Chickens possess mannose-binding lectin (MBL) which could be vital in managing pathogenic bacteria in chickens. MBL is one of the soluble proteins secreted by the chicken’s innate immune system which can be activated when chickens are exposed to chicken-related diseases.
- chickens
- use of antibiotics
- innate immunity
- lectin pathway
- complement system
- mannose-binding lectin quantification method
1. Introduction
Chicken production plays a vital role in meeting the increasing demand for chicken products (such as meat and eggs) by using adequate management practices and structured breeding programs [1]. Despite the improvement in poultry management practices, bacterial disease is still a big threat to the chicken production industry [1,2,3][1][2][3]. For instance, countries like United States of America, China, Southern Europe and Portugal accounted for up to 50% loss in poultry product due to bacterial infections [2,3][2][3]. This problem has led to the incessant use of antibiotics due to their ability to improve feed efficiency, weight and poultry health. However, the use of antibiotics has been found to cause the emergence of antimicrobial resistant genes in chickens [3]. These antimicrobial resistant genes are then transferred between bacteria in the environment and into human pathogens [2,3][2][3] thereby causing serious health issues. For this reason, antibiotic use for growth promotion has been banned in some countries. Froebel et al. [4] stated that some government policies such as the United States Veterinary Feed Directive and the European Union are discouraging use of antibiotic growth promoters. There is, therefore, an urgent need to find alternative measures to manage bacterial diseases without compromising the health of chickens, humans or the environment.
The defense mechanism of chickens against pathogenic diseases entails the activity of both the innate and adaptive immune systems. Bacterial diseases are combated by an innate immune system (IIS) before the intervention of the adaptive immune system [5,6,7][5][6][7]. The IIS response is the host’s first line defense mechanism against any microbial infection; it functions by fighting pathogens at the point of entry via both innate immune cells and innate immune proteins [8,9][8][9]. On the other hand, the adaptive immune system ensures protection against subsequent infections by the action of both T and B lymphocytes [10] with activity against specific pathogens.
2. Brief Overview of the Chicken Innate Immune System
The IIS response is the host’s first line defense mechanism against microbial infection. For example, it functions by fighting pathogens at the point of entry via both innate immune cells and secreted soluble effector proteins [9]. The main innate immune cells include phagocytes such as macrophages and heterophils (comparable to the mammalian neutrophils), but also epithelial cells play an important role in the immediate response towards invading pathogens. The specific innate immune proteins are multiple and include complement system proteins, cytokines and chemokines (often secreted by the innate immune cells) and secreted soluble effector proteins, such as collectins and antimicrobial peptides, that can also kill or neutralize microbes [9].
An important first step in the innate immune response is recognition of the pathogen. There are specific structures on the microbe called pathogen-associated molecular patterns (PAMPs) which are recognized by membrane bound, or soluble pattern recognition receptors (PRRs) of the host. Larsen et al. [11] and Faghfouri et al. [12], both showed that toll-like receptors (TLRs), retinoic acid-inducible gene-I-like receptors (RLRs) and C-type lectin receptors (CLRs) are an important group of PRRs that can recognize PAMPs from microbial DNA/RNA to protein and membrane components of microbes. When PAMPs bind to PRRs, it leads to a signaling cascade which in turn increases cytokine and chemokine production; these molecules can activate immune cells. In some cases, through chemotaxis, the numbers of immune cells are increased at the site of infection. Signaling can also increase the production and secretion of antimicrobial compounds, such as defensins or other antimicrobial proteins that can neutralize the pathogens [13,14][13][14].
3. Mannose-Binding Lectin
Mannose-binding lectin (MBL) is also considered a member of the PRR family. In contrast to other PRRs such as TLRs, it is not membrane bound but a soluble-type protein which can also be considered as an effector molecule. Binding of MBL to PAMPs on the pathogen cell wall can initiate the lectin pathway of complement activation, which further neutralizes pathogens by aggregating them, (denying attachment of bacteria to epithelial cells of host). MBL can also bind apoptotic cells via ligation (joining of two DNA strands by a phosphate ester linkage) on the phagocyte membrane by macrophages [15]. The removal of apoptotic cells is necessary for organogenesis, tissue maintenance and proper activity of the immune system. MBL is also regarded as an acute-phase protein (proteins which respond to inflammatory signals by increasing its plasma concentration by 25% or more). These proteins participate in host defense and adaptation and also act as transport proteins with antioxidant activity [15,16][15][16]. Mannose-binding lectin is an innate host defense molecule, with great affinity to initiate the lectin pathway of complement via attached mannose-binding lectin-associated serine protease (MASP-2) [17,18,19][17][18][19]. The MBL structure provides a wide array of defenses against pathogens’ physiochemical activities as mentioned in the study of Takahashi [7]. Several authors have indeed discussed the impact of MBL on bacterial infection in chickens [15,16,17,18][15][16][17][18].
4. Complement System
The complement system (CS) plays a very important role in defending the host against diseases and inflammation. It is known that the CS consists of more than 30 soluble or membrane proteins that upon activation interact (the complement cascade), eventually leading to formation of an antibacterial complex of proteins and formation of several signaling molecules that stimulate further immune responses (Figure 1) [6,31][6][20].
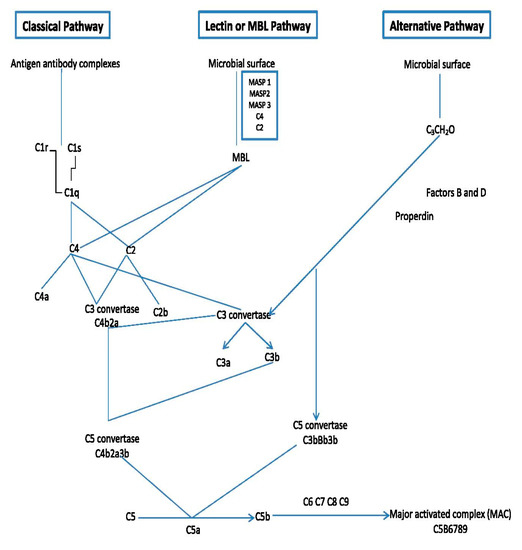
Figure 1. Outline of the major components and mode of action of the complement system.
The order of events of the complement cascade is as follows: Firstly, the complement cascade is activated via one of three different but eventually merging pathways: the lectin pathway, the classical pathway and the alternative pathway [6,31,32,33,34,35][6][20][21][22][23][24]. These pathways bring forth the formation of convertase enzymes which form several chemokine and other important signaling factors including the complement factor (C5b).
All these pathways converge at C3, which is the most abundant complement protein found in the blood. At the point of converging, there is a formation of activation products such as C3a, C3b, C5a and the MAC or C5b-9 (Figure 1). The membrane attack complex (MAC) is a protein complex that can bind and lyse Gram-negative bacteria. It becomes triggered within a few minutes upon entry of Gram-negative bacteria in the host cells [31,36][20][25]. More details on how the complement system functions can be further seen in excellent reviews of Sarma and Ward, [6] and Heesterbeek et al. [37][26].
5. MBL Localization/Synthesis
The cMBL and other members of the collectin family are all located at the end of the chromosome 6 [32][21] and the human collectin cluster is located on chromosome 10 [33][22]. Oka et al. [21][27] described MBL as the first C-type lectin member to be isolated among chicken species from the chicken liver. Several authors reported that cMBL synthesis is highest in the liver (hepatocytes) area while some lower quantities of MBL were seen in the larynx, infundibulum and abdominal sac [16,18,19,32][16][18][19][21]. Additionally, an infinitesimal amount of MBL mRNA was detected in the thymus, ovary and uterus region [32][21]. Likewise, Laursen and Nielsen [37][26] and Ulrich-Lynge et al. [18] detected the presence of cMBL in the germinal region of ceca, thymus, spleen and lungs of healthy chickens. In conclusion, cMBL can be seen in the reproductive, circulatory, digestive and respiratory system of chickens but at varying quantities.
6. MBL Concentration vs. Bacterial Disease Protection
As stated above, cMBL synthesis is known to occur mostly in the hepatocytes’ region, while limited quantities are found in the other organs. However, upon infection, higher MBL levels can be observed in specific organs due to local up-regulation. When there is an adequate quantity of MBL synthesized in the liver, the host organism will have the ability to combat bacterial pathogens at entry [17,18][17][18].
For example, Schou et al. [17] described that a low concentration of cMBL caused high disease prevalence in Pasteurella multocida-infected chickens, while Norup et al. [16] observed that high concentrations of MBL reduced chickens’ susceptibility to Escherichia coli [16]. Similar effects were observed for infections with Salmonella enterica and Salmonella infantis [42][28], while also, the outcome of viral infections seems to be related to serum levels of MBL [36][25]. Interestingly, there seems to be a strong genetic component that determines the concentration of MBL in chickens, with differences in average MBL levels between breeds [17]. Chicken strains specifically selected for high (L10H) and low (L10L) serum concentration of MBL have been bred and used to study the role of MBL in the chickens’ immune response. These studies consistently show that higher levels of MBL are correlated to lower bacterial disease in chicken. In one study, the amount of Salmonella shed in L10L chickens was higher than L10H chicken on day 4 pi. In chickens, it was shown that there was a higher level of Salmonella binding to MBL in chickens with high MBL levels (L10H strain) than in low-MBL chickens (L10L) at different days post infection (pi) [18]. This was suggested to be due to the huge role MBL plays in safeguarding chickens against Salmonella. Additionally, the L10L chicken excreted Salmonella for more days than the L10H chicken; this could be due to the presence of higher Salmonella counts in L10L chickens’ intestines than in L10H chickens. Schou et al. [17] also showed that chickens with low MBL concentrations were more prone to other pathogenic diseases. Different levels of binding of MBL to S. aureus NCT6571 strain (strong binding), C. lusitanae (strong binding) and E. faecalis (low binding) have been demonstrated [34][23] while Ulrich-Lynge et al. [18] and Seliger et al. [31][20] both observed that high cMBL levels and high monocyte numbers influence the clearance of Escherichia coli and some Salmonella spp of the C1 serotype. The lists of some of the reported cases of MBL affinity to bind bacterial diseases in chickens are presented in Table 1.
Table 1. Selected reports of cMBL (chicken mannose-binding lectin) bacterial binding.
|
Bacteria Strain |
Site of Binding |
References |
|---|---|---|
|
Pasteurella multocida |
Liver, spleen, serum |
[17] |
|
Escherichia coli |
Liver, serum |
|
|
Salmonella enterica |
Liver, serum |
[18] |
|
Staphylococcus aureus |
Ceca, serum |
|
|
Klebsiella oxytoca |
Serum |
|
|
Klebsiella pneumoniae |
Serum |
|
|
Pseudomonas aeruginosa |
Serum |
|
|
Yersinia pseudotuberculosis |
Serum |
|
|
Salmonella Typhimurium |
Serum |
[19] |
|
Salmonella Montevideo |
Serum |
|
|
Enterobacter cloacae |
Serum |
References
- Idowu, P.A.; Mpayipheli, M.; Muchenje, V. Practices, Housing and Diseases within Indigenous Poultry Production in Eastern Cape, South Africa. J. Agric. Sci. 2018, 10, p111.
- Lei, C.-W.; Zhang, Y.; Kang, Z.-Z.; Kong, L.-H.; Tang, Y.-Z.; Zhang, A.-Y.; Yang, X.; Wang, H.-N. Vertical transmission of Salmonella Enteritidis with heterogeneous antimicrobial resistance from breeding chickens to commercial chickens in China. Vet. Microbiol. 2020, 240, 108538.
- Hawkey, P.M. The growing burden of antimicrobial resistance. J. Antimicrob. Chemother. 2008, 62, i1–i9.
- Froebel, L.; Jalukar, S.; Lavergne, T.; Lee, J.; Duong, T. Administration of dietary prebiotics improves growth performance and reduces pathogen colonization in broiler chickens. Poult. Sci. 2019, 98, 6668–6676.
- Wallis, R. Interactions between mannose-binding lectin and MASPs during complement activation by the lectin pathway. Immunobiology 2007, 212, 289–299.
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2010, 343, 227–235.
- Takahashi, K. Mannose-binding lectin and the balance between immune protection and complication. Expert Rev. Antiinfect. Ther. 2011, 9, 1179–1190.
- Ali, Y.M.; Lynch, N.J.; Haleem, K.S.; Fujita, T.; Endo, Y.; Hansen, S.; Holmskov, U.; Takahashi, K.; Stahl, G.L.; Dudler, T.; et al. The Lectin Pathway of Complement Activation Is a Critical Component of the Innate Immune Response to Pneumococcal Infection. PLoS Pathog. 2012, 8, e1002793.
- Silva, P.M.D.S.; De Oliveira, W.F.; Albuquerque, P.B.S.; Correia, M.T.D.S.; Coelho, L.C.B.B. Insights into anti-pathogenic activities of mannose lectins. Int. J. Biol. Macromol. 2019, 140, 234–244.
- Lowenthal, J.; Bean, A.; Kogut, M. What’s so special about chicken immunology? Dev. Comp. Immunol. 2013, 41, 307–309.
- Larsen, F.T.; Bed’Hom, B.; Guldbrandtsen, B.; Dalgaard, T.S. Identification and tissue-expression profiling of novel chicken c-type lectin-like domain containing proteins as potential targets for carbohydrate-based vaccine strategies. Mol. Immunol. 2019, 114, 216–225.
- Faghfouri, A.H.; Zarrin, R.; Maleki, V.; Payahoo, L.; Bishak, Y.K. A comprehensive mechanistic review insight into the effects of micronutrients on toll-like receptors functions. Pharmacol. Res. 2020, 152, 104619.
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via toll-like receptor 4 signalling. Cell. Microbiol. 2016, 18, 424–436.
- Loh, S.H.; Park, J.-Y.; Cho, E.H.; Nah, S.-Y.; Kang, Y.-S. Animal lectins: Potential receptors for ginseng polysaccharides. J. Ginseng Res. 2017, 41, 1–9.
- Nielsen, O.L.; Jensenius, J.C.; Jørgensen, P.H.; Laursen, S.B. Serum levels of chicken mannan-binding lectin (MBL) during virus infections; indication that chicken MBL is an acute phase reactant. Vet. Immunol. Immunopathol. 1999, 70, 309–316.
- Norup, L.; Dalgaard, T.; Friggens, N.; Sørensen, P.; Juul-Madsen, H. Influence of chicken serum mannose-binding lectin levels on the immune response towards Escherichia coli. Poult. Sci. 2009, 88, 543–553.
- Schou, T.; Permin, A.; Christensen, J.; Cu, H.; Juul-Madsen, H. Mannan-binding lectin (MBL) in two chicken breeds and the correlation with experimental Pasteurella multocida infection. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 183–195.
- Ulrich-Lynge, S.L.; Dalgaard, T.S.; Norup, L.R.; Song, X.; Sørensen, P.; Juul-Madsen, H.R. Chicken mannose-binding lectin function in relation to antibacterial activity towards Salmonella enterica. Immunobiology 2015, 220, 555–563.
- Zhang, W.; Van Eijk, M.; Guo, H.; Van Dijk, A.; Bleijerveld, O.B.; Verheije, M.H.; Wang, G.; Haagsman, H.P.; Veldhuizen, E.J. Expression and characterization of recombinant chicken mannose binding lectin. Immunobiology 2017, 222, 518–528.
- Seliger, C.; Schaerer, B.; Kohn, M.; Pendl, H.; Weigend, S.; Kaspers, B.; Härtle, S. A rapid high-precision flow cytometry based technique for total white blood cell counting in chickens. Vet. Immunol. Immunopathol. 2012, 145, 86–99.
- Hogenkamp, A.; Van Eijk, M.; Van Dijk, A.; Van Asten, A.J.; Veldhuizen, E.J.; Haagsman, H.P. Characterization and expression sites of newly identified chicken collectins. Mol. Immunol. 2006, 43, 1604–1616.
- Juul-Madsen, H.R.; Norup, L.R.; Jørgensen, P.H.; Handberg, K.J.; Wattrang, E.; Dalgaard, T.S. Crosstalk between innate and adaptive immune responses to infectious bronchitis virus after vaccination and challenge of chickens varying in serum mannose-binding lectin concentrations. Vaccine 2011, 29, 9499–9507.
- Seiler, B.T.; Cartwright, M.; Dinis, A.L.M.; Duffy, S.; Lombardo, P.; Cartwright, D.; Super, E.H.; Lanzaro, J.; Dugas, K.; Super, M.; et al. Broad-spectrum capture of clinical pathogens using engineered Fc-mannose-binding lectin enhanced by antibiotic treatment. F1000Research 2019, 8, 108.
- Eisen, D.P.; Minchinton, R.M. Impact of Mannose-Binding Lectin on Susceptibility to Infectious Diseases. Clin. Infect. Dis. 2003, 37, 1496–1505.
- Beltrame, M.H.; Catarino, S.J.; Goeldner, I.; Boldt, A.B.W.; De Messias-Reason, I.J. The Lectin Pathway of Complement and Rheumatic Heart Disease. Front. Pediatr. 2015, 2, 148.
- Heesterbeek, D.A.; Angelier, M.L.; Harrison, R.A.; Rooijakkers, S.H. Complement and Bacterial Infections: From Molecular Mechanisms to Therapeutic Applications. J. Innate Immun. 2018, 10, 455–464.
- Oka, S.; Kawasaki, T.; Yamashina, I. Isolation and characterization of mannan-binding proteins from chicken liver. Arch. Biochem. Biophys. 1985, 241, 95–105.
- Ulrich-Lynge, S.L.; Dalgaard, T.S.; Norup, L.R.; Kjærup, R.M.; Olsen, J.E.; Sørensen, P.; Juul-Madsen, H.R. The consequence of low mannose-binding lectin plasma concentration in relation to susceptibility to Salmonella Infantis in chickens. Vet. Immunol. Immunopathol. 2015, 163, 23–32.
- Kjærup, R.M.; Norup, L.R.; Skjødt, K.; Dalgaard, T.S.; Juul-Madsen, H.R. Chicken mannose-binding lectin (mannose-binding lectin) gene variants with influence on mannose-binding lectin serum concentrations. Immunogenetics 2013, 65, 461–471.
