Touch surfaces made of copper-based alloys such as brasses are used in healthcare settings in an attempt to reduce the bioburden and limit environmental transmission of nosocomial pathogens.
- copper
- brass
- antibacterial activity
- hospital acquired infections
- antibiotic resistance
- antibacterial surfaces
1. Introduction
Brasses are alloys composed of copper and zinc, as opposed to bronze which are alloys of copper and tin. The zinc percentage within brass alloys can vary from 5 to 40% [1]. Below 35% of Zn, the alloy is termed copper-rich and forms a single solution of face-centered cubic (fcc) copper matrix called α brass [1][2]. Above 35% of Zn, an ordered body-centered-cubic (bcc) phase called β phase is stably formed in addition to the α phase [1][2]. From a layman point of view, copper-rich brass alloys are called red brasses, while brasses with a higher Zn content are termed yellow brasses [3]. Over time, other chemical elements such as manganese, nickel, aluminum, lead or silicium, to name a few, have been added to brass to create the High Entropy Alloys (HEA) used nowadays, improving their technological properties and resistance to corrosion [2][4].
Along with bronze, brass has been one of the first alloys forged by mankind with reports of brass artifacts dating back to the 3rd millennium BC [5]. In Europe, the first intentional productions of brass in the Greek, Etruscan and Roman civilizations are thought to have taken place sometime during the 1st millennium BC [6]. Brass was then used to create everyday objects such as handles, coins, arms (daggers, axes, etc.) or decorative objects such as fibulae, rings and statuettes [6]. In addition to these early mundane uses of brass alloys, and more in line with the subject of this paper, copper, the main component of brass, has also long been employed to prevent water fouling or treat infections, with a first written trace of such uses found in the Smith papyrus (around 1500 BC) [7]. Practices such as the storage of Ganges’ water in brass or copper utensils in accordance with ancient texts of Ayurveda in India or the addition of copper coins in water canteens by Second World War Japanese soldiers to keep the water sanitary have been documented, indirectly pointing at the bactericidal activity of copper [8][9][10]. This bactericidal activity is concentration-dependent. Indeed, at low concentrations, copper plays an important role in the life of bacteria as it is involved in a number of their metalloenzymes called cuproenzymes [11][12]. However, at high concentrations, copper ions overcome the bacterial handling/detoxification systems and are able to generate a wide array of detrimental perturbations for the bacterial cell such as mismetalation of metalloenzymes induced by the ionic imbalance or excessive production of reactive oxygen species (ROS) initiated through a Fenton-like reaction. Those ROS can in turn lead to membrane, protein and DNA damages and eventually to cell death [12][13].
In healthcare settings, the initial observations on the potential of brass to limit the bacterial contamination of touch surfaces, as compared to stainless steel, were published in the early eighties [14]. These observations paved the way towards a possible mean of reducing indirect transmission of bacteria originating from touch surfaces, especially in healthcare facilities. It thus offered an additional preventive tool in the fight against hospital acquired infections (HAIs).
2. Antimicrobial Mechanism of Action of Copper-Containing Surfaces
Observational descriptions of the bactericidal effect of surfaces made out of copper or copper alloys were reviewed as early as in the seventies [15]. Many laboratory studies on various bacterial species followed, showing a rapid (within minutes) bactericidal effect of copper-containing surfaces against most of the strains tested.
This biocide activity has been termed contact killing [16]. Contact killing implies a direct and mandatory contact between bacteria and the surface for the antibacterial activity to take place. This point was demonstrated by the lack of antibacterial activity of copper coupons coated with a honeycomb-like polymer grid preventing direct contact with a wet Enterococcus hirae inoculum [17]. Meanwhile, similar but uncoated copper coupons were able to induce a reduction in the E. hirae inoculum greater than 106 bacteria in 30 min. Importantly, copper ion release was found to be similar for both the coated and uncoated coupons. Also, Zheng et al. [18] reported that CuSO4 in solution was less effective in killing Candida albicans planktonic or biofilm cells than copper ions released from the surface of coupons, implying that direct contact was instrumental in the killing process [18]. A few years later, Solioz proposed a 4-step chronological order for contact killing by copper-containing surfaces [19]. The first and crucial step consists in the dissolution of copper ions from the surface and their accumulation in the small aqueous space between the material surface and bacterial membrane, reaching the mM range [19][20]. These copper ions can lead to (i) the generation of reactive oxygen species (ROS) [21], (ii) the inhibition of the respiratory chain [22], (iii) lipid peroxidation [23][24] damages to cell membrane [24][25][26][27], (iv) DNA degradation [28][29], (v) modified protein expression [30], and (vi) displacement of iron-sulfur clusters and inactivation of metalloproteins [31][32] (Figure 1). The actual mechanism(s) as well as the precise order in which these mechanisms are involved in copper-mediated contact killing is expected to be dependent on factors such as the bacterial physiology and environmental conditions (e.g., presence of moisture or buffering/proteinaceous agents). Nevertheless, copper ions are instrumental in the contact-killing process, as demonstrated by the notable reductions/delays in bacterial deaths registered when copper chelators such as bichinchoninic acid, ethylenediaminetetraacetic acid (for Cu2+) or bathocuproine disulfonic acid (for Cu+) are used [21][32][33].
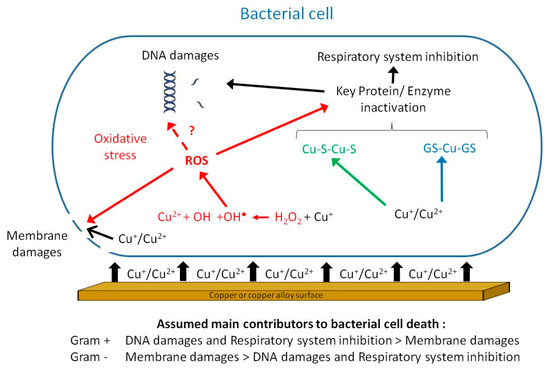
Figure 1. Putative mechanisms involved in contact killing: direct destruction of the membrane by cupric and cuprous ions (black pathway); hydrogen peroxide-dependent oxidation of Cu+ in the cell under aerobic conditions generating reactive oxygen species (ROS) (red pathway); interactions between copper ions and glutathione under anaerobic conditions (blue pathway) and displacement of iron from iron-sulfur clusters (green pathway). The final steps leading to bacterial cell death include inactivation of key proteins/enzymes, among which are those involved in the respiratory system as well as membrane and DNA damages.
3. In Vitro Antibacterial Activity of Copper and Brass Alloys
3.1. Vegetative Forms
In order to demonstrate the antimicrobial efficacy of copper and copper alloy surfaces, a multitude of techniques have been used such as bacterial enumeration by culture, live/dead staining, fluorescence in situ hybridization or bioluminescent strains [34]. Despite varying experimental protocols, most studies relate a good efficacy of copper surfaces on vegetative forms of a wide range of bacterial species. The antibacterial efficacy is usually assessed after a time of exposure to the copper-containing surface using reduction in bacterial counts either compared to the initial inoculum and/or to the counts obtained on a control surface deprived of antibacterial properties such as glass, plastic or stainless steel (Figure 2).

Figure 2. Antibacterial efficacy of copper-containing surfaces: general procedure and calculations. Reduction cutoff values compared to stainless steel for the validation of an anti-microbial efficacy according to Association Française de NORmalisation (AFNOR) NFS90-700 (in red) and US Environmental Protection Agency (EPA) (in green).
These tests were first held on food pathogens and collection strains susceptible to antibiotics [35][36][37], but publications soon moved on to report significant reductions in bacterial counts on copper and copper alloy surfaces for a range of multidrug-resistant (MDR) bacteria and/or bacteria originating from clinical settings [32][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53]. Indeed, the importance of testing clinical strains to evaluate whether any co-selected, cross-selected and/or co-regulated resistance between copper and antibiotics and/or detergent-disinfectants could occur soon appeared as mandatory [46]. Most of these studies gave encouraging results as to the efficiency of brasses on Vancomycin Resistant Enterococci (VRE), MDR or Extremely drug-resistant (XDR) Acinetobacter baumannii, Meticillin-Resistant Staphylococcus aureus (MRSA), MDR Pseudomonas aeruginosa, Escherichia coli, Mycobacterium tuberculosis and Carbapenem-producing enterobacteria (CPE) (Table 1). However, within a given bacterial genus, some species (and even within a given species, some strains) could display slightly different susceptibility to copper and brass alloys.
Table 1. Antibacterial activity of brass surfaces on selected nosocomial pathogens.
|
Bacterial Species |
Copper Alloy (% Cu) |
Efficacy a |
References |
|
|---|---|---|---|---|
|
Exposure Time |
||||
|
Short (≤1 h) |
Long (>1 h) |
|||
|
S. aureus |
C26000 (70) |
98.8–100 |
ND–100 |
|
|
MRSA |
C24000 (80) C26000 (70) NP (62–63) |
74–100 6.7–97.3 6.7–99.85 |
84.1–100 ND–100 ND–100 |
|
|
E. faecalis |
C26000 (70) C28000 (60) |
~20 to 50 ~20 |
100 100 |
[28] |
|
E. faecium |
C26000 (70) C28000 (60) |
~20 to 84 ~20 |
99.99–100 99.94–99.99 |
[28] |
|
VRE |
NP (62.5) |
99.92 |
ND |
[45] |
|
C. difficile (vegetative cells and spores) |
C26000 (70) |
36.9 |
68.4 |
[47] |
|
A. baumannii |
C26000 (70) |
0–98.3 |
98.1–100 |
|
|
MDR/XDR A. baumannii |
C27400 (63.2) NP (62–63) |
0–99.85 20–99.95 |
0–99.94 ND–100 |
|
|
XDR A. lwoffii |
C27400 (63.2) |
91.63 |
58.36 |
[44] |
|
A. pittii |
C27400 (63.2) |
68.89 |
99.79 |
[44] |
|
MDR Enterobacter spp. |
NP (62.5–63) |
26–99.34 |
ND–100 |
|
|
E. coli |
C21000 (95) C23000 (85) C26000 (70) C28000 (60) C83300 (93) C83600 (85) C85700 (61) |
>90 100 96.3–100 65.5–99.99 33.3–86.7 33.3 33.3 |
100 100 97.2–100 76.5–100 100 99.97–100 99.97–99.99 |
|
|
MDR E. coli |
NP (62.5-63) |
0–99.44 |
ND–98.4 |
|
|
K. pneumoniae |
C26000 (70) C28000 (60) NP (62) |
100 99.86–100 100 |
100 100 100 |
|
|
MDR K. pneumoniae |
NP (62.5–63) |
39.3–99.77 |
ND–73.2 |
|
|
P. aeruginosa |
C26000 (70) NP (62) |
15.6–77.5 5.4 |
96.9–100 100 |
[39] |
|
MDR P. aeruginosa |
NP (62.5–63) |
ND-100 |
97.2–100 |
|
Note: MRSA, Meticillin-Resistant Staphylococcus aureus; MDR, Multi-Drug-Resistant; ND, Not Determined; NP, Not Provided; VRE, Vancomycin-Resistant Enterococcus, XDR, extremely drug-Resistant; a: % reduction against stainless steel (S30400) control (stated or calculated from data given in the references). Short exposure times ranged from 5 min to 60 min and long exposure times from 75 min to 24 h.
For example, within the Acinetobacter genus, the reduction in Acinetobacter lwoffii counts on brass obtained after a 5 h exposure were lower (around 99% reduction) than those obtained for Acinetobacter pittii and most A. baumannii strains (99.9% reduction and above) [44]. At the species level, one XDR A. baumannii strain count was unchanged after a 5 h exposure to brass, while the two other A. baumannii strains (1 MDR and 1 XDR) registered a count reduction of at least 99.9% [44]. Similarly, out of three MRSA strains, two were readily eradicated from the surface of brass while only a one log (90%) reduction was registered for the third one after a 6 h exposure [38].
A likely explanation for those behavioral discrepancies between strains of a single species is the development of adaptation mechanisms to cope with copper toxicity. Recent reports have shown that laboratory mutants of E. coli and S. aureus are able to survive on copper surfaces much longer than their parent strains [54]. However, the precise mechanism(s) leading to this increased survival could not be determined by proteomic and genomic analyses [54]. Upregulation of copper efflux systems such as Cus system in E. coli or transmission of plasmids harboring copper-resistance genes by Horizontal Gene Transfer (HGT) have been proposed to explain copper-tolerance in some strains [55]. Also, in A. baumannii, the presence of copRS in strains was recently linked with copper-tolerance [56]. All these mechanisms increase the survival time of strains on copper surfaces but do not prevent contact killing from eventually taking place. From an ecological point of view, no causal relationship between exposure to copper-containing surfaces and the de novo development of antibiotic-resistant strains has been evidenced so far. The likelihood of a widespread emergence of bacterial strains resistant to copper-induced contact killing and its possible contribution to overall antibiotic-resistance can be evaluated as low on the basis of the following considerations: (i) copper and copper alloys have been used for a very long time without enabling the rise of complete resistance to copper-induced contact killing, (ii) contact killing is a rapid process, especially in dry conditions, and does not leave bacterial cells the time to divide, and (iii) DNA damages, which are part of the contact-killing process, also target plasmidic DNA [16]. However, a point of concern is the possible contribution of these surfaces in the selection of strains already resistant to antibiotics and also carrying copper-tolerance/resistance genes through co-selection [55][57][58].
Other concerns regarding the use of copper-containing surfaces in general and brasses in particular as a mean to reduce the microbial bioburden in healthcare settings are the long-term persistence of a suitable antimicrobial effect, especially after repeated applications of disinfectants on these surfaces, and the effectiveness of disinfective treatments. A recent report indicated that antimicrobial efficacy of a solid copper alloy was better retained than those of a copper-containing polymer or a copper alloy-coated stainless steel [59]. A lower effectiveness of peracetic acid aerosol disinfection targeting Geobacillus stearothermophilus spores on copper and brass as opposed to ceramics, stainless steel or polyvinylchloride was also reported [60]. Thus, the impact of cleaning protocols and disinfectants on the antibacterial effectiveness of copper alloys warrants further work.
3.2. Sporulated Forms
On spore-forming bacteria, the results obtained by copper and brass surfaces were less clear-cut. For example, Weaver et al. [47] showed that copper and copper alloys containing at least 70% of copper were able to significantly reduce the number ofClostridium (now Clostridioides) difficile vegetative forms and spores compared to stainless steel (Table 1). Similarly, no vegetative forms and less than 0.2% of germinating spores of two C. difficile strains were recovered from copper coupons after a 60 min exposure. However, no reduction in C. difficile dormant spores was recorded after a 3 h exposure [53]. Aerobic spore-forming Bacillus subtilis was also employed as a test species to explore the sporicidal activity of copper and of a silver nickel copper alloy (solid or sprayed forms). It showed a significant reduction in dormant and germinating spores [61]. These results were not in accordance with an earlier report that only registered a 1 log reduction in B. subtilis counts after a 45 min exposure. By contrast, a B. subtilis mutant strain unable to form endospores was eradicated from copper and brass after a 20–30 min exposure, indicating that endospores were instrumental in the survival of B. subtilis on the surface [24]. Bacillus anthracis vegetative forms were found to be slightly reduced (−1 log) on copper as compared to stainless steel after a 10 min exposure but B. anthracis counts remained steady afterwards during the full 24 h exposure time [62]. This persistence was once more demonstrated to be linked with the presence of B. anthracis endospores [62]. Espirito-Santo et al. also showed that endospores from Bacillus cereus were able to survive after a short exposition to copper [25]. Taken together, those results imply that mechanisms involved in contact killing are less efficient on endospores than on vegetative forms.
3.3. Factors Influencing the Antibacterial Effectiveness of Copper Alloys
The experiments reported above use varying sets of parameters (inoculum density and volume, wet vs. dry inoculation, incubation temperature and relative humidity (RH), and so on). It is therefore difficult to draw fair comparisons and give a ruling on the relative efficacy of different copper alloys from published results, if these alloys are not tested within the same framework. While 99.9% “pure” copper is widely confirmed and used as the “gold standard” of antibacterial efficacy, the ranking of antibacterial activity according to the copper content of alloys is not as straightforward. Looking at the results of a number of laboratory studies published so far, the greater the copper content of a copper surface, the better and/or quicker the reported antimicrobial efficacy appears to be [23][36][43][50][63][64]. However, MacDonald et al. showed that bronze containing 95% copper was less efficient than 70% copper brass and 99.9% copper in reducing S. aureus viable counts [52]. Similarly, Noyce et al. pointed out that aluminum-bronze C95,500 (78% Cu) and nickel-aluminum-bronze C9,5800 (9% Al, 81% Cu) demonstrated poor antimicrobial effectiveness in spite of their relatively high copper contents [38]. Also, similar antibacterial activities against C. albicans and K. pneumoniae were reported for brass alloys containing 62.5 and 70% copper [39] while Cronobacter sakazakii was as readily eradicated on brass and copper nickel containing 70% and 88.6% copper as on 99.9% copper under dry conditions [65]. The antibacterial activity against Salmonella strains was also reported as being greater on brass containing 60% copper than on nickel silver containing 65% copper [63]. Therefore, copper content might not be the only parameter driving the antibacterial effectiveness of copper alloys. Pointing out the occasional better antibacterial properties of nickel-silver C75200 (nickel-silver) against cartridge brass C26000 with a higher copper content, Warnes et al. suggested that other metal constituents and physical properties (especially copper-release rates) may have a role in the bactericidal activity of copper alloys [28].
Other parameters have also been shown to influence the antibacterial effectiveness of copper-containing surfaces such as:
-
Surface structure
-
Surface structure
Cold spray deposition of copper led to a higher reduction in MRSA than those witnessed for plasma and wire arc depositions [66]. Also, a copper surface generated by electroplating has been shown to generate a twofold higher copper release in the medium compared to rolled and polished coppers [67]. This higher rate of copper release was correlated with a swifter antibacterial activity of the electroplated copper [67]. The authors also hypothesized that the grooves generated through electroplating allowed for an enhanced contact surface with the bacteria that could, in turn, favor the contact-killing process.
-
Surface oxidization
-
Surface oxidization
Under biospheric conditions, both CuO and Cu2O can be formed by oxidization on the surface of copper-containing materials [68]. Cu2O production is favored by a reducing environment (e.g., in the presence of organic matter or bacteria) while CuO is mainly generated in an oxidizing environment. Both cuprous and cupric oxides have been shown to display antibacterial activity but CuO was reported as less effective than Cu2O, which has an antibacterial activity similar to that of copper [69]. Also, treatments lowering the corrosion process resulted in a lower antibacterial effectiveness of copper surfaces [70].
-
Temperature and RH
-
Temperature and RH
MRSA were shown to be as readily killed under high temperature and Relative Humidity (RH) (35 °C and 90% RH) as under low temperature and RH (20 °C, 24% RH) [48]. However, pointing out that the 37 °C and 100% RH conditions recommended in the ISO 22196 [71] antimicrobial surface efficacy test were far from reflecting actual healthcare environmental conditions, Ojeil et al. proposed to test the antimicrobial activity of copper surfaces at either 20 °C, 50% RH or 20 °C, 40% RH [51]. They showed that the higher temperature and RH conditions allowed for a greater antimicrobial efficacy on a S. aureus strain of all copper alloys tested than those closer to actual environmental conditions. Noyce et al. also reported the differences in antibacterial activities against E. coli after exposures at 4 °C and 22 °C [37]. In this work, most copper alloys (including three brass alloys) allowed for a faster complete kill at 22 °C than at 4 °C.
-
Wet or dry inoculum/exposure
-
Wet or dry inoculum/exposure
One of the most influencing features in the way antibacterial testing is performed for copper-containing surfaces is the inoculum volume, whether it is spread or not and whether it is kept moist. One the one hand, dry inoculation of the bacteria (small volume, spread and dried) on the surface is generally thought to be more representative of real-life conditions [72]. It is also a better way to take into account the susceptibility of bacterial strains to desiccation on non-porous surfaces. On the other hand, wet exposure is more conducive to bacterial growth on surfaces than dry exposure and would represent a worst-case scenario.
Therefore, wet and dry conditions have been tested for the inoculation of bacteria on copper-containing surfaces and the results compared in several papers. Espirito Santo et al. were among the first to report that a reduction in inoculum moisture led to a decreased survival of E. coli on copper-containing surfaces as compared to previous studies using wet inoculums conditions. This reduction could not be linked to desiccation or osmotic stress as the strain displayed good survival rates on stainless steel when dry inoculum conditions were applied [21]. However, the same team showed that Gram-positive bacteria survived longer than Gram-negative ones under dry inoculum conditions [73]. This pattern was later confirmed on E. faecium [70]. Using C. sakazakii strains, another study showed that dry inoculation conditions (2 µL of the bacterial suspension spread on the surface and left to dry in open air) enabled a swifter reduction in viable bacterial counts than under wet inoculation conditions (25 µL of the bacterial suspension spread on the surface kept in a closed container to maintain moisture) [65]. Also, comparing a 9 µL spread inoculum with a 1 µL non-spread one, Dauvergne et al. showed that the bacterial recovery was greater with the later technique [45].
-
Presence of organic compounds
-
Presence of organic compounds
As the main mechanism of action for copper-containing surfaces is contact killing, which is thought to be driven by the release of copper ions, the presence of organic molecules on the surface could interfere with the action cupric and cuprous ions on bacteria. Indeed, the addition of liquid beef extract reduced the antimicrobial effect of several copper alloys including brass against E. coli [37]. The authors of this study hypothesized that beef extract might act as a protective matrix against copper exposure, especially because of its fat content. C. sakazakii reduction rates were also found to be lower when bacteria were suspended in an infant formula as compared to Tryptic Soy Broth (TSB) [65]. Using repeated soiling with S. aureus suspended in 1% Bovine Serum Albumin (BSA), a study showed that despite cleaning procedures, the efficiency of a copper surface against S. aureus was reduced [74]. However, Ojeil et al. later showed that the addition of BSA at 3 g/L did not significantly modify the antibacterial activity of a series of copper alloys against S. aureus [51]. Some copper alloys even displayed a better efficacy when the BSA soil load was present [51]. Another work demonstrated that the presence of organic compounds such as those contained in TSB impaired the antibacterial activity of copper and brass against E. coli and S. aureus [75].
All these influencing parameters led to the publication of various standardized or normalized protocols to assess the antimicrobial activity of non-porous surfaces and allow fairer comparisons of efficacies between studies, such as:
-
International Organization for Standardization (ISO) 22196:2011 [72]
-
International Organization for Standardization (ISO) 22196:2011 [72]
As described above, this guideline recommends antibacterial activity testing in temperature and RH conditions that are far from healthcare settings’ ones. Also, a wet inoculum of 0.4 mL is used in this protocol, which is not in line with the usual volumes of droplets bearing microbial contaminations in the environment. Moreover, this protocol was originally designed to test plastic surfaces and has an exposure end point of 24 h. Such a distant end point might not be adapted to simulate real-life conditions for metal surfaces present in healthcare settings and more especially for copper and brass because of their rapid antibacterial activity.
-
Environmental Protection Agency (EPA) protocols [76]
-
Environmental Protection Agency (EPA) protocols [76]
In 2008, the American EPA published a list of copper alloys validated as suitable for claiming antimicrobial properties on the basis of a series of tests. The first test to be passed in order to be coined as a sanitizer is a reduction of at least 99.9% (3 log) of a dry (20 µL) bacterial inoculum on said surface after a 2 h exposure (Figure 2). Two additional protocols have also been validated by the EPA: the residual self-sanitizing activity test (held after cycles of wet and dry wear) and the continuous reduction of bacterial contamination test (including 8 repeat inoculations on the metal surface over a 24 h period).
-
Association Française de NORmalisation (AFNOR) NF S90–700 [77]
-
Association Française de NORmalisation (AFNOR) NF S90–700 [77]
Recently, a French norm has been published on the bactericidal effectiveness of non-porous surfaces [77]. The method described therein mentions a dry inoculum of 1 µL, an exposure time of 1 h and a cutoff for the validation of antibacterial properties of a 99% (2 log) reduction (Figure 2). This cutoff value is less stringent than that of EPA but NF S90-700 only allows one hour before evaluating the residual viable bacteria whereas EPA has a 2 h exposure time. These two protocols therefore do not agree on the reduction to be attained and other authors have proposed [41] that a reduction ranging from 2 to 3 log (99 to 99.9%) would mean bacteriostatic properties for the antimicrobial surface while a reduction of over 3 log (>99.9%) would stand for bactericidal properties.
4. Brass alloys to reduce hospital acquired infections?
The first report of the antibacterial properties of brass in hospital settings was published by Kuhn in 1983 [14]. This paper highlighted that, even though the door hardware made of brass looked dirtier than the stainless steel one, it limited the bacterial bioburden and could be a mean of reducing HAIs. There is a gap of over 20 years between this first observational study and the publication of further field trials attempting to ascertain the efficacy of copper and brass alloys in reducing the bacterial bioburden on commonly touched surfaces. The possibility of transmission of nosocomial pathogens from environmental sources and more specifically from frequently touched surfaces had indeed gained credibility in the meantime [78][79]. To this day, trials on bioburden reduction outnumber the ones seeking to establish a link between this reduction and a possible decrease in the prevalence and/or incidence of HAIs, as will be seen below.
Most of the studies using copper alloys and presented below were implemented in intensive care units (ICUs) to limit the inherent biases linked with field trials. For example, critically ill patients are generally not ambulatory. Therefore, interactions of these patients with other environmental environmental surfaces within and outside the room are limited. Additionally, ICU patients are at further risk of HAI because of the severity of illness, invasive procedures, and frequent interactions with healthcare workers [80].
The first proper attempt at evaluating a possible reduction in the incidence of HAIs using copper-containing alloys was published by Salgado et al.
[81]
. The authors registered a significant reduction in HAIs and/or colonization by MRSA and VRE in ICUs in a prospective, intention-to-treat study in room containing 6 objects made of copper-based alloys
vs.
rooms with control objects. No influence of the length of stay on the occurrence of HAI/colonization in either type of rooms. A significant relation between environmental burden and the occurrence of HAI was also witnessed
[81]
. Limitations were nevertheless highlighted such as the removal of some copper-containing objects from patient rooms or the introduction of such objects in control rooms. Also, the study was not double-blinded but such a study design would be difficult to achieve because of the distinctive aspects of copper alloys and their control counterparts. Modifications in hand hygiene routines following the introduction of copper-containing objects have also been pointed out as a possible interfering factor. However, in this case, a 9-month gap existed between the first implementation of these copper-containing objects and the start of the study, hence mitigating the influence of this putative interfering factor. Questions about the possibility of selective reporting and the biological plausibility of the findings were also raised
[82]
.
Another prospective study on the possible reduction of HAIs incidence was held in Chile
[83]
in an adult intensive care unit. Despite a lower rate of catheter-associated bacteremia in copper-fitted rooms, no significant difference in HAI global incidence was found between rooms fitted with copper and control rooms. However, several limitations have been put forward by the authors themselves such as the limited number of included patients compared to the one forecast to get a significant statistical result or the fact that infections not related with invasive devices were not taken into account. Others can be added such as a minimum length of stay to be included in the study of 24h, when HAIs are usually diagnosed after a 48h stay. Also, different cleaning protocols were used for copper-based devices (0.6% citric acid) and their counterparts (quaternary ammoniums).
In another study, the authors failed to find a significant reduction in HAI rates after the implementation of brass devices in a pediatric ICU
[84]
. Nevertheless, HAI incidence rates decreased from 13.0 per 1,000 patient days for patients treated in the control settings to 10.6 per 1,000 patient days for patients treated in intervened rooms. Patients who developed HAI events in copper-containing rooms more frequently presented pre-existing conditions and had longer lengths of stay than the ones in control rooms
[84]
. Despite the occurrence of several interfering factors such as temporary overcrowding of the pediatric ICU and assumptions for the statistical analysis that were unmet, these results of this trial are still interesting. The authors point out the methodological difficulties encountered in this kind of environmental interventions and also the potential economical interest in implementing antimicrobial copper surfaces in addition to the public health one
[84]
.
Lastly, a study held in a long-term care facility fitted with copper alloy door handles and handrails showed no significant differences in the relative risk of infections during nosocomial outbreaks between the copper-fitted wing and the control wing[85]. However, when results were split according to the transmission mode of the causative agents (suspected to mostly be viruses), a significant reduction of the relative risk of infection was witnessed in the copper outfitted wing for hand-transmitted infectious agents (p<0.001), which appears as logical.
Most of the above mentioned studies are objectionable, mainly because randomized double-blinded studies are impossible to achieve when replacing a surface by another one displaying distinguishable features. However, to strengthen the evidence for the use of antibacterial brass surfaces in healthcare settings, further field works taking into account the TREND and ORION statements should be carried out [86].
5. Conclusion
The mechanisms underlying the antimicrobial effects of copper-containing surfaces are now fairly well described, even if the prevalence and order in which these mechanisms take place are still a matter of debate. Many laboratory studies have been held on copper-containing surfaces. They show a broad spectrum of activity for these surfaces against bacteria. Only against bacterial spores is their effectiveness limited. However, these laboratory trials point out that various parameters related to the surface structure, environmental conditions, inoculums and presence of organic soils on the surface can mitigate the antibacterial effectiveness of brass and copper surfaces.
Field trials using brass and copper surfaces consistently report reductions in the bacterial bioburden but evidence is still sparse as to a significant impact on HAIs. To better establish the impact of those surfaces on HAIs, further studies are warranted. Similarly, further work is still needed to assess the long-term effects of chemical/physical wear on the antimicrobial effectiveness copper and brass surfaces. Indeed, not all copper-containing surfaces are equal. Depending on the copper alloy composition, soiling and tarnishing might occur at varying speeds, sometimes causing acceptance problems [87]. Also, the advantages of integral brass alloys against CuO containing resins should be justified by additional studies. The latter ones have recently been developed to benefit from the antimicrobial activity of copper ions with a less costly material. However, some experiments already showed that their effectiveness decreases with time and wear as compared to integral copper alloys [88][89]. Brass touch-surfaces should therefore be considered a complement to, not a substitute for, hand hygiene practices, disinfection operations and other standard cleaning methods.
References
- Choucri, J.; Zanotto, F.; Grassi, V.; Balbo, A.; Ebn Touhami, M.; Mansouri, I.; Monticelli, C. Corrosion behavior of different brass alloys for drinking water distribution systems. Metals 2019, 9, 649.
- Laws, K.J.; Crosby, C.; Sridhar, A.; Conway, P.; Koloadin, L.S.; Zhao, M.; Aron-Dine, S.; Bassman, L.C. High entropy brasses and bronzes—Microstructure, phase evolution and properties. J. Alloys Compd. 2015, 650, 949–961.
- Red Brass vs. Yellow Brass: What Are the Differences? Available online: (accessed on 8 December 2020).
- Nagase, T.; Shibata, A.; Matsumuro, M.; Takemura, M.; Semboshi, S. Alloy design and fabrication of ingots in Cu-Zn-Mn-Ni-Sn high-entropy and Cu-Zn-Mn-Ni medium-entropy brasses. Mat. Design 2019, 181, 107900.
- Thornton, C.P. Of Brass and Bronze in Prehistoric Southwest Asia. In Metals and Mines: Studies in Archaeometallurgy; La Niece, S., Hook, D., Craddock, P.T., Eds.; Archetype Publications: London, UK, 2007; pp. 123–135.
- Craddock, P.T. The composition of the copper alloys used by the Greek, Etruscan and Roman civilizations. J. Archaeol. Sci. 1978, 5, 1–16.
- Dawson, W.R. The Egyptian Medical Papyri. In Diseases in Antiquity; Brothwell, D., Sandison, A.T., Eds.; Thomas, C.C.: Springfield, IL, USA, 1967; pp. 98–114.
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175.
- Sudha, V.P.; Ganesan, S.; Pazhani, G.; Ramamurthy, T.; Nair, G.; Venkatasubramanian, P. Storing drinking-water in Copper pots kills contaminating diarrhoeagenic bacteria. J. Health Popul. Nutr. 2012, 30, 17–21.
- Tandon, P.; Chhibber, S.; Reed, R.H. Inactivation of Escherichia coli and coliform bacteria in traditional brass and earthernware water storage vessels. Ant. Van. Leeuwen. 2005, 88, 35–48.
- Andrei, A.; Öztürk, Y.; Khalfaoui-Hassani, B.; Rauch, J.; Marckmann, D.; Trasnea, P.-I.; Daldal, F.; Koch, H.-G. Cu homeostasis in bacteria: The Ins and Outs. Membranes 2020, 10, 242.
- Giachino, A.; Waldron, K.J. Copper tolerance in bacteria requires the activation of multiple accessory pathways. Mol. Microbiol. 2020, 114, 377–390.
- Mikolay, A.; Huggett, S.; Tikana, L.; Grass, G.; Braun, J.; Nies, D.H. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl. Microbiol. Biotechnol. 2010, 87, 1875–1879.
- Kuhn, P.J. Doorknobs: A source of nosocomial infection? Diagn. Med. 1983, 6, 62–63.
- Dick, R.J.; Wray, J.A.; Johnston, H.N. A Literature and Technology Search on the Bacteriostatic and Sanitizing Properties of Copper and Copper Alloy Surfaces; Phase 1 Final Report, INCRA Project No. 212; Battelle Columbus Laboratories: Columbus, OH, USA, 1973.
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2010, 77, 1541–1547.
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611.
- Zheng, S.; Chang, W.; Li, C.; Lou, H. Als1 and Als3 regulate the intracellular uptake of copper ions when Candida albicans biofilms are exposed to metallic copper surfaces. FEMS Yeast Res. 2016, 16, 29.
- Solioz, M. Copper Toxicity. In Copper and Bacteria; Solioz, M., Ed.; Springer: Cham, Switzerland, 2018; pp. 11–19.
- Molteni, C.; Abicht, H.K.; Solioz, M. Killing of bacteria by copper surfaces involves dissolved copper. Appl. Environ. Microbiol. 2010, 76, 4099–4101.
- Santo, C.E.; Taudte, N.; Nies, D.H.; Grass, G. Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 2007, 74, 977–986.
- Warnes, S.L.; Keevil, C.W. Lack of involvement of Fenton chemistry in death of methicillin-resistant and methicillin-sensitive strains of Staphylococcus aureus and destruction of their genomes on wet or dry copper alloy surfaces. Appl. Environ. Microbiol. 2016, 82, 2132–2136.
- Hong, R.; Kang, T.Y.; Michels, C.A.; Gadura, N. Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 1776–1784.
- San, K.; Long, J.; Michels, C.A.; Gadura, N. Antimicrobial copper alloy surfaces are effective against vegetative but not sporulated cells of gram-positive Bacillus subtilis. Microbiologyopen 2015, 4, 753–763.
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2010, 77, 794–802.
- Santo, C.E.; Quaranta, D.; Grass, G. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. Microbiologyopen 2012, 1, 46–52.
- Warnes, S.L.; Caves, V.; Keevil, C.W. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ. Microbiol. 2011, 14, 1730–1743.
- Warnes, S.L.; Green, S.M.; Michels, H.T.; Keevil, C.W. Biocidal efficacy of copper alloys against pathogenic enterococci involves degradation of genomic and plasmid DNAs. Appl. Environ. Microbiol. 2010, 76, 5390–5401.
- Nandakumar, R.; Santo, C.E.; Madayiputhiya, N.; Grass, G. Quantitative proteomic profiling of the Escherichia coli response to metallic copper surfaces. BioMetals 2011, 24, 429–444.
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349.
- Quaranta, D.; Krans, T.; Santo, C.E.; Elowsky, C.G.; Domaille, D.W.; Chang, C.J.; Grass, G. Mechanisms of contact-mediated killing of yeast cells on dry metallic copper surfaces. Appl. Environ. Microbiol. 2010, 77, 416–426.
- Warnes, S.L.; Keevil, C.W. Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry surface contact. Appl. Environ. Microbiol. 2011, 77, 6049–6059.
- Mathews, S.; Kumar, R.; Solioz, M. Copper reduction and contact killing of bacteria by iron surfaces. Appl. Environ. Microbiol. 2015, 81, 6399–6403.
- Sjollema, J.; Zaat, S.A.; Fontaine, V.; Ramstedt, M.; Luginbuehl, R.; Thevissen, K.; Li, J.; Van Der Mei, H.C.; Busscher, H.J. In vitro methods for the evaluation of antimicrobial surface designs. Acta Biomater. 2018, 70, 12–24.
- Faúndez, G.; Troncoso, M.; Navarrete, P.; Figueroa, G. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 2004, 4, 19.
- Wilks, S.; Michels, H.; Keevil, C. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005, 105, 445–454.
- Noyce, J.O.; Michels, H.; Keevil, C.W. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl. Environ. Microbiol. 2006, 72, 4239–4244.
- Noyce, J.; Michels, H.; Keevil, C. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297.
- Mehtar, S.; Wiid, I.; Todorov, S. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: An in-vitro study. J. Hosp. Infect. 2008, 68, 45–51.
- Gould, S.W.J.; Fielder, M.D.; Kelly, A.F.; Morgan, M.; Kenny, J.; Naughton, D.P. The antimicrobial properties of copper surfaces against a range of important nosocomial pathogens. Ann. Microbiol. 2009, 59, 151–156.
- Souli, M.; Galani, I.; Plachouras, D.; Panagea, T.; Armaganidis, A.; Petrikkos, G.; Giamarellou, H. Antimicrobial activity of copper surfaces against carbapenemase-producing contemporary Gram-negative clinical isolates. J. Antimicrob. Chemother. 2012, 68, 852–857.
- Eser, O.K.; Ergin, A.; Hasçelik, G. Antimicrobial activity of copper alloys against invasive multidrug-resistant nosocomial pathogens. Curr. Microbiol. 2015, 71, 291–295.
- Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Bulanda, M.; Walkowicz, M.; Osuch, P.; Knych, T. Antibiotic resistance, ability to form biofilm and susceptibility to copper alloys of selected staphylococcal strains isolated from touch surfaces in Polish hospital wards. Antimicrob. Resist. Infect. Control. 2017, 6, 1–12.
- Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Majka, G.; Bulanda, M. Antimicrobial effect of copper alloys on Acinetobacter species isolated from infections and hospital environment. Antimicrob. Resist. Infect. Control. 2018, 7, 10.
- Dauvergne, E.; Lacquemant, C.; Adjidé, C.; Mullié, C. Validation of a worst-case scenario method adapted to the healthcare environment for testing the antibacterial effect of brass surfaces and implementation on hospital antibiotic-resistant strains. Antibiotics. 2020, 9, 245.
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.G.J.; Hobman, J.L. Metal resistance and its association with antibiotic resistance. Adv. Microb. Physiol. 2017, 70, 261–313.
- Weaver, L.; Michels, H.T.; Keevil, C.W. Survival of Clostridium difficile on copper and steel: Futuristic options for hospital hygiene. J. Hosp. Infect. 2008, 68, 145–151.
- Michels, H.T.; Noyce, J.O.; Keevil, C.W. Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Lett. Appl. Microbiol. 2009, 49, 191–195.
- Elguindi, J.; Wagner, J.; Rensing, C. Genes involved in copper resistance influence survival of Pseudomonas aeruginosa on copper surfaces. J. Appl. Microbiol. 2009, 106, 1448–1455.
- Michels, H.T.; Anderson, D.G. Antimicrobial Regulatory Efficacy Testing of Solid Copper Alloy Surfaces in the USA. In Metal Ions in Biology and Medicine; Collery, P., Maymard, I., Theophanides, T., Khassanova, L., Collery, T., Eds.; John Libbey Eurotext: Paris, France, 2008; Volume 10, pp. 185–190.
- Ojeil, M.; Jermann, C.; Holah, J.; Denyer, S.; Maillard, J.-Y. Evaluation of new in vitro efficacy test for antimicrobial surface activity reflecting UK hospital conditions. J. Hosp. Infect. 2013, 85, 274–281.
- McDonald, M.; Wesgate, R.; Rubiano, M.; Holah, J.; Denyer, S.; Jermann, C.; Maillard, J.-Y. Impact of a dry inoculum deposition on the efficacy of copper-based antimicrobial surfaces. J. Hosp. Infect. 2020, 106, 465–472.
- Wheeldon, L.J.; Worthington, T.; Lambert, P.A.; Hilton, A.C.; Lowden, C.J.; Elliott, T.S.J. Antimicrobial efficacy of copper surfaces against spores and vegetative cells of Clostridium difficile: The germination theory. J. Antimicrob. Chemother. 2008, 62, 522–525.
- Bleichert, P.; Bütof, L.; Rückert, C.; Herzberg, M.; Francisco, R.; Morais, P.V.; Grass, G.; Kalinowski, J.; Nies, D.H. Mutant strains of Escherichia coli and Methicillin-resistant Staphylococcus aureus obtained by laboratory selection to survive on metallic copper surfaces. Appl. Environ. Microbiol. 2020, 87, 1788–1820.
- Pietsch, F.; O’Neill, A.; Ivask, A.; Jenssen, H.; Inkinen, J.; Kahru, A.; Ahonen, M.; Schreiber, F. Selection of resistance by antimicrobial coatings in the healthcare setting. J. Hosp. Infect. 2020, 106, 115–125.
- Thummeepak, R.; Pooalai, R.; Harrison, C.; Gannon, L.; Thanwisai, A.; Chantratita, N.; Millard, A.D.; Sitthisak, S. Essential gene clusters involved in copper tolerance identified in Acinetobacter baumannii clinical and environmental isolates. Pathogens 2020, 9, 60.
- Andrade, L.N.; Siqueira, T.E.S.; Martinez, R.; Darini, A.L.C. Multidrug-resistant CTX-M-(15, 9, 2)- and KPC-2-producing Enterobacter hormaechei and Enterobacter asburiae isolates possessed a set of acquired heavy metal tolerance genes including a chromosomal sil operon (for acquired silver resistance). Front. Microbiol. 2018, 9, 539.
- Arredondo-Alonso, S.; Top, J.; McNally, A.; Puranen, S.; Pesonen, M.; Pensar, J.; Marttinen, P.; Braat, J.C.; Rogers, M.R.C.; Van Schaik, W.; et al. Plasmids shaped the recent emergence of the major nosocomial pathogen Enterococcus faecium. mBio 2020, 11, 03284–03319.
- Bryce, E.A.; Velapatino, B.; Khorami, H.A.; Donnelly-Pierce, T.; Wong, T.; Dixon, R.; Asselin, E. In vitro evaluation of antimicrobial efficacy and durability of three copper surfaces used in healthcare. Biointerphases 2020, 15, 011005.
- Horn, H.; Niemeyer, B. Aerosol disinfection of bacterial spores by peracetic acid on antibacterial surfaces and other technical materials. Am. J. Infect. Control. 2020, 48, 1200–1203.
- Shafaghi, R.; Mostaghimi, J.; Pershin, V.; Ringuette, M. Sporicidal efficacy of thermal-sprayed copper alloy coating. Can. J. Microbiol. 2017, 63, 384–391.
- Bleichert, P.; Santo, C.E.; Hanczaruk, M.; Meyer, H.; Grass, G. Inactivation of bacterial and viral biothreat agents on metallic copper surfaces. BioMetals 2014, 27, 1179–1189.
- Zhu, L.; Elguindi, J.; Rensing, C.; Ravishankar, S. Antimicrobial activity of different copper alloy surfaces against copper resistant and sensitive Salmonella enterica. Food Microbiol. 2012, 30, 303–310.
- Liu, J.; Li, F.; Liu, C.; Wang, H.; Ren, B.; Yang, K.; Zhang, E. Effect of Cu content on the antibacterial activity of titanium-copper sintered alloys. Mater. Sci. Eng. C 2014, 35, 392–400.
- Elguindi, J.; Alwathnani, H.A.; Rensing, C. Rapid inactivation of Cronobacter sakazakii on copper alloys following periods of desiccation stress. World J. Microbiol. Biotechnol. 2012, 28, 1837–1841.
- Champagne, V.K.; Helfritch, D.J. A demonstration of the antimicrobial effectiveness of various copper surfaces. J. Biol. Eng. 2013, 7, 8.
- Zeiger, M.; Solioz, M.; Edongué, H.; Arzt, E.; Schneider, A.S. Surface structure influences contact killing of bacteria by copper. Microbiologyopen 2014, 3, 327–332.
- Hans, M.; Mathews, S.; Mücklich, F.; Solioz, M. Physicochemical properties of copper important for its antibacterial activity and development of a unified model. Biointerphases 2016, 11, 018902.
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166.
- Elguindi, J.; Moffitt, S.; Hasman, H.; Andrade, C.; Raghavan, S.; Rensing, C. Metallic copper corrosion rates, moisture content, and growth medium influence survival of copper ion-resistant bacteria. Appl. Microbiol. Biotechnol. 2010, 89, 1963–1970.
- International Standard Organization. ISO 22196:2011 Measurement of Antibacterial Activity on Plastics and other Non-Porous Surfaces. 2016. Available online: (accessed on 4 October 2018).
- Kotay, S.M.; Donlan, R.M.; Ganim, C.; Barry, K.; Christensen, B.E.; Mathers, A.J. Droplet rather than aerosol-mediated dispersion is the primary mechanism of bacterial transmission from contaminated hand-washing sink traps. Appl. Environ. Microbiol. 2019, 18, 1049–1056.
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348.
- Airey, P.; Verran, J. Potential use of copper as a hygienic surface; problems associated with cumulative soiling and cleaning. J. Hosp. Infect. 2007, 67, 271–277.
- Różańska, A.; Chmielarczyk, A.; Romaniszyn, D.; Sroka-Oleksiak, A.; Bulanda, M.; Walkowicz, M.; Osuch, P.; Knych, T. Antimicrobial properties of selected copper alloys on Staphylococcus aureus and Escherichia coli in different simulations of environmental conditions: With vs. without organic contamination. Int. J. Environ. Res. Public Health 2017, 14, 813.
- United States Environmental Protection Agency. Test Method for Efficacy of Copper Alloy Surfaces as a Sanitizer. 2008. Available online: (accessed on 4 October 2018).
- Association Française de Normalisation. Surfaces à Propriétés Biocides—Méthode d’évaluation de l’activité Bactéricide de Base d’une Surface Non Poreuse NF S90–700. 2019. Available online: (accessed on 20 May 2019).
- Kramer, A.; Schwebke, I.; Kampf, G.; How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. . BMC Infect. Dis. 2006, 6, 130.
- Dancer, S.J.; The role of environmental cleaning in the control of hospital-acquired infection. Journal of Hospital Infection 2009, 73, 378-385, 10.1016/j.jhin.2009.03.030.
- Sharpe, P.A.; Schmidt, M.G.; Control and Mitigation of Healthcare-Acquired Infections: Designing Clinical Trials to Evaluate New Materials and Technologies. HERD: Health Environments Research & Design Journal 2011, 5, 94-115, 10.1177/193758671100500109.
- Salgado, C.D.; Sepkowitz, K.A.; John, J.F.; Cantey, J.R.; Attaway, H.H.; Freeman, K.D.; Sharpe, P.A.; Michels, H.T.; Schmidt, M.G.; Copper Surfaces Reduce the Rate of Healthcare-Acquired Infections in the Intensive Care Unit. Infection Control & Hospital Epidemiology 2013, 34, 479-486, 10.1086/670207.
- Harbarth, S.J.; Maiwald, M.; Dancer S.J.; The Environment and Healthcare-Acquired Infections: Why Accurate Reporting and Evaluation of Biological Plausibility Are Important. Infection Control & Hospital Epidemiology 2013, 34, 996-997, 10.1086/671741.
- Rivero, P.; Brenner, P.; Nercelles, P.; Impacto del cobre en la reducción de infecciones intrahospitalarias, mortalidad y gasto en antimicrobianos en una Unidad de Cuidados Intensivo de adultos. Revista chilena de infectología 2014, 31, 274-279, 10.4067/s0716-10182014000300004.
- von Dessauer, B.; Navarrete, M.S.; Benadof, D.; Benavente, C.; Schmidt, M.G.; Potential effectiveness of copper surfaces in reducing health care–associated infection rates in a pediatric intensive and intermediate care unit: A nonrandomized controlled trial. American Journal of Infection Control 2016, 44, e133-e139, 10.1016/j.ajic.2016.03.053.
- Zerbib, S.; Vallet, L.; Muggeo, A.; de Champs, C.; Lefebvre, A.; Jolly, D.; Kanagaratnam, L.; Copper for the Prevention of Outbreaks of Health Care–Associated Infections in a Long-term Care Facility for Older Adults. Journal of the American Medical Directors Association 2020, 21, 68-71., 10.1016/j.jamda.2019.02.003.
- Stone, S.P.; Cooper, B.S.; Kibbler, C.C.; Cookson, B.D.; Roberts, J.A.; Medley, G.F.; Duckworth, G.; Lai, R.; Ebrahim, S.; Brown, E.M.; et al.Wiffen, P.J.Davey, P.G. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. The Lancet Infectious Diseases 2007, 7, 282-288, 10.1016/s1473-3099(07)70082-8.
- Hinsa-Leasure, S.M.; Nartey, Q.; Vaverka, J.; Schmidt, M.G.; Copper alloy surfaces sustain terminal cleaning levels in a rural hospital. American Journal of Infection Control 2016, 44, e195-e203, 10.1016/j.ajic.2016.06.033.
- Bryce, E.A.; Velapatino, B.; Akbari Khorami, H.; Donnelly-Pierce, T.; Wong, T.; Dixon, R.; Asselin, E.; In vitro evaluation of antimicrobial efficacy and durability of three copper surfaces used in healthcare. Biointerphases 2020, 15, 011005, 10.1116/1.5134676.
- Coppin, J.D.; Villamaria, F.C.; Williams, M.D.; Copeland, L.A.; Zeber, J.E.; Jinadatha, C.; Self-sanitizing copper-impregnated surfaces for bioburden reduction in patient rooms. American Journal of Infection Control 2017, 45, 692-694, 10.1016/j.ajic.2017.01.012.
