The use of photodynamic therapy (PDT) to eradicate microorganisms has been regarded as a promising alternative to anti-infective therapies, such as those based on antibiotics, and more recently, is being considered for skin wound-healing. Among the several molecules exploited as photosensitizers (PS), porphyrinoids exhibit suitable features for achieving those goals efficiently. The capability that these macrocycles display to generate reactive oxygen species (ROS) gives a significant contribution to the regenerative process. ROS are responsible for avoiding the development of infections by inactivating microorganisms such as bacteria but also by promoting cell proliferation through the activation of stem cells which regulates inflammatory factors and collagen remodeling. The PS can act solo or combined with several materials, such as polymers, hydrogels, nanotubes, or metal-organic frameworks (MOF), keeping both the microbial photoinactivation and healing/regenerative processes’ effectiveness.
- Photodynamic therapy
- photosensitizer
- ALA
- protoporphyrin-IX
- porphyrins
- chlorins
- phthalocyanines
- photodynamic inactivation
- reactive oxygen species
- wound healing
- tissue regeneration
1. Introduction
N-heterocyclic macrocycles well-known for their mediation in important biological functions, such as respiration and photosynthesis, but also by their role in a wide range of clinical and non-clinical applications that have high impacts on human life [1][2][3]. Natural tetrapyrrolic macrocycles like heme (iron(II) complex of PP-IX) and chlorophylls (
Figure 1), but also the synthetic porphyrins and analogs ones (e.g., benzoporphyrins, chlorins, phthalocyanines) [4][5] are recognized to present unique structural, physicochemical, and photochemical features to be used in the development of dyes for dye-sensitized solar cells (DSSC) [6][7][8][9][10][11][12][13], (chemo) sensors [14][15][16][17][18][19][20], (photo)catalysts [21][22][23][24][25][26][27], biomarkers [14][28][29] or as therapeutic photosensitizers (PS) [30][31][32][33][34][35][36][37][38][39][40], among other applications [1].
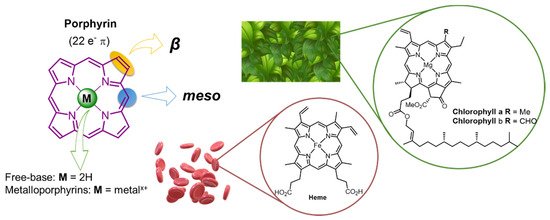
Figure 1.
a
b
1
2
Figure 2B) are particularly relevant for their success to mediate the photodynamic process in photodynamic therapy (PDT) against tumoral cells [33][36][41][42], but also toward non-oncological pathologies, such as age-related macular degeneration. In the last two decades, porphyrinoids (either as free-base or coordinated with different metals) have been recognized as highly efficient PS to eradicate a broad range of microorganisms including drug-resistant strains using the same concept of PDT [31][37][43][44][45][46][47][48][49][50].
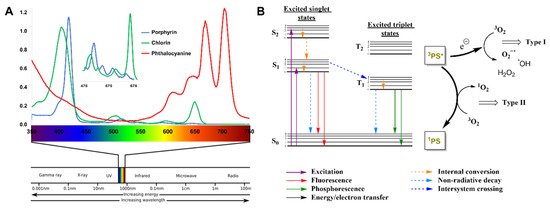
Figure 2.
A
B
2. PDT
1
3
3
2
1
2−
2
2
Figure 2B) [51][52][53]. Such radicals are then responsible for the elimination of undesirable bacteria.
Recently, the potential of PDT for tissue regeneration has caught the attention of many research groups concerning the role of non-porphyrinoid PS for skin wound healing, as was recently highlighted in our previous review [54]. In that review article, it is discussed that the PDT process not only enhances the mitochondrial activity, respiratory chain, and ATP synthesis [52][55], but also recruits important metalloproteinases (MMP)/growth factors (GF) and activate signal-regulated kinases [56][57] that are critical for the differentiation and proliferation of skin cells (such as fibroblasts) and extracellular matrix (ECM) components (such as elastin and collagen) [53][56][57][58] (
in situ,
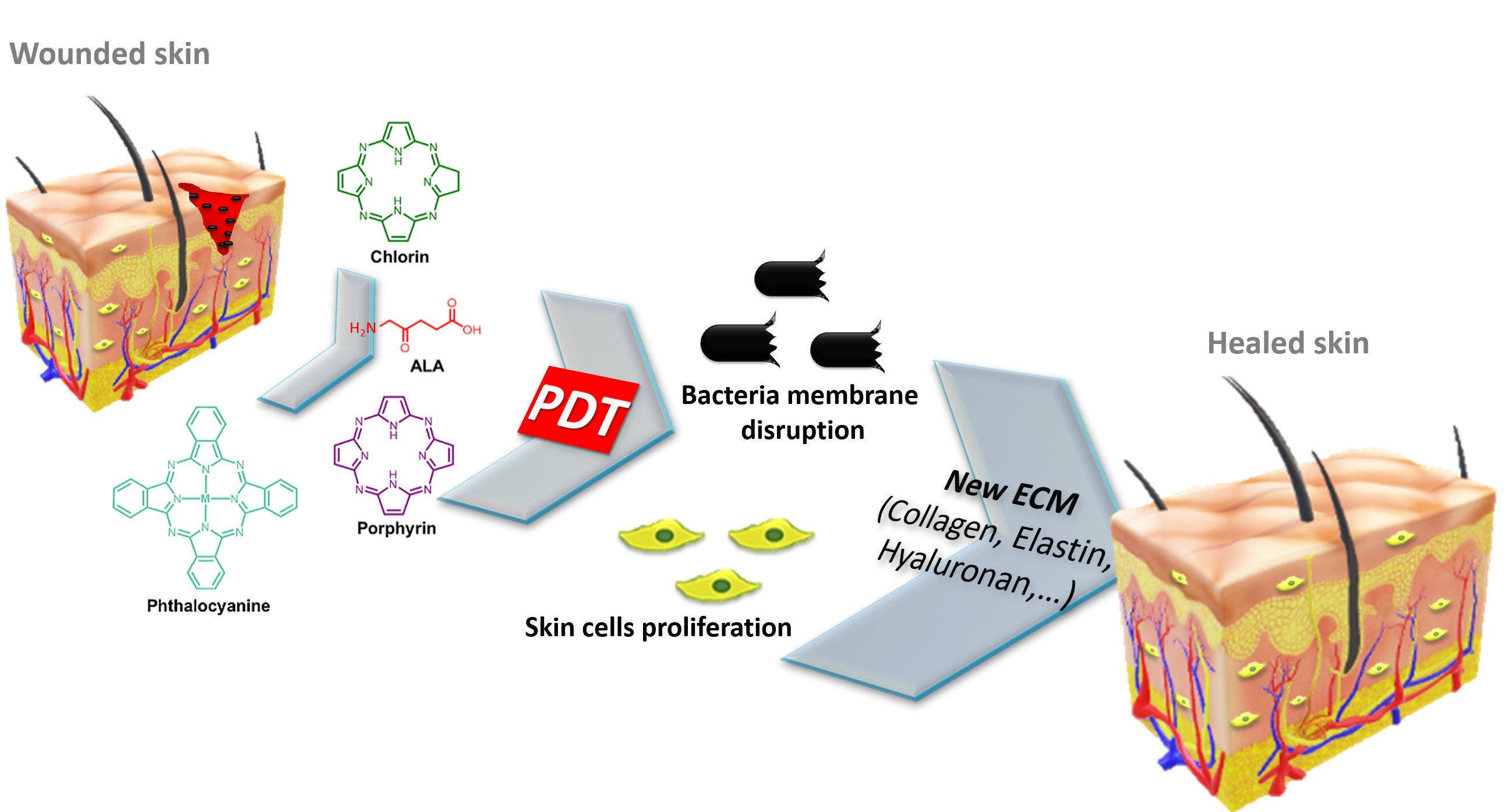
Figure 3.
The PS studies comprised (i) first-generation, consisting of mixtures obtained from natural occurring porphyrins (e.g., hematoporphyrin derivative, Photofrin); (ii) second-generation, such as porphyrins, chlorins, benzoporphyrins and phthalocyanines with pure structures; and (iii) third-generation, resulting from the combination of second-generation PS with specific delivery carriers, such as antibodies, liposomes, polymers, nanoparticles, and micelles to overcome the drawbacks of the other two PS generations, such as low accumulation on the desired site, self-aggregation and low solubility in aqueous media [46][59][60].
®
®
®
®
®
®
®
3. Final Remarks
In summary, PDI with porphyrinoids-based photosensitizers exhibits great potential for the treatment of skin wounds, by enhancing healing in bacteria-infected wounds. Noticeably, in recent years, significant efforts were performed by the scientific community to improve the use of tetrapyrrolic macrocycles in skin wound healing. The capability of porphyrinoids to act as efficient PS (Table 1) relies on their capability to generate ROS when irradiated at wavelengths comprised in the “therapeutic window”.
2) relies on their capability to generate ROS when irradiated at wavelengths comprised in the “therapeutic window”.Table 12.
| Wound Type | Infection | PS | Log Reduction |
Improved Healing | Ref. | |
|---|---|---|---|---|---|---|
| Ex Vivo | Wound healing organ culture | Not infected | ALA | n.a. | YES | [61][68] |
| In vivo animal studies | Excisions | Not infected | ALA | n.a. | YES | [62][72] |
| AlPcCl-PEG | n.a. | YES | [55] | |||
| E. coli | pLCe6 | YES | inconclusive | [63][98] | ||
| Ce6-IL | YES | YES | [64][108] | |||
| TCPP-PB@MOF | YES | YES | [65][118] | |||
| TCPP-PCN224 | YES | YES | [66][119] | |||
| MOF-TCPP-Dopamine | YES | YES | [67][121] | |||
| PGA-TMPyP nanofibrous mat | YES | YES | [68][122] | |||
| AgNP/ZnPcCO2H fabric | YES | YES | [69][132] | |||
| P. aeruginosa | pL-Ce6 | YES | YES | [70][99] | ||
| pL–Cp6 | YES | NO | [71][110] | |||
| S. aureus | Ce6-IL | YES | YES | [64][108] | ||
| TCPP-PB@MOF | YES | YES | [65][118] | |||
| TCPP-PCN224 | YES | YES | [66][119] | |||
| MOF-TCPP-dopamine | YES | YES | [67][121] | |||
| PGA-TMPyP nanofibrous mat | YES | YES | [68][122] | |||
| MRSA; P. aeruginosa |
pL–Cp6 | n.d. | NO | [72][111] | ||
| TMPyP | YES | inconclusive | [73][117] | |||
| MRSA; E. coli; P. aeruginosa |
TDAHPP | YES | YES | [74][116] | ||
| MREC; MRAB |
ZnPc-(Lys)5 | YES | YES | [75][130] | ||
| MRSA | RLP068/Cl-cellulose gel | YES | YES | [76][126] | ||
| Abscesses | S. aureus | pLCe6 | YES | inconclusive | [77][106] | |
| E. coli | YES | inconclusive | [77][106] | |||
| P. aeruginosa | YES | inconclusive | [77][106] | |||
| A. baumannii | YES | inconclusive | [78][100] | |||
| Burns | Not infected | MAL | n.a. | YES | [79][89] | |
| MRSA | PEI-Ce6 | YES | YES | [80][101] | ||
| RLP068/Cl | YES | YES | [81][127] | |||
| MDR S. aureus | Sinoporphyrin derivative | YES | YES | [82][112] | ||
| S. aureus | PTMPyP | YES | NO | [83][113] | ||
| Ulcers | MRSA | ALA | YES | YES | [84][79] | |
| Skin wounds (not specified) |
Not infected | Pheophorbide a | n.a. | YES | [85][97] | |
| Fotoditazin–Pluronic F127 | n.a. | YES | [86][107] | |||
| P. aeruginosa | ALA | YES | YES | [87][80[88],90] | ||
| S. aureus | HTCC-Ce6-Mg-EGCG NP | YES | YES | [89][109] | ||
| Incisions | Not infected | m-THPC-collagen scaffolds | n.a. | YES | [90][114] | |
| CASP | n.a. | YES | [67][121] | |||
| MPD-MA | n.a. | NO | [68][122] | |||
| In vivo human studies | Cutaneous Leishmaniasis lesions | Leishmania major; Leishmania tropica |
ALA | n.d. | YES | [91][75] [92][76] |
| Leg Ulcer | MRSA | ALA | n.d. | YES | [93][78] | |
| P. aeruginosa | ALA | n.d. | YES | [94][69] | ||
| RLP068/Cl | YES | YES | [95][128] | |||
| S. aureus; P. aeruginosa E. coli |
TPP | YES | YES | [96][115] | ||
| S. aureus | RLP068/Cl | YES | YES | [95][128] | ||
| Chronic Venous Ulcers |
E. faecalis | ALA | n.d. | YES | [97][81] | |
| MAL | n.d. | YES | [98][86] | |||
| S. aureus | ALA | n.d. | YES | [97][81] | ||
| MAL | n.d. | YES | [98][86] | |||
| Actinic Keratosis | Not infected | MAL | n.a. | YES | [99][82] | |
| Excisional Wounds | Not infected | MAL | n.a. | inconclusive | [100][67] |
n.a.—not applicable; n.d.—not determined.
meso
-tetraarylporphyrins or phthalocyanines. Porphyrinoids display high synthetic versatility, allowing the preparation of several different PS delivery systems in the target wound site. Tetrapyrrolic can be directly attached to the matrix through covalent bonds, encapsulated in polymeric nanocarriers, incorporated by a physical mixture with hydrogels, or used to decorate polymeric systems throughout non-covalent approaches. The symbiotic effect of PS and biocompatible polymeric systems improve not only the healing rate, but also the PS efficiency against Gram-negative bacteria (bacteria that are less affected by PDI) growth and consequent biofilm formation, the optimization of the PS into the wound, and the PS degradation rates slow down. Moreover, this approach allows topical application at the wound site which is a significant advantage for the regenerative and skin wound healing processes.Considering that the limited clinical success of wound healing [101] is associated with the lack of (i) funding, (ii) centers of excellence concerning wound care management, (iii) methodologic consistency in clinical trials, and (iv) the appearance of more complex pathologies due to the populations aging, and too many drugs with limited efficacies, we believe that PDI/healing can have an opportunity to overcome some of these drawbacks. This approach can improve the local asepsis due to its antimicrobial effect, which includes areas with diminished blood irrigation, and where the conventional antimicrobials do not work. Consequently, it will reduce patient pain and increase cosmetic effects due to enhanced cell proliferation. Additionally, it can be performed in ambulatory care environments, requiring low-cost light sources, and demanding easy clinical staff training. Furthermore, the treatment can be repeated without resistance development.
Considering that the limited clinical success of wound healing [133] is associated with the lack of (i) funding, (ii) centers of excellence concerning wound care management, (iii) methodologic consistency in clinical trials, and (iv) the appearance of more complex pathologies due to the populations aging, and too many drugs with limited efficacies, we believe that PDI/healing can have an opportunity to overcome some of these drawbacks. This approach can improve the local asepsis due to its antimicrobial effect, which includes areas with diminished blood irrigation, and where the conventional antimicrobials do not work. Consequently, it will reduce patient pain and increase cosmetic effects due to enhanced cell proliferation. Additionally, it can be performed in ambulatory care environments, requiring low-cost light sources, and demanding easy clinical staff training. Furthermore, the treatment can be repeated without resistance development.