The commercial availability of inorganic/organic precursors for sol-gel formulation is very high and increase day by day. By using the sol-gel technology, it is possible to provide materials with functional/multi-functional characteristics including flame retardant, anti-mosquito, water-repellent oil-repellent, anti-bacterial, anti-wrinkle, ultraviolet (UV) protection, self-cleaning and other properties. Some of these properties are discussed here, describing basic chemistry, factors affecting the sol-gel process, as well as progress and parameters controlling sol-gel technology for thin coatings.
- Sol-gel technology
1. Introduction
Sol-gel technology has tremendous potential due to the combination of novel materials with a high degree of homogeneity at the molecular level with excellent physical and chemical properties [1][2][3][4][5][6].
In ancient times in China, tofu was prepared by utilizing this technology and hence it is not a new technology [7]. In the mid-19th century, sol-gel technology was used for preparing one-component compounds using sols and gels [5][8]. In the process of making glass, the sol-gel process requires less temperature compared to the conventional high-temperature melting method [5]. The method of forming a gel by mixing SiCl4 and ethanol and hydrolysis in humid air was discovered by Ebelmen in 1846 [9][10]. The sol-gel method was successfully used to produce SiO2-B2O3-Al2O3 -Na2O-K2O multi-component glasses which were analyzed by Dislich [11] thus creating great curiosity towards sol-gel technology. In 1981, the first international workshop on glasses [6][7][12][13] and ceramics from gels was held, which helped to further develop sol-gel technology. Sol-gel technology is being used widely since 1980 in the process of synthesizing superconducting materials [14], functional ceramic materials [15] nonlinear optical materials [16], catalysts and enzyme carriers [17][18], porous glass materials and other materials [19][20]. It was a milestone in the history of materials science in which many papers and patents broadly utilizing surface coating and other aspects were published [21]. The solution, sol or gel, solidifies the compounds of metal-organic or inorganic (precursor) to form a sol or gel state, followed by the development of an oxide by heat treatment. The polycondensation reaction transforms Si-OR- and Si-OH-comprising species into siloxane compounds which is the basic chemical principle of sol-gel treatment of silica-based materials. Corner sharing connects this to SiO4 tetrahedra (or RSiO3 tetrahedra in hybrid materials) from a structural point of view. In order to achieve a stable gel, it is essential to maximize the number of siloxane bonds (-Si-O-Si-) and subsequently minimize the number of silanol (Si-OH) and alkoxo (Si-OR) groups [22][23][24][25].
Sol-gel is a wet treatment process, and for sol-gel synthesis, a homogeneous solution is formed by dissolving the precursor in a solvent reaction (water or an organic solvent) which is the foremost step, irrespective of whether the raw material is either an inorganic salt or metal alkoxide [5][23]. Here this process is oriented towards commercial coatings.

Figure 1. Various applications of sol-gel finishing products.
Coatings are widely used in industry for many different purposes. They have permeated practically all areas of manufacturing, and it would be difficult to find a finished product that has escaped the application of a coating. Coatings are applied to protect bulk materials from corrosion and other detrimental effects of the ambient atmosphere. They are used to change surface properties and colour, gloss, and general appearance. Adhesive coatings are used in laminating and in the preparation of composites. Coatings are used as barriers for gases and liquids, and for many other reasons. Application techniques, coatings types, and their purposes make coating technology an extremely diverse field.
Although the literature on coatings can be traced back to the era of electrodeposition in general, and aluminising of iron in the early forties, much of the information on coatings relevant to achievement of optimum performance production, control and replacement economics has accumulated significantly in the seventies and to date.
The sol-gel process has been suggested as an alternative to conventional methods such as sputtering, chemical vapour deposition (CVD), and plasma spray for applying thin ceramic coatings. Sol-gel thin films are in a few notable cases technically sound alternatives to these methods as well as commercially viable alternatives [26][27][28][29][30][31].
The technology of sol-gel thin films has been around for more than 50 years. The process is surprisingly simple. A solution containing the desired alkoxide is prepared with a solvent and water. It is applied to a substrate by spinning, dipping, or draining. A tacky film forms that is typically 1 micron thick, uniform over large areas and adherent to the substrate. The sol gel process is able to apply a coating simultaneously to the inside and outside of complex shapes. The film dries quickly to a microporous and mesoporous coating of oxide [7][32][33][34][35][36].
In summary, this topic review describes the sol-gel coating technique and gives examples of sol-gel processed materials with prospects of successful commercial exploitation in many technologies.
2. The sol-gel method
There are four stages in the sol-gel coating process:
(i) preparation of the sol, (ii) spraying of the sol on the substrate, (iii) drying the sol to a gel, and finally, (iv) firing to obtain a consolidated and adherent coating [23][37][38][39][40][41]. Colloidal dispersions are not new. Their preparation is well characterized but not in the context of high temperature coatings. There can be aqua-sols or organo-sols, and these can be aggregated or non-aggregated in nature. Essentially, they are stable dispersions of particles in a fluid of colloidal units of hydrous oxides or hydroxides with particle sizes ranging from 20 Angstroms to 1 micron. Non-aggregated colloidal units are less than 100 Angstroms and such oxide-sols can form deposits of almost theoretical density even with low temperature firing (less than 850 °C). The same oxide in aggregated colloidal units of 200-300 Angstroms can form a porous layer of a high effective surface area. The degree of aggregation is controlled by the properties of the sol.
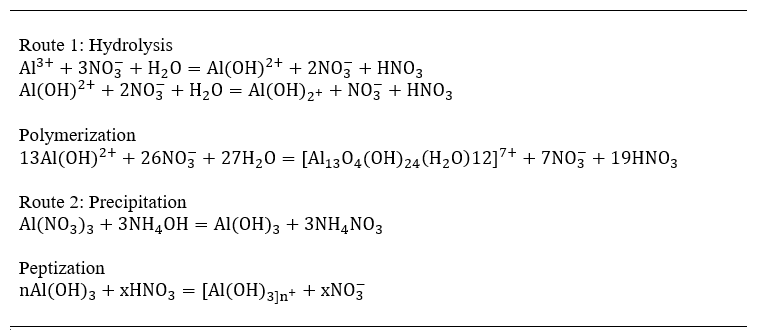
Sol-preparation can take two routes. Route 1 is hydrolysis followed by polymerization. Route 2 adopts precipitation and then peptization. Although only oxides have been considered for coating so far, it is possible that other ceramics may also have been attempted, e.g. Si3N4, AIN etc. It seems possible that cermets can be tried if they can be prepared as very fine aggregates. Oxide-sols of ceria, silica, zirconia, titania, thoria and alumina and gel-glass have been reported by the A.E.R.E, Harwell group. Table 1 shows the formation of hydrous alumina sols. It is probable that many of these are mesoporous materials. The extent of aggregation of the sol and its stability depends on factors such as the nature of the precursor material and the ratio of the anion to metal oxide. Absorption of H+ on the metal oxide hydrates causes positive charging of the colloidal units. Anions in the solution associate with these and thus establish dipoles in the sol thus repelling each colloidal unit from its neighbours. The degree of aggregation depends on this inter- colloidal unit separation, and the anion to metal oxide ratio is a critical factor. A mole ratio of 0.32 for HNO3/CeO2 results in a gel density of 2.4 while a ratio of 1 yields a gel with 4.0 g cm-3 density. Sols can be applied by immersion of the substrate, or by spinning, electrophoresis or spraying. Any drying or thickening of the sol during spraying has to be compensated for, so that the aggregation optimized for the purpose remains so. The method of application of the sol depends on the shape of the substrate which must be thoroughly cleaned and abraded at the outset.
The sol-to-gel transformation is done by careful, gradual drying; an over-rapid drying can cause gel-crazing. At this stage the process is reversible. The gel can be "refloated" to become the sol by merely adding the vehicle, e.g. water for aquo-sols.
Consolidation to a coating occurs when the gel-coated substrate is fired to high temperatures (440-1200°C) where sintering can occur, but the heating times are short, usually 15 minutes. A single sol-application gives a 0.1-2 micron thick coating which can be modified by controlling the rheology of the sol or by additives.
Unlike in slurry coats, a sol-gel material on a substrate may not have a distinct diffusion zone at the substrate-coat interface. But the coating oxide and the growing oxide do get interdispersed. Also, there may be mixed phases of the solid oxide. For instance, sol-gel silica
Table 1. Formation of hydrous alumina sols.
results in a mixture of beta-cristobalite with some alpha-quarts. TiO2 forms coatings of density 4.07 and 4.11 g cm-3 when dried at 800 and 1000ºC respectively, while the theoretical densities of anatase and rutile are 3.85 and 4.26 g cm-3. CeO2, on the other hand forms single phase structured coating. Table 2 lists the densification effects of four sol-gel coating products.
Table 1. Formation of hydrous alumina sols.

Except for their oxidation resistance data sol-gel coatings have not been characterized in the published literature. No information can be given here on micrographs of the cross section, adhesion and cyclic thermal effects, and studies on the substrate-sol-gel-coated system.
Table 2. Densification effects of four sol-gel coating materials.

|
Calcination Temp., ºC |
Density, (g cm-3) Silica Titania Ceria Zirconia Non-agg Agg Non-agg Agg Non-agg Non-agg |
|||||
|
Dry Gel |
1.8 |
0.5 |
2.6 |
1.5 |
4.3 |
3.6 |
|
400 |
1.5 |
|
3.2 |
1.5 |
5.2 |
3.9 |
|
600 |
1.5 |
0.4 |
3.4 |
1.6 |
6.0 |
4.8 |
|
800 |
2.1 |
0.5 |
4.1 |
2.6 |
6.9 |
5.2 |
|
1000 |
2.0 |
0.5 |
4.1 |
3.7 |
6.9 |
5.2 |
|
1200 |
2.0 |
1.8 |
|
|
|
|
|
Theoretical density g cm-3 |
2.2 |
|
4.3* |
|
7.1 |
5.6 |
|
Melting pt, ºC |
1600 1840 2600 2715 |
|||||
(*Rutile; Anatase 3.9 g cm-3); Agg - Aggregate; Non-Agg - Non Aggregate
3. Sol-gel coatings
The range of substrates that can be coated by the sol-gel process is not limited to those capable of withstanding a high firing temperature because of the associated low temperature for processing the gel. Thus, it is possible to put an inorganic sol-gel coating on a plastic. The majority of coatings have been applied by this technique in order to achieve some specific optical quality. Much of the progress leading to commercial coatings has been pioneered by Schott in Germany [42][43]. Their process for coating architectural glass is simple. Large panes (4x3m) are chemically cleaned then dipped into a bath containing a hydrolysable metal compound. The pane is slowly withdrawn into a moist atmosphere which hydrolyses the film forming a transparent metal oxide layer on the surface.
A simple relationship shows that the thickness of the film is proportional to the drawing speed to a power of 2/3:
d = k V2/3 d = thickness
V = drawing speed
k = constant
The process is such that the film flows down the plate after wetting the surface. Because it wets the surface, it partly adheres and when the film reacts with the moisture in the atmosphere it undergoes hydrolysis and condensation. As mentioned previously, this film can be densified by a thermal treatment.
One major problem that arises here is anisotropic shrinkage. If the gel film attempts to shrink, it is prevented from doing so in the lateral direction by adhesion to the substrate.
One distinct advantage of this technique over others is the homogeneity and uniformity of films that can be obtained. Schott produce window panes known as Calorex and these show an excellent uniformity of coating. The technique is versatile and allows multilayer coatings to be produced which enhances the range of optical properties that can be produced. Glasses produced from alkoxides can also be doped with transition metals such as Co, Ni, Cr and Cu. Coatings have been developed for:
- anti-reflective purposes such as coating the surface of silicon solar cells
- partially reflective coatings
- filters for ultraviolet and colour cut-off
- oxidation resistance on metals such as iron and brass
- protection of moisture sensitive optical glasses
- strengthening of normal glass items by application of an appropriate sol-gel coating.
The important parameters in film formation for optical purposes are the refractive index of the film and its adsorption characteristics. Some examples of metal oxide coatings that absorb in the U.V. and visible are listed in Table 3.
There are several inherent advantages and disadvantages of using a solution method for coating. Firstly, it is possible to coat all over, inside and outside the component. The solution can coat everywhere uniformly which is a distinct advantage over vacuum coating but inside and outside coating may not be desirable. The capital equipment needed is not very sophisticated although the withdrawal from the solution needs to be as smooth and vibration free as possible. But nowadays this is easily achieved using high resolution stepping motors to drive frictionless lead screws and mounting the dip coater on an antivibration table. Commercial products that are made by the dip coat process are:
- Sun Shielding Glass (IROX) - The interference layers allow some parts of the incident
Table 3. Metal oxide coatings that absorb in the UV and visible regions.
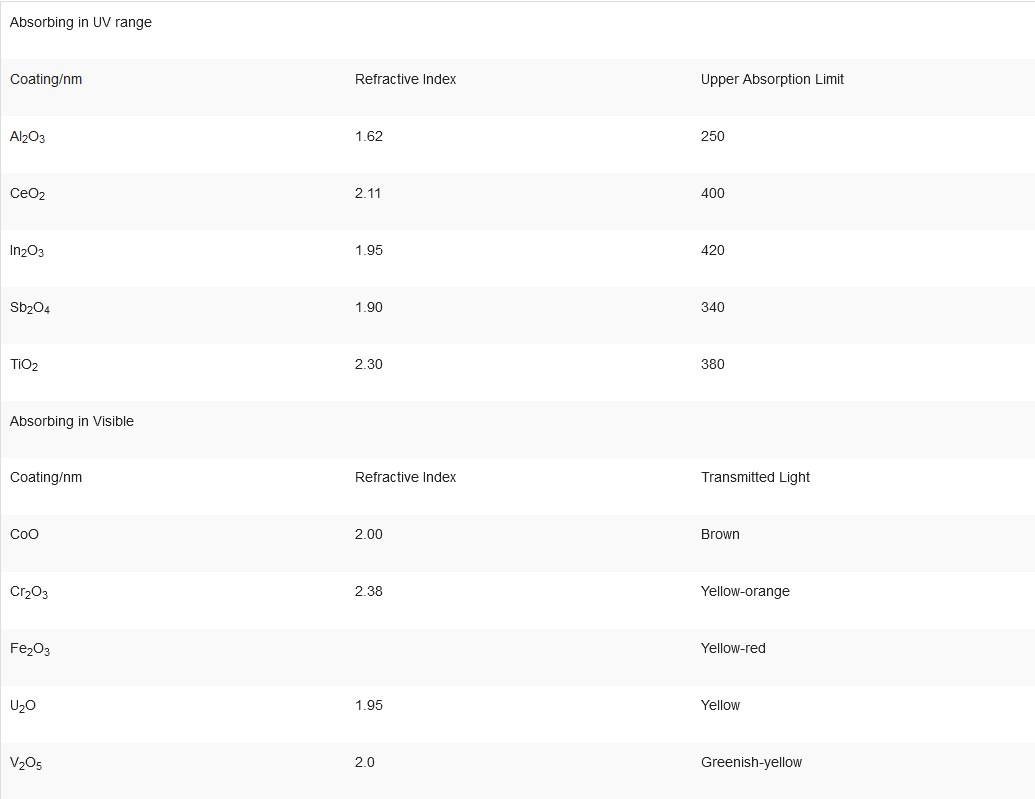
|
Absorbing in UV range |
||
|
Coating/nm |
Refractive Index |
Upper Absorption Limit |
|
Al2O3 |
1.62 |
250 |
|
CeO2 |
2.11 |
400 |
|
In2O3 |
1.95 |
420 |
|
Sb2O4 |
1.90 |
340 |
|
TiO2 |
2.30 |
380 |
|
Absorbing in Visible |
||
|
Coating/nm |
Refractive Index |
Transmitted Light |
|
CoO |
2.00 |
Brown |
|
Cr2O3 |
2.38 |
Yellow-orange |
|
Fe2O3 |
|
Yellow-red |
|
U2O |
1.95 |
Yellow |
|
V2O5 |
2.0 |
Greenish-yellow |
light spectrum to be transmitted whilst others are reflected. The coating is a colloidal titania in which certain colloidal metal particles are incorporated. This gives a scratch resistant coating which does not change colour in terms of transmitted and reflected light.
- Heat Mirrors - High intensity lamps such as spot lights and projector lamps use halogen lamps which not only generate a light of high intensity but also a great deal of I.R. (infra-red) radiation and it is desirable to reduce the heat produced. Filters can be made using sol-gel coatings which will transmit most of the visible light but reflect away the I.R.
- Cold Mirrors - This is the opposite to the above in that it transmits the I.R. region and reflects the visible. It too can be used to remove heat.
- Anti-Reflective Coatings - As the name implies, these are coatings that reduce the amount of light reflected from a surface. The panel glasses on instruments in air-craft often reflect shafts of sunlight and this is where such coatings are helpful. This is also helpful in automotive rear view mirrors.
Anti-reflective coatings are of three types:
- Single layer coatings where nc=ng; nc=refractive index of coating and ng=refractive index of glass.
- Multi-layer made from nc1 and nc2 where the two indices are different.
- Graded index where there is progressive change of index from the glass substrate to the external surface, usually air. This is achieved using a phase separating glass on the coating then etching one phase out to give a material the porosity of which varies with depth.
Anti-reflective coatings on silicon have been of considerable interest in solar cell technology. One great advantage is that the coating can be densified at 400°C, a temperature low enough not to cause excessive damage to the solar cell.
Further work has been done in spraying a mixture of colloidal silica and a partially hydrolysed silicon alkoxide onto glass surfaces. The alkoxide hydrolyses and condenses and helps form a bond between the colloidal silica particles as shown below:
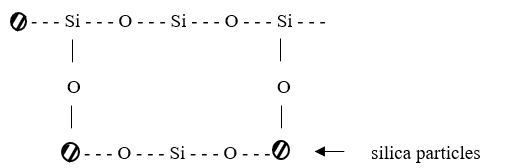
Colour conversion filters or interference layer coatings are another way of achieving colour filters on lamps. These coatings are applied to glasses with low thermal expansion coefficient which gives the filter thermal shock resistance.
With the advent of higher power lasers being used in many technologies, both lenses and mirrors require special characteristics if they are not to absorb light energy in such quantity as to cause irreversible damage. This requires materials of very high purity. Many other devices can be produced for high energy laser optics including heat reflecting filters, dichroic beam splitters and polarizers. These coatings have been produced from colloidal titania and titania/silica films from colloidal gels.
Absorptive coatings are coatings that are absorptive but show no interference effects. This is achieved by incorporating finely divided platinum or palladium in a silica or alumina matrix. They give "grey" absorbtive coatings i.e. absorption is not really wavelength dependent. Less expensive alternatives have been provided using nickel oxide. They have found use in applications where glare is a problem.
Other coating methods can be employed for gels. Spin coating is a technology that has been well developed by the micro-electronics industry for the application of thin films of photoresist onto silicon wafers. This method can be applied here and consists of dropping a quantity of sol onto the substrate which is spinning at high speed, e.g. 3000 r.p.m. This gives uniform films and is ideal for a sol which hydrolyses very rapidly since it can be kept away from moisture prior to being dropped onto the surface. As it hits the surface and spreads, it can then react very quickly with water in the atmosphere and gel. Further advantages are that it is economical in usage of solution and only one surface need be coated. Disadvantages are edge effects on non axi- symmetric substrates and the mechanical problems associated with spinning large substrates. The thickness control is a little easier than dip coating and is governed by:
- spin speed
- viscosity of the solution
A final method is spray coating. In the past, the literature has criticized this technique as the least effective of the three but more recently advances in spraying and atomizing equipment have brought this technique into line with others. In particular, it is possible to spray onto a hot surface, e.g. glass below tg and obtain a very rapid reaction between the sol and the glass which gives coatings that develop excellent adherence and form in a very short time. The improvements to this technique have been brought about by modern techniques that enable very finely atomized jets to be produced by the use of ultrasonics or high pressure hydraulic atomisers. Very recently develoments have taken place in producing "molecular sprays".
Spraying has potential as a hot coating technique for applying coatings to glass surfaces in order to strengthen the object. Work by Schmidt at the Fraunhofer Institute and Uhlmann at M.I.T. has shown that conventional sol-gel coatings have a strengthening effect on glass. The mechanisms are far from understood but it is suggested that the coating penetrates existing flaws and that the gel forms bridges across the faces of the cracks. Uhlmann [44][45] has also reported nitriding the gel glass coating to increase its inherent strength. Ceramic Developments (Midlands) Ltd. has shown a strength increase on glass rods and containers coated by a hot process. A partially hydrolysed alkoxide is sprayed onto the hot glass surface between 400 and 600°C and this instantly hydrolyses and forms a coating which gives a significant strength increase. Further work is being carried out in this area. Apart from optical properties and strength improvement, it is also possible to apply electrically conducting layers to glass by sol-gel. Gonzalez-Oliver and Kato [46] have reported work on deposition of antimony doped tin oxide on various glass panels. Electroconductive layers are finding applications in display panel technology, and it is suggested that they will find other applications such as in transparent ovens or could be used to heat up the glass to prevent ice or moisture build up.
The techniques of dip coating and spray coating were contrated and in particular it was discovered that the spray coatings were more crystalline than dip coated films and that the spray coating showed preferred orientation.
There are many reported sol-gel coatings and some of them are listed in Table 4.
Table 4. Examples of sol-gel coatings.
4. Conclusions
Acceptance of the sol-gel process for thin mesoporous films is mostly a question of educating the user of thin films and coatings on how to apply these films [47]. The number of commercial products available to the general public in this area is small. There are the spin-on coatings for doping silicon wafers (Accuspin from Allied-Signal Electronic Chemicals and Liquicoat from - Chemicals). There are the liquid oxides for various applications (Atolon from Nippon Soda). Many industries may have internal uses for what can be classified as sol-gel coatings or sputtered coatings. To summarize sol-gel coatings, there are limitations. The main limitation is that only films around 1 micron thick can be applied with ease by a sol-gel process. Thinner films can be applied, but thicker films suffer from the same problems of drying, shrinkage, and cracking that plague bulk samples. However, for thin films there are the applications cited above and probably others that have been overlooked.
An important feature to emphasize is the mesoporous nature of the films in the early stages. It appears that mesoporosity has not been exploited for applications in which a high surface area coating is desired. Some applications to look for in sol-gel coatings are catalytic surfaces, membrane properties, and self-lubricating surfaces. In this context, it is our opinion that many opportunities exist for mesoporous inorganic structures used as advanced sol-gel coatings.
References
- Vihodceva, S.; Kukle, S.; Muter, O. Adv. Mater. Res. 2015, 1117: 213-216.
- Cao, N.; Lyu, Q.; Li, J.; Wang, Y.; Yang, B.; Szunerits, S.; Boukherroub, R. Chem. Eng. J. 2017, 326: 17-28.
- Liang, S., Neisius, N. M.; Gaan, S. Prog. Org. Coat. 2013; 7 6: 1642-1665.
- Alongi, J.; Ciobanu, M.; Tata, J.; Carosio, F.; Malucelli, G. J. Appl. Polym. Sci. 2011, 119: 1961-1969.
- Pierre, A. C. ed. Introduction to Sol-Gel Processing. Springer, Boston, MA, USA, 1998.
- Sakka, S.; Kamiya, K. J. Non-Cryst. Solids 1982, 48: 31-46.
- Dislich, H.; Hinz, P. J. Non-Cryst. Solids 1982, 48: 11-16.
- Danks, A. E.; Hall, S. R.; Schnepp, Z. Mater. Horizons. 2016, 3: 91-112.
- Kursawe, M.; Hilarius, V.; Pfaff, G.; Anselmann, R. Modern Surface Technology. John Wiley & Sons, Hoboken, NJ, USA, 2006.
- Baccile, N.; Babonneau, F.; Thomas, B.; Coradin, T. J. Mater. Chem. 2009, 19: 8537-8559.
- Dislich, H. Angew. Chem. Int. Ed. Engl. 1971, 10: 363-370.
- Zarzycki, J. J. Non-Cryst, Solids 1982, 48: 105-116.
- Klein, L.C.; Garvey, G.J. J. Non-Cryst. Solids 1982, 48: 97-104.
- Kondas, G. J. Non-Cryst. Solids 1990, 121: 436-442
- Vincenzini, P. High Performance Ceramic Films and Coatings. North-Holland Publishing, Co. Amsterdam, The Netherlands, 1991.
- Kim, H. K.; Kang, S.-J.; Choi, S.-K.; Min, Y.-H.; Yoon, C.-S. Chem. Mater. 1999, 11: 779-788.
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C. -M.; Mahapatra C.; Kim, H.-M.; Knowles, J. C. Prog. Mater. Sci. 2016, 77: 1-79.
- Avnir, D.; Braun, S.; Lev, O.; Ottolenghi, M. Chem. Mater. 1994, 6: 1605-1614.
- Inayat, A.; Reinhardt, B., Herwig, J.; Küster, C.; Uhlig, H.; Krenkel, S.; Raedlein, E.; Enke, D. New J. Chem. 2016, 40: 4095-4114.
- Feinle, A.; Elsaesser, M.S.; Hüsing, N. Chem. Soc. Rev. 2016, 45: 3377-3399.
- Buschow, K. H. J.; Cahn, R.W.; Flemings, M.C.; Ilschner, B.; Kramer, E. J.; Mahajan, S.; Veyssière, P. B., eds. Encyclopedia of Materials: Science and Technology. Elsevier, Oxford, UK, 2001.
- Mahltig, B.; Haufe, H.; Böttcher, H. J. Mater. Chem. 2005, 15: 4385-4398.
- Hench, L.L.; West, J.K. Chem. Rev. 1990, 90: 33-72.
- Brinker, C. J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing. Elsevier, Amsterdam, The Netherlands, 1990.
- Brinker, C. J.; Frye, J.C.; Hurd, A.J.; Ashley, C.S. Thin Solid Films 1991, 201: 97-108.
- Klein, L. C. Sol-Gel Technology for Thin films, Fibers, Preforms, Electronics and Specialty Shapes, Noyes Publications, Park Ridge, NJ, USA, 1989.
- Laine, R. M., ed. Transformation of Organometallics into Common and Exotic Materials: Design and Activation. Springer, Dordrecht, The Netherlands, 1988.
- Schrope, M. Nature 2011, 472: 152-154.
- Xie, A.; Cui, J.; Chen, Y., Lang, J.; Li, C.; Yan , Y.; Dai, J. Surf. Coat. Technol. 2019, 361: 19-26.
- Wei, Q. B., ed. Surface Modification of Textiles. Woodhead Publishing, Sawston, UK, 2009.
- Montazer, M.; Harifi, T.B., eds. Nanofinishing of Textile Materials. Woodhead Publishing, Sawston, UK, 2018.
- Pénard, A.-L.; Gacoin, T.; Boilot, J.-P. Acc, Chem. Res. 2007, 40: 895-902.
- Haas-Santo, K.; Fichtner, M.; Schubert, K. Appl. Catal. A: General 2001, 220: 79-92.
- Adraider, Y.; Pang, Y. X.; Nabhani, F.; Hodgson, S. N.; Sharp, M.C.; Al-Waidh, A. Ceramics International 2013, 39, 9665-9670.
- Yarbrough, R.; Davis, K.; Dawood, S.; Rathnayake, H. RSC Adv. 2020, 10: 14134-14146.
- Figueira, R.B. Polymers 2020, 12, 689.
- Hanaor , D.A.H.; Chironi, I.; Karatchevtseva, I; Triani, G.; Sorrell. C.C. Adv. Appl. Ceramics 2012, 111: 149-158.
- Brinker, C. J.; Hurd, A.J. J. Phys. III France 1994, 4: 1231-1242.
- Carrera-Figueiras, C.; Pérez-Padilla, Y.; Estrella-Gutiérrez, M.A.;Uc-Cayetano, E.G.; Juárez-Moreno, J.A.; Avila-Ortega, A. Surface Science Engineering Through Sol-Gel Process, Applied Surface Science, IntechOpen Limited, London, UK, 2019.
- Jimenez, R.; Sobrados, I.; Martinez, S.; Criado, M.; Perea, B.; Sanz, J. J. Alloys and Compounds 2020, 844: 156051-156059.
- Wang H.; Liu, X.; Niu, P.; Wang, Sh. L.; Shi, J.; Li,L. Matter 2020, 2:1377-1413.
- Mackenzie, J.D.; Bescher, E.P. J Sol-Gel Sci. & Technology 2000, 19: 23-29.
- Aegerter, M.A.; Mennig, M., eds. Sol-Gel Technologies for Glass Producers and Users. Springer Science + Business Media, New York, USA, 2004.
- Zelinski, B.J.J.; Uhlmann, D.R. J. Phys. Chem, Solids 1984, 10: 1069-1090.
- Uhlmann, D. R.; Ulrich, D., eds. Ultrastructure Processing of Advanced Materials, John Wiley & Sons, Inc., New York, USA, 1992.
- Gonzalez, C. J. R.; Kato, I. J. Non- Cryst. Solids 1986, 82: 400-410.
- Sequeira, C.A.C.; Hudson, M.J., eds. Multifunctional Mesoporous Inorganic Solids. Kluwer Academic Publishers, Dordrecht, The Netherlands, 1993.
