Transdermal vaccination route using biodegradable microneedles is a rapidly progressing field of research and applications. The fear of painful needles is one of the primary reasons most people avoid getting vaccinated. Therefore, developing an alternative pain-free method of vaccination using microneedles has been a significant research area. Microneedles comprise arrays of micron-sized needles that offer a pain-free method of delivering actives across the skin. Apart from being pain-free, microneedles provide various advantages over conventional vaccination routes such as intramuscular and subcutaneous. Microneedle vaccines induce a robust immune response as the needles ranging from 50 to 900 μm in length can efficiently deliver the vaccine to the epidermis and the dermis region, which contains many Langerhans and dendritic cells. The microneedle array looks like band-aid patches and offers the advantages of avoiding cold-chain storage and self-administration flexibility. The slow release of vaccine antigens is an important advantage of using microneedles. The vaccine antigens in the microneedles can be in solution or suspension form, encapsulated in nano or microparticles, and nucleic acid-based. The use of microneedles to deliver particle-based vaccines is gaining importance because of the combined advantages of particulate vaccine and pain-free immunization.
- microneedles
- microneedle vaccine
- vaccine delivery
- skin vaccination
- transdermal
- immune response
- microparticles
1. Introduction
Infectious diseases have been prevalent in human history for centuries. The discovery and ongoing use of vaccines to prevent diseases have greatly benefited human health. As a result, many ailments have been eradicated, controlled, or deemed irreverent, allowing many generations of children to survive into adulthood, consequently increasing human life expectancy [1]. Vaccines mimic infections and utilize the immune system to produce immunity against the invading pathogen without succumbing to the pathogenesis of the disease. Traditionally, vaccines fall into three groups: whole pathogen vaccine, subunit vaccine, and nucleic acid vaccine [2]. First, whole pathogen vaccines have further subtypes; live attenuated vaccines are a type of whole pathogen vaccine that utilizes a weakened version of the pathogen to induce immunity while not being able to cause the disease. Inactivated vaccines are also whole pathogen vaccines that involve the inactivation of the pathogen using chemical or high-temperature treatments. Second, subunit vaccines focus on isolating and purifying specific components from the pathogen (or synthetic production) to induce immunity. Finally, nucleic acid vaccines involve introducing genetic material in the form of a plasmid DNA or messenger RNA that encodes for the antigens [2].
Vaccination is regarded as one of the most cost-effective medical interventions ever introduced. A publication from the Centers for Disease Control and Prevention estimates that between 1994–2013, vaccines prevented over 320 million illnesses, 21 million hospitalizations, and 732,000 premature deaths, saving at least $295 billion in medical costs [3]. However, the lack of licensed vaccines for emerging diseases, especially during epidemics and pandemics, can have a drastic impact on a country’s health and economy. The most recent example is the SARS-CoV-2 pandemic that has infected about 90.4 million people resulting in over 1.94 million deaths worldwide as of December 2020. The year 2020 saw the desperate need for an effective vaccine against SARS-CoV-2. Vaccines have always played an essential role in reducing the prevalence of infectious diseases worldwide. Therefore, continuous research and development on vaccine antigens and vaccine delivery are necessary for combatting future pandemics caused by novel and fast-evolving infectious diseases.
2. Microneedles for Transdermal Delivery
Microneedle arrays are minimally invasive micron-sized needles that penetrate the stratum corneum, which is the skin’s primary barrier to delivering a therapeutic through the skin. Microneedles vary between 50–900 microns in height (
2. Microneedles for Transdermal Delivery
Microneedle arrays are minimally invasive micron-sized needles that penetrate the stratum corneum, which is the skin’s primary barrier to delivering a therapeutic through the skin. Microneedles vary between 50–900 microns in height (
Figure 1) and are fabricated using various geometries and various metals, silicones, and polymers. The application of microneedle patches into the skin forms microscopic aqueous pores to allow the diffusion of drugs to the skin’s epidermal layer [4]. The concept of microneedles was first established decades ago but only became prominent in significant research in the mid-1990s. In contrast to hypodermic needles, microneedles are more patient compliant as they are pain-free and can be self-administered. Microneedles are micron-sized as to be able to deliver almost any drug or small particle formulation but not long enough to cause any pain during administration (
3) and are fabricated using various geometries and various metals, silicones, and polymers. The application of microneedle patches into the skin forms microscopic aqueous pores to allow the diffusion of drugs to the skin’s epidermal layer [30]. The concept of microneedles was first established decades ago but only became prominent in significant research in the mid-1990s. In contrast to hypodermic needles, microneedles are more patient compliant as they are pain-free and can be self-administered. Microneedles are micron-sized as to be able to deliver almost any drug or small particle formulation but not long enough to cause any pain during administration (
Table A1 could be found in https://www.mdpi.com/2072-666X/12/4/435). Additionally, microneedle delivery allowed delivery to precise tissues such as within the skin or the eye. There are several types of microneedles: solid, coated, dissolving, and hollow [5].
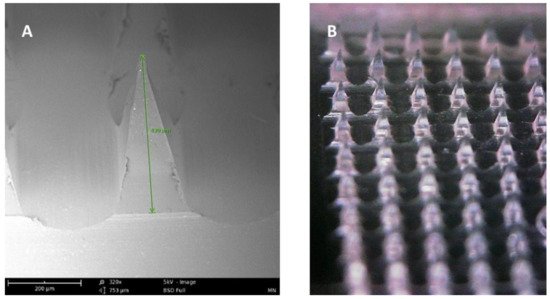
). Additionally, microneedle delivery allowed delivery to precise tissues such as within the skin or the eye. There are several types of microneedles: solid, coated, dissolving, and hollow [31].

Figure 1. (A) SEM image of a dissolving polymeric microneedle (size: 430 μm, scale 200 μm). (B) Optical microscope image of the same microneedle array patch.
2.1. Solid Microneedles
Solid microneedles are often used as a skin pretreatment. They are inserted into the skin and then removed to form micron-sized pores on the skin surface. Drug solutions within a patch can then be applied to the surface, which contains the micropores. Another variation utilized a roller containing solid microneedles, which pokes holes in the stratum corneum multiple times as the roller moves across the skin [6].
2.2. Hollow Microneedles
Hollow microneedles are miniature versions of the conventional hypodermic needles. Drug delivery through hollow microneedles is achieved through a pressure-driven flow of a liquid formulation. In contrast to other types, hollow microneedles are challenging to produce due to their structure and fragility [6]. However, hollow microneedles can deliver large, continuous amounts of actives compared to the other microneedle types [4].
2.3. Dissolving Microneedles
Dissolving microneedles are made using biodegradable materials such as various polymers and sugars loaded with therapeutics. After the needle is applied to the skin, the needles dissolve to release the payload into the skin. The advantage of dissolving microneedles in contrast to solid and hollow microneedles includes the ease of fabrication and single-step application of the patch. Dissolving microneedles have been looked at extensively for delivery vaccines through the skin [6].
2.4. Coated Microneedles
Coated microneedles consist of solid microneedles that have been coated with a drug solution or dispersion. There are various methods to produce coated microneedles, including dip coating, in which the microneedles are “dipped” into the coating solution. Spray coating can also be used to coat the needles. Coated microneedles are not as commonly used as solid, hollow, and dissolving microneedles since they offer a minimal amount of surface area for drug absorption.
3. Composition of Microneedles
Various materials can be used to produce microneedle arrays and these materials are all FDA approved [7]. The first material used was silicon. Advantages of using silicon include high flexibility to allow needles with customizable shapes and sizes. However, silicon microneedles’ main limitations include high cost, long fabrication times, and multi-step processing [4]. Metals can also be used for microneedle fabrication. Some common materials include stainless steel and titanium. Metal microneedles are considered biocompatible as metals have been used in medical devices for decades. Additionally, metals produce desirable mechanical properties. Ceramics have also been used to make microneedles. They can be produced at a lower cost compared to other materials. Silica glass can be used to make microneedle patches with varying needle geometries quickly in a small-scale setting.
However, silica glass can be brittle and generally has be to be produced by hand. Carbohydrates can be easily formulated into microneedle patches through molding of hot melts/slurries of the carbohydrates. These patches dissolve upon skin insertion and are cheap to manufacture and safe for use in humans. Maltose, trehalose, sucrose, and mannitol are some of the sugars that have commonly been used to formulate dissolving microneedles. The main disadvantage of these types of needles is the high-heat treatment needed to produce these needles, limiting therapeutics that can be incorporated. Additionally, the integrity of these needles can be drastically affected by temperature and humidity. More commonly, FDA approved polymers are used to fabricate microneedles. Moreover, polymers such as polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP), hyaluronic acid, polylactic acid are some of the few polymers used extensively in the fabrication of dissolving microneedles [7][8]. Many polymers are biocompatible, biodegradable, possess low toxicity, are mechanically strong enough to penetrate the skin, and are low cost [4].
4. Vaccination Using Microneedles
Vaccination via the skin or the intradermal route of administration was the original concept of immunization. Edward Jenner, who discovered the first vaccine (against smallpox), administered the vaccine by scratching it onto the first vaccine recipient’s skin [9]. The most common vaccination routes are the IM route or the SC route employing a painful needle for administration. Currently, only the Bacille Calmette-Guérin (BCG) and rabies vaccines are administered via the intradermal route [10]. The skin has an abundant presence of antigen-presenting cells (APCs) in the form of Langerhans cells and dermal dendritic cells (
3. (A) SEM image of a dissolving polymeric microneedle (size: 430 μm, scale 200 μm). (B) Optical microscope image of the same microneedle array patch.
2.1. Solid Microneedles
Solid microneedles are often used as a skin pretreatment. They are inserted into the skin and then removed to form micron-sized pores on the skin surface. Drug solutions within a patch can then be applied to the surface, which contains the micropores. Another variation utilized a roller containing solid microneedles, which pokes holes in the stratum corneum multiple times as the roller moves across the skin [32].
2.2. Hollow Microneedles
Hollow microneedles are miniature versions of the conventional hypodermic needles. Drug delivery through hollow microneedles is achieved through a pressure-driven flow of a liquid formulation. In contrast to other types, hollow microneedles are challenging to produce due to their structure and fragility [32]. However, hollow microneedles can deliver large, continuous amounts of actives compared to the other microneedle types [30].
2.3. Dissolving Microneedles
Dissolving microneedles are made using biodegradable materials such as various polymers and sugars loaded with therapeutics. After the needle is applied to the skin, the needles dissolve to release the payload into the skin. The advantage of dissolving microneedles in contrast to solid and hollow microneedles includes the ease of fabrication and single-step application of the patch. Dissolving microneedles have been looked at extensively for delivery vaccines through the skin [32].
2.4. Coated Microneedles
Coated microneedles consist of solid microneedles that have been coated with a drug solution or dispersion. There are various methods to produce coated microneedles, including dip coating, in which the microneedles are “dipped” into the coating solution. Spray coating can also be used to coat the needles. Coated microneedles are not as commonly used as solid, hollow, and dissolving microneedles since they offer a minimal amount of surface area for drug absorption.
3. Composition of Microneedles
Various materials can be used to produce microneedle arrays and these materials are all FDA approved [33]. The first material used was silicon. Advantages of using silicon include high flexibility to allow needles with customizable shapes and sizes. However, silicon microneedles’ main limitations include high cost, long fabrication times, and multi-step processing [30]. Metals can also be used for microneedle fabrication. Some common materials include stainless steel and titanium. Metal microneedles are considered biocompatible as metals have been used in medical devices for decades. Additionally, metals produce desirable mechanical properties. Ceramics have also been used to make microneedles. They can be produced at a lower cost compared to other materials. Silica glass can be used to make microneedle patches with varying needle geometries quickly in a small-scale setting.
However, silica glass can be brittle and generally has be to be produced by hand. Carbohydrates can be easily formulated into microneedle patches through molding of hot melts/slurries of the carbohydrates. These patches dissolve upon skin insertion and are cheap to manufacture and safe for use in humans. Maltose, trehalose, sucrose, and mannitol are some of the sugars that have commonly been used to formulate dissolving microneedles. The main disadvantage of these types of needles is the high-heat treatment needed to produce these needles, limiting therapeutics that can be incorporated. Additionally, the integrity of these needles can be drastically affected by temperature and humidity. More commonly, FDA approved polymers are used to fabricate microneedles. Moreover, polymers such as polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP), hyaluronic acid, polylactic acid are some of the few polymers used extensively in the fabrication of dissolving microneedles [33,34]. Many polymers are biocompatible, biodegradable, possess low toxicity, are mechanically strong enough to penetrate the skin, and are low cost [30].
4. Vaccination Using Microneedles
Vaccination via the skin or the intradermal route of administration was the original concept of immunization. Edward Jenner, who discovered the first vaccine (against smallpox), administered the vaccine by scratching it onto the first vaccine recipient’s skin [35]. The most common vaccination routes are the IM route or the SC route employing a painful needle for administration. Currently, only the Bacille Calmette-Guérin (BCG) and rabies vaccines are administered via the intradermal route [28]. The skin has an abundant presence of antigen-presenting cells (APCs) in the form of Langerhans cells and dermal dendritic cells (
Figure 2). As a result, the skin as a site of vaccination is gaining importance. Microneedles as a form of transdermal administration were developed in 1976; however, this concept was used for immunization only in recent years [11]. Soluvia™ was the first intradermal vaccine developed by Becton, Dickinson, and Company. The microneedle system is comprised of a 30-gauge metallic microneedle that is inserted 1.5 mm into the skin, which allows the delivery of the vaccine antigen into the dermis. This system was used to deliver the influenza vaccine [12] (
4). As a result, the skin as a site of vaccination is gaining importance. Microneedles as a form of transdermal administration were developed in 1976; however, this concept was used for immunization only in recent years [36]. Soluvia™ was the first intradermal vaccine developed by Becton, Dickinson, and Company. The microneedle system is comprised of a 30-gauge metallic microneedle that is inserted 1.5 mm into the skin, which allows the delivery of the vaccine antigen into the dermis. This system was used to deliver the influenza vaccine [37] (
). Since then, microneedles have been explored extensively for vaccination against various viral and bacterial infections and immunotherapy for cancers (
Table A3).
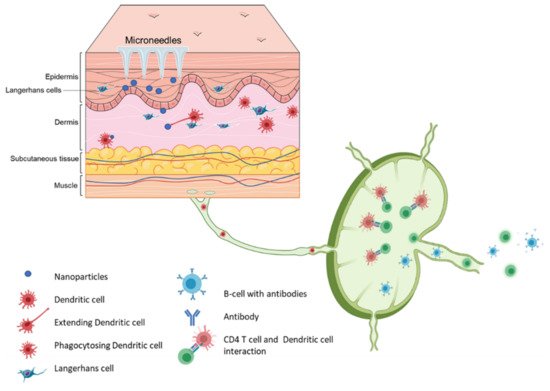
).

Figure 2. Schematic representation of immune cells activation post immunization using microneedle vaccines.
4.1. Solid Microneedles for Vaccine Delivery
As described earlier, there are different kinds of microneedles; solid, hollow, and dissolving. Each of these microneedle systems has been investigated as immunizations tools. Stainless steel is the most common material used to make solid microneedles for vaccine delivery [5][13]. These stainless-steel microneedles dip-coated with various antigens, including antigen solutions as well as antigens encapsulated in nanoparticles [14][15]. The coated antigen gets released into the skin layers upon administration of metal microneedles. As a result, the coating of the microneedles in one of the key aspects which determines the efficacy of metal microneedle vaccine. Thus, there have been advancements to improve the efficiency of stainless steel microneedles, such as nanopatterning the stainless-steel microneedle surface [16]. This nanopatterning has been shown to enhance the dip coating of antigens onto the microneedle tips by improving the hydrophilicity of the microneedle surface. Such a nano-patterned microneedle system showed enhanced plasmid DNA loading. This enhanced loading was responsible for the improved immune response observed in the in vivo study compared to conventional metal microneedles [16]. Different methods have been used to enhance the coating of antigens on the surface of solid microneedles. Solid microneedles coated with multilayers of charge reversal pH-sensitive copolymers enhanced the delivery of the DNA vaccine to antigen-presenting cells, thus producing enhanced immune response [17]. The Mantoux technique is the traditional intradermal administration method; however, an improved or first-generation intradermal administration technique involving solid microneedles in a Nanopatch™ comprising 10,000 micro projections/cm
4.1. Solid Microneedles for Vaccine Delivery
2 each 250 µm long enhanced the antigenicity of the HPV vaccine administered. Moreover, this method allowed administering the HPV vaccine without the adjuvant with a transfer efficiency of almost 20% [18]. The Nanopatch
TM was also used in a clinical trial to deliver an influenza vaccine coated on the microneedles. The randomized, partly-blinded, placebo-controlled study reported that most of the subjects preferred the microneedle vaccine over their past IM injection experience [19]. Moreover, other novel materials have also been explored to fabricate solid microneedles for administering vaccines for infectious diseases and cancer immunotherapy [20]. A solid microneedle system was developed using silk fibroin for immunization against influenza, Clostridium difficile, and Shigella. A pre-clinical study in mice demonstrated that silk fibroin was able to form solid microneedles, which provided long-term protection with dose sparing effect in case of influenza and provided moderate protection from challenge with Clostridium difficile [21]. The authors note that the amount of antigen delivered to the mice upon administration of the vaccine is less than the coated dose. The difference in dose highlights one of the challenges of vaccination using microneedles [21].
4.2. Hollow Microneedles for Vaccine Delivery
Efficient targeting of APCs is imperative to achieve an effective immune response. It is noted that particulate vaccines are more efficiently taken up by the APCs [22]. Combining the effective targeting of APCs using the particulate vaccine and pain-free administration of the vaccine offers a powerful tool for immunization. As a result, a lot of research is now focused on delivering antigens encapsulated in a polymeric particle using microneedles. Hollow microneedles are studied extensively to deliver particle-based vaccines. These microneedles contain the vaccine antigen, filled inside the hollow needles which upon administration, deliver the vaccine antigens in the skin. A microneedle system comprising of applicator-controlled silica hollow microneedles facilitated the delivery model antigen ovalbumin with and without adjuvant encapsulated in various optimized nanoparticles, namely poly (lactic-co-glycolic) (PLGA) nanoparticles, liposomes, mesoporous silica nanoparticles (MSNs), and gelatin nanoparticles (GNPs). Penetrating a depth of 120 microns, the microneedle delivery of PLGA nanoparticles and liposomes induced an excellent humoral and cellular immune response [23]. Similar results were observed when ovalbumin with and without adjuvant encapsulated in PLGA nanoparticles was administered using 3M plastic hollow microneedles attached to an applicator [24].
Hollow microneedles have also been used to deliver DNA vaccines encapsulated in a nanoparticle system. The DNA vaccine encoding for ovalbumin was encapsulated in cationic niosomes to produce a better and more robust immune response than the naked DNA. Moreover, the DNA vaccine encapsulated in the niosome resulted in a better immune response than the SC route [25]. Therapeutic cancer vaccine administered using a digitally controlled hollow microneedle injection system required significantly less antigen as compared to traditional intradermal injection. This unique hollow microneedle system composed of silica achieved automated micro-injections (0.25–10μL) to deliver a synthetic long peptide HPV E7 [18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38] derived from HPV encapsulated in cationic liposomes. There microinjected antigen induced a robust cell CD8+ cytotoxic and CD4+ T-helper cell response [26].
4.3. Dissolving Microneedles for Vaccine Delivery
Even though hollow microneedles are efficient in inducing a robust immune response, they still leave material on the skin and are not made of biodegradable material. Dissolving microneedles offer advantages over these shortcomings. Dissolving microneedles are composed of FDA approved polymers and can be loaded with the vaccine antigen or nanoparticles containing the vaccine antigen. Upon administration, these microneedles dissolve completely to release the vaccine into the skin. Dissolving microneedles loaded with microparticles have the advantage of the slow release of antigens to as to achieve sustained release of antigen which helps in achieving a robust adaptive immune response. Dissolving microneedles for immunization was first developed for the administration of the influenza vaccine. The microneedle system was composed of polyvinyl pyrrolidone and could deliver the encapsulated lyophilize antigen in 5 minutes [27]. Since then, several dissolving microneedles for vaccines have been fabricated with various polymers and sugars [28]. Dissolving microneedles have also been proven to maintain the antigen’s stability at room temperature (25 °C) for more than one year. Influenza vaccine in dissolving microneedles composed of polyvinyl alcohol (PVA) and sucrose maintained its stability at 25 °C and 60 °C for up to 24 months and four months, respectively [29]. Similarly, a malaria antigen had superior stability in a dissolving microneedle patch composed of sugars as compared to the vaccine in its liquid form [30]. Furthermore, Dissolving microneedles made of PVA have been widely studied and have proven to elucidate significantly robust immune responses upon challenge. They have also been an efficient delivery system for prophylactic DNA vaccine for cervical cancer [31][32]. Protective immune responses in pregnancy have also been studied upon challenge with tetanus toxin compared to traditional routes such as IM [33]. PVA microneedles have demonstrated induction of TH1 cytokines (IFN-γ and IL-12) when challenged with Streptococcus suis bacteria and confer long-term protection by induction IgG2a antibodies specific to S. suis bacteria [34]. Dissolving microneedles have proven to induce superior antibody response against adenovirus-based Plasmodium falciparum malaria vaccine, AdHu5–PfRH5. A study proved that low prime dose given using dissolving microneedles, and a boost dose given intramuscularly gave a robust immune response as compared prime dose given intramuscularly. Thus, microneedle aid in achieving a superior immune response as compared to the conventional intramuscular route [30]. Additionally, microneedles composed of PVA and sugars demonstrated also enhanced immune response upon challenge with Neisseria gonorrhoeae [39]. Like hollow microneedles, dissolving microneedles have also been used to study the delivery of vaccine antigens encapsulated in cationic liposomes. The cationic liposome-based microneedle vaccine protected against a challenge with the bacteria Leishmania donovani [35]. The BCG vaccine is an intradermal vaccine; however, it is administered using a hypodermic needle. A study involving coated BCG vaccine coated on dissolving microneedles made using sodium alginate and sugars revealed that the microneedle system produced an immune response comparable to the injected vaccine [36].
Hyaluronic acid is another biodegradable polymer that is used a lot for the formulation of dissolving microneedles for vaccine delivery for infectious diseases and cancer immunotherapy [37]. A canine influenza vaccine was successfully coated on hyaluronic acid microneedle tips. The microneedle had separable tips in a system called insertion-responsive microneedles. The microneedle coated with freeze-dried vaccine provided thermal stability to the vaccine when stored at 50 °C for three weeks compared to the liquid form and protected from challenge with H3N2 wild type virus [38]. Another study for insertion responsive microneedle system for canine influenza vaccine proved that the system could be used to vaccinate dogs without the need to shave their hair and provided better compliance for the dog and owners [40]. Hyaluronic acid microneedle induced a robust immune response with just one dose in a B16F10 mouse melanoma model compared to microneedles without degradation trigger or intratumoral injection of free, programmed death-1 with the same dose for skin cancer [41]. Sustained-release polymers have also been employed for the formulation of microneedles for vaccine delivery. A microneedle system comprising poly (lactic-co-glycolic acid) was used to prepare vaccine cores and shells using the micro-molding technique. This system was used to deliver the clinically approved vaccine Prevnar-13 against the bacterium Streptococcus pneumoniae. It was observed that the microneedle vaccine was able to induce an immune response comparable to that obtained on multiple SC injections [42].
Additionally, a hepatitis B vaccine formulated in a dual release pattern using polylactic acid and carboxymethylcellulose (CMC) could function as a prime and booster in one microneedles system. The microneedle vaccine was again able to produce an immune response similar or even higher than two shots given using the conventional administration method [43]. As a result, microneedles made using sustained-release polymers with various degradation kinetics can be adopted to achieve vaccines that require multiple boosters. Additionally, dissolving microneedles have been extensively explored as administration systems for antigens encapsulated in micro and nanoparticles [44]. Microneedles have also been used to deliver combination vaccines in the form of a compartmental microneedle array (CMA). This CMA formulated with polylactic acid consisted of two separate sections in the same microneedle patch. The two sections were coated with B-Y influenza vaccine for B/Yamagata virus B-V influenza vaccine for B/Victoria virus. The in vivo studies in mice indicated that the mice vaccinated with the CMA had higher neutralizing antibodies and better survival rates than the traditional IM route. The dissolving microneedle influenza vaccine has also been reported to have better patient compliance than the conventional IM route in Phase I clinical study [45]. Dissolving microneedles have also been studied as a potential vaccination system to tackle the COVID-19 pandemic. Researchers found that microneedles formulated using CMC coated with the SARS-CoV-2-S1 protein produced significant levels of antigen-specific antibodies [46].
4.2. Hollow Microneedles for Vaccine Delivery
4.3. Dissolving Microneedles for Vaccine Delivery
Even though hollow microneedles are efficient in inducing a robust immune response, they still leave material on the skin and are not made of biodegradable material. Dissolving microneedles offer advantages over these shortcomings. Dissolving microneedles are composed of FDA approved polymers and can be loaded with the vaccine antigen or nanoparticles containing the vaccine antigen. Upon administration, these microneedles dissolve completely to release the vaccine into the skin. Dissolving microneedles loaded with microparticles have the advantage of the slow release of antigens to as to achieve sustained release of antigen which helps in achieving a robust adaptive immune response. Dissolving microneedles for immunization was first developed for the administration of the influenza vaccine. The microneedle system was composed of polyvinyl pyrrolidone and could deliver the encapsulated lyophilize antigen in 5 minutes [52]. Since then, several dissolving microneedles for vaccines have been fabricated with various polymers and sugars [53]. Dissolving microneedles have also been proven to maintain the antigen’s stability at room temperature (25 °C) for more than one year. Influenza vaccine in dissolving microneedles composed of polyvinyl alcohol (PVA) and sucrose maintained its stability at 25 °C and 60 °C for up to 24 months and four months, respectively [54]. Similarly, a malaria antigen had superior stability in a dissolving microneedle patch composed of sugars as compared to the vaccine in its liquid form [55]. Furthermore, Dissolving microneedles made of PVA have been widely studied and have proven to elucidate significantly robust immune responses upon challenge. They have also been an efficient delivery system for prophylactic DNA vaccine for cervical cancer [56,57]. Protective immune responses in pregnancy have also been studied upon challenge with tetanus toxin compared to traditional routes such as IM [58]. PVA microneedles have demonstrated induction of TH1 cytokines (IFN-γ and IL-12) when challenged with Streptococcus suis bacteria and confer long-term protection by induction IgG2a antibodies specific to S. suis bacteria [59]. Dissolving microneedles have proven to induce superior antibody response against adenovirus-based Plasmodium falciparum malaria vaccine, AdHu5–PfRH5. A study proved that low prime dose given using dissolving microneedles, and a boost dose given intramuscularly gave a robust immune response as compared prime dose given intramuscularly. Thus, microneedle aid in achieving a superior immune response as compared to the conventional intramuscular route [55]. Additionally, microneedles composed of PVA and sugars demonstrated also enhanced immune response upon challenge with Neisseria gonorrhoeae [29]. Like hollow microneedles, dissolving microneedles have also been used to study the delivery of vaccine antigens encapsulated in cationic liposomes. The cationic liposome-based microneedle vaccine protected against a challenge with the bacteria Leishmania donovani [60]. The BCG vaccine is an intradermal vaccine; however, it is administered using a hypodermic needle. A study involving coated BCG vaccine coated on dissolving microneedles made using sodium alginate and sugars revealed that the microneedle system produced an immune response comparable to the injected vaccine [61].
Hyaluronic acid is another biodegradable polymer that is used a lot for the formulation of dissolving microneedles for vaccine delivery for infectious diseases and cancer immunotherapy [62]. A canine influenza vaccine was successfully coated on hyaluronic acid microneedle tips. The microneedle had separable tips in a system called insertion-responsive microneedles. The microneedle coated with freeze-dried vaccine provided thermal stability to the vaccine when stored at 50 °C for three weeks compared to the liquid form and protected from challenge with H3N2 wild type virus [63]. Another study for insertion responsive microneedle system for canine influenza vaccine proved that the system could be used to vaccinate dogs without the need to shave their hair and provided better compliance for the dog and owners [64]. Hyaluronic acid microneedle induced a robust immune response with just one dose in a B16F10 mouse melanoma model compared to microneedles without degradation trigger or intratumoral injection of free, programmed death-1 with the same dose for skin cancer [65]. Sustained-release polymers have also been employed for the formulation of microneedles for vaccine delivery. A microneedle system comprising poly (lactic-co-glycolic acid) was used to prepare vaccine cores and shells using the micro-molding technique. This system was used to deliver the clinically approved vaccine Prevnar-13 against the bacterium Streptococcus pneumoniae. It was observed that the microneedle vaccine was able to induce an immune response comparable to that obtained on multiple SC injections [66].
Additionally, a hepatitis B vaccine formulated in a dual release pattern using polylactic acid and carboxymethylcellulose (CMC) could function as a prime and booster in one microneedles system. The microneedle vaccine was again able to produce an immune response similar or even higher than two shots given using the conventional administration method [67]. As a result, microneedles made using sustained-release polymers with various degradation kinetics can be adopted to achieve vaccines that require multiple boosters. Additionally, dissolving microneedles have been extensively explored as administration systems for antigens encapsulated in micro and nanoparticles [68]. Microneedles have also been used to deliver combination vaccines in the form of a compartmental microneedle array (CMA). This CMA formulated with polylactic acid consisted of two separate sections in the same microneedle patch. The two sections were coated with B-Y influenza vaccine for B/Yamagata virus B-V influenza vaccine for B/Victoria virus. The in vivo studies in mice indicated that the mice vaccinated with the CMA had higher neutralizing antibodies and better survival rates than the traditional IM route. The dissolving microneedle influenza vaccine has also been reported to have better patient compliance than the conventional IM route in Phase I clinical study [69]. Dissolving microneedles have also been studied as a potential vaccination system to tackle the COVID-19 pandemic. Researchers found that microneedles formulated using CMC coated with the SARS-CoV-2-S1 protein produced significant levels of antigen-specific antibodies [70].
