained using the sol–gel method with titanium (IV) isopropoxide and zirconium (IV) propoxide as precursors. Two sols were produced and then mixed with silver nitrate and dimehtylformamide in order to form silver nanoparticles. The substrate was able to detect a concentration of R6G as low as 10
-18 M, as referenced by the article, as a single R6G molecule. Ji et al. [124] used zirconium nitrate as the precursor for producing ZrO
M, as referenced by the article, as a single R6G molecule. Ji et al. [42] used zirconium nitrate as the precursor for producing ZrO
2
nanoparticles through the sol–gel method. Sintering temperatures ranging from 450 to 650 °C were used, and two crystalline phases were obtained, tetragonal and monoclinic, with the latter in the highest proportion. The diameter of the particles ranged from 8.1 to 17.6 nm, or an average of 10.5 nm, and such sizes increased with the increase in sintering temperature. The highest enhancement factor of 4.32 × 10
3 was achieved through the adsorption of 4-MBA on the surface of the particles and calcination at 500 °C. Hu et al. [125] produced through templating a nanoarray consisting of silver nanopillars in a flower-like disposition covered with anodized aluminum oxide for corrosion protection of the silver. In order to fashion such a material, sol–gel silica was used in one step of a multi-step process as corrosion protection for the aluminum metal sheet used as a substrate. A Raman enhancement test was carried out on the material, detecting R6G concentrations from 10
was achieved through the adsorption of 4-MBA on the surface of the particles and calcination at 500 °C. Hu et al. [43] produced through templating a nanoarray consisting of silver nanopillars in a flower-like disposition covered with anodized aluminum oxide for corrosion protection of the silver. In order to fashion such a material, sol–gel silica was used in one step of a multi-step process as corrosion protection for the aluminum metal sheet used as a substrate. A Raman enhancement test was carried out on the material, detecting R6G concentrations from 10
−10
M to 10
−7 M. Li et al. [126] produced a composite of α-Fe
M. Li et al. [44] produced a composite of α-Fe
2
O
3
–SiO
2
–Ag for the detection of pesticides. The iron and silver nanoparticles were obtained using iron (III) hexachloride and silver nitrate, respectively. The silica was obtained through the sol–gel method using TEOS as a precursor and ammonia as a catalyzer. The iron nanoparticles had a cube-like morphology and were covered by a layer of silica; then, the silver nanoparticles coated the exterior of the Fe–SiO
2
nanoparticles. The final composite was tested for signal enhancement in analyzing different concentrations of
p
-aminothiophenol ranging from 1 × 10
−4
M to 1 × 10
−4
M, and concentrations of thiram ranging from 1 × 10
−3
M to 1 × 10
−7
M. The composite achieved a detection of thiram in concentrations lower than 7 ppm, which is lower than the limit set by the United States Environmental Protection Agency as of 2016 for such a fungicide.
Shi et al. [127] developed a ZrO
Shi et al. [45] developed a ZrO
2
–Ag–SiO
2
composite for SERS applications. Silica nanoparticles were prepared using TEOS as a precursor and ammonia as a catalyzer. Silver nanoparticles were deposited on top of the SiO
2
as an intermediate layer, and then, the SiO
2
–Ag composite was covered in a ZrO
2
layer obtained through the sol–gel method and using zirconium (IV) propoxide as a precursor. The estimated diameter of the silica nanoparticles was 340 nm and that of the silver nanoparticles was 30 nm. The composite had a SERS activity capable of detecting concentrations of 4-ATP and R6G in the ranges of 10
−9
M and 10
−8 M, respectively. Soto-Nieto et al. [128] produced a fibrous composite of silica, hydroxyapatite, and silver for the enhancement of spectroscopic signals in SEIRAS and SERS. Silica and hydroxyapatite were obtained using the sol–gel method with TEOS and triethyl phosphite–calcium nitrate as precursors, respectively. Fibrous mats were fabricated through electrospinning by mixing the sols with PVP and by sintering at temperatures from 200 to 1150 °C. The fibers had an average diameter of 304 nm (
M, respectively. Soto-Nieto et al. [46] produced a fibrous composite of silica, hydroxyapatite, and silver for the enhancement of spectroscopic signals in SEIRAS and SERS. Silica and hydroxyapatite were obtained using the sol–gel method with TEOS and triethyl phosphite–calcium nitrate as precursors, respectively. Fibrous mats were fabricated through electrospinning by mixing the sols with PVP and by sintering at temperatures from 200 to 1150 °C. The fibers had an average diameter of 304 nm (
Figure 7b). The fibrous mats were doped with silver nanoparticles using electrodeposition and AgNO
2b). The fibrous mats were doped with silver nanoparticles using electrodeposition and AgNO
3
as the precursor. The SEIRAS enhancement factor was estimated as 2.01 × 10
6
, and the SERS enhancement factor was estimated as 3.46 × 10
8
.
References
- van der Put, P.J. Solid State Reactions. In The Inorganic Chemistry of Materials; Springer: Boston, MA, USA, 1998; pp. 87–110.
- Subasri, R.; Raju, K.S. Multifunctional Sol-Gel Nanocomposite Coatings for Aerospace, Energy, and Strategic Applications: Challenges and Perspectives. In Handbook of Advanced Ceramics and Composites: Defense, Security, Aerospace and Energy Applications; Springer: Cham, Switzerland, 2020; pp. 1413–1442.
- Cavalheiro, A.A.; Cabeza, N.A.; Anjos, A.D.; Rodrigues, D.C.M. Synthesis of Silver Embedded Nanoparticles Immobilized within Silica Alumina Porous Matrix to Sensor Applications. In Proceedings of the Brazilian SBPMat meeting, João Pessoa, Brazil, 28 September–2 October 2014.
- Sakka, S.; Kamiya, K. The sol-gel transition in the hydrolysis of metal alkoxides in relation to the formation of glass fibers and films. J. Non-Cryst. Solids 1982, 48, 31–46.
- Sinha, A.K.; Seelan, S.; Okumura, M.; Akita, T.; Tsubota, S.; Haruta, M. Three-dimensional mesoporous titanosilicates prepared by modified sol− gel method: Ideal gold catalyst supports for enhanced propene epoxidation. J. Phys. Chem. B 2005, 109, 3956–3965.
- Aguilar, G.V.; Fonseca, M.R.J.; Ramírez, Á.M.; Gracia, A.G.J. Photoluminescence studies on ZnO thin films obtained by sol-gel method. Recent Appl. Sol.-Gel Synth. 2017, 195.
- Sun, M.; Zhao, T.; Ma, Z.; Li, Z. Facile preparation of macro-mesoporous zirconium titanate monoliths via a sol–gel reaction accompanied by phase separation. J. Mater. Res. 2019, 34, 4066–4075.
- Ashrafi-Shahri, S.M.; Ravari, F.; Seifzadeh, D. Smart organic/inorganic sol-gel nanocomposite containing func-tionalized mesoporous silica for corrosion protection. Prog. Org. Coat. 2019, 133, 44–54.
- Kayani, Z.N.; Riaz, S.; Naseem, S. Magnetic and antibacterial studies of sol-gel dip coated Ce doped TiO2 thin films: Influence of Ce contents. Ceram. Int. 2020, 46, 381–390.
- Sharifianjazi, F.; Parvin, N.; Tahriri, M. Synthesis and characteristics of sol-gel bioactive SiO2-P2O5-CaO-Ag2O glasses. J. Non-Cryst. Solids 2017, 476, 108–113.
- Singh, J.; Palsaniya, S.; Soni, R. Mesoporous dark brown TiO2 spheres for pollutant removal and energy storage applications. Appl. Surf. Sci. 2020, 527, 146796.
- Pant, B.; Park, M.; Park, S.J. Recent advances in TiO2 films prepared by sol-gel methods for photocatalytic degradation of organic pollutants and antibacterial activities. Coatings 2019, 9, 613.
- Zhu, H.; Jing, Y.; Pal, M.; Liu, Y.; Liu, Y.; Wang, J.; Zhao, D. Mesoporous TiO2@ N-doped carbon composite nan-ospheres synthesized by the direct carbonization of surfactants after sol–gel process for superior lithium storage. Nanoscale 2017, 9, 1539–1546.
- Kumar, A. Sol gel synthesis of zinc oxide nanoparticles and their application as nano-composite electrode material for supercapacitor. J. Mol. Struct. 2020, 1220, 128654.
- Sonia, M.M.L.; Anand, S.; Vinosel, V.M.; Janifer, M.A.; Pauline, S.; Manikandan, A. Effect of lattice strain on structure, morphology and magneto-dielectric properties of spinel NiGdxFe2−xO4 ferrite nano-crystallites synthesized by sol-gel route. J. Magn. Magn. Mater. 2018, 466, 238–251.
- Zhang, Z.; Yu, J.; Ma, L.; Sun, Y.; Wang, P.; Wang, T.; Peng, S. Preparation of the plasmonic Ag/AgBr/ZnO film sub-strate for reusable SERS detection: Implication to the Z-scheme photocatalytic mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117381.
- Wan, M.; Zhao, H.; Peng, L.; Zou, X.; Zhao, Y.; Sun, L. Loading of Au/Ag Bimetallic Nanoparticles within and Out-side of the Flexible SiO2 Electrospun Nanofibers as Highly Sensitive, Stable, Repeatable Substrates for Versatile and Trace SERS Detection. Polymers 2020, 12, 3008.
- Shamitha, C.; Senthil, T.; Wu, L.; Kumar, B.S.; Anandhan, S. Sol–gel electrospun mesoporous ZnMn2O4 nano-fibers with superior specific surface area. J. Mater. Sci. Mater. Electron. 2017, 28, 15846–15860.
- Zhang, W.; Liu, W.; Liu, Y.; Wang, C. Tribological behaviors of single and dual sol–gel ceramic films on Ti–6Al–4V. Ceram. Int. 2009, 35, 1513–1520.
- La Monaca, A.; Paolella, A.; Guerfi, A.; Rosei, F.; Zaghib, K. Electrospun ceramic nanofibers as 1D solid electrolytes for lithium batteries. Electrochem. Commun. 2019, 104, 106483.
- Cho, Y.-S.; Roh, S.H. Sol–gel synthesis of porous titania fibers by electro-spinning for water purification. J. Dispers. Sci. Technol. 2017, 39, 33–44.
- Methaapanon, R.; Chutchakul, K.; Pavarajarn, V. Photocatalytic zinc oxide on flexible polyacrylonitrile nanofibers via sol–gel coaxial electrospinning. Ceram. Int. 2020, 46, 8287–8292.
- Ansari, M.A.; Albetran, H.M.; Alheshibri, M.H.; Timoumi, A.; Algarou, N.A.; Akhtar, S.; Slimani, Y.; Almessiere, M.A.; AlAhmari, F.S.; Baykal, A.; et al. Synthesis of Electrospun TiO2 Nanofibers and Characterization of Their Antibacterial and Antibiofilm Potential against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 572.
- Yuan, K.; Jin, X.; Xu, C.; Wang, X. Manipulation of electrospun mesoporous zirconia nanofiber with enhanced surface area and catalytic property. Ceram. Int. 2019, 45, 13414–13421.
- Nagrath, M.; Alhalawani, A.; Yazdi, A.R.; Towler, M.R. Bioactive glass fiber fabrication via a combination of sol-gel process with electro-spinning technique. Mater. Sci. Eng. C 2019, 101, 521–538.
- Ávila-Martínez, A.K.; Roque-Ruiz, J.H.; Torres-Pérez, J.; Medellín-Castillo, N.A.; Reyes-López, S.Y. Allura Red dye sorption onto electrospun zirconia nanofibers. Environ. Technol. Innov. 2020, 18, 100760.
- Koo, J.Y.; Lim, Y.; Kim, Y.B.; Byun, D.; Lee, W.-Y. Electrospun yttria-stabilized zirconia nanofibers for low-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2017, 42, 15903–15907.
- Pescador-Rojas, J.A.; Jiménez-Pérez, J.L.; Sánchez-Ramírez, J.F.; Gutiérrez-Fuentes, R.; Correa-Pacheco, Z.N.; Zúñiga-Zarco, J.P.; Orozco-Flores, L.D. Synthesis of Electrospun Titania Nanofibers for Thermal Lens Study in Heat Transport Applications. Mater. Sci. Forum 2018, 936, 58–62.
- De Dicastillo, C.L.; Patiño, C.; Galotto, M.J.; Palma, J.L.; Alburquenque, D.; Escrig, J. Novel Antimicrobial Titanium Dioxide Nanotubes Obtained through a Combination of Atomic Layer Deposition and Electrospinning Technologies. Nanomaterials 2018, 8, 128.
- Garibay-Alvarado, J.A.; Espinosa-Cristóbal, L.F.; Reyes-López, S.Y. FIBROUS SILICA-HYDROXYAPATITE COMPOSITE BY ELECTROSPINNING. Int. J. Res. Granthaalayah 2017, 5, 39–47.
- Roque-Ruiz, J.H.; Garibay-Alvarado, J.A.; Medellín-Castillo, N.A.; Reyes-López, S.Y. Preparation of Electrospun Hydroxyapatite-Glass Fibers for Removal of Cadmium (Cd+2) and Lead (Pb+2) from Aqueous Media. Water Air Soil Pollut. 2020, 231, 1–13.
- Garibay-Alvarado, J.A.; Farías, R.; Reyes-López, S.Y. Sol-Gel and Electrospinning Synthesis of Lithium Niobate-Silica Nanofibers. Coatings 2019, 9, 212.
- Wang, Y.; Li, W.; Xia, Y.; Jiao, X.; Chen, D. Electrospun flexible self-standing γ-alumina fibrous membranes and their potential as high-efficiency fine particulate filtration media. J. Mater. Chem. A 2014, 2, 15124–15131.
- Choi, Y.S.; Oh, K.; Koh, H.-L. Electrospun Alumina-Nanofiber-Supported Pt–Sn Catalyst for Propane Dehydrogenation. J. Nanosci. Nanotechnol. 2020, 20, 6897–6903.
- Xie, Y.; Jin, Y.; Zhou, Y.; Wang, Y. SERS activity of self-cleaning silver/titania nano-array. Appl. Surf. Sci. 2014, 313, 549–557.
- Xie, Y.; Meng, Y. SERS performance of graphene oxide decorated silver nanoparticle/titania nanotube array. RSC Adv. 2014, 4, 41734–41743.
- Roque-Ruiz, J.H.; Martínez-Máynez, H.; Zalapa-Garibay, M.A.; Arizmendi-Moraquecho, A.; Farias, R.; Reyes-López, S.Y. Surface enhanced Raman spectroscopy in nanofibers mats of SiO2-TiO2-Ag. Results Phys. 2017, 7, 2520–2527.
- Singh, J.; Manna, A.K.; Soni, R.K. Bifunctional Au–TiO2 thin films with enhanced photocatalytic activity and SERS based multiplexed detection of organic pollutant. J. Mater. Sci. Mater. Electron. 2019, 30, 16478–16493.
- Prakashan, V.; Gejo, G.; Sanu, M.; Sajna, M.; Subin, T.; Biju, P.; Cyriac, J.; Unnikrishnan, N. Novel SPR based fiber optic sensor for vitamin A using core-shell nanoparticles doped SiO2-TiO2-ZrO2 ternary matrix. Appl. Surf. Sci. 2019, 484, 219–227.
- Prakashan, V.; Georgeab, G.; Sanu, M.S.; Sajnaac, M.S.; Saritha, A.; Sudarsanakumar, C.; Biju, P.; Josepha, C.; Unnikrishnan, N. Investigations on SPR induced core shell doped SiO2-TiO2-ZrO2 fiber optic sensor for mercury detection. Appl. Surf. Sci. 2020, 507, 144957.
- Hareesh, S.; Simon, S.M.; Jose, T.A.; Gopinath, M.; Saritha, A.; Joseph, C.; Biju, P.; Unnikrishnan, N. Highly sensitive and stable Ag nanoparticles decorated TiO2-ZrO2 composite SERS substrates for Rhodamine 6G detection. Mater. Today Proc. 2020, 33, 1396–1401.
- Ji, P.; Wang, Z.; Shang, X.; Zhang, Y.; Liu, Y.; Mao, Z.; Shi, X. Direct Observation of Enhanced Raman Scattering on Nano-Sized ZrO2 Substrate: Charge-Transfer Contribution. Front. Chem. 2019, 7, 245.
- Hu, Y.; Wang, X.; Zhang, M.; Wang, S.; Li, S.; Chen, G. A Hierarchical Anodic Aluminum Oxide Template. Nano Lett. 2021, 21, 250–257.
- Li, L.; Zhao, A.; Wang, D.; Guo, H.; Sun, H.; He, Q. Fabrication of cube-like Fe3O4@SiO2@Ag nanocomposites with high SERS activity and their application in pesticide detection. J. Nanoparticle Res. 2016, 18, 178.
- Shi, T.; Tang, Z.; Liang, P.; Zhang, X.; Zhang, D.; Shao, Q.; Chen, H. ZrO2@ SiO2 Sandwich Structure with High SERS Enhancement Effect and Stability. J. Phys. Chem. C 2020, 124, 25967–25974.
- Soto-Nieto, F.; Farías, R.; Reyes-López, S.Y. Sol–Gel and Electrospinning Synthesis of Silica–Hydroxyapatite–Silver Nanofibers for SEIRAS and SERS. Coatings 2020, 10, 910.
