Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs that play an essential role in various cellular activities, such as differentiation, proliferation, and apoptosis.
- lncRNAs
- natural products
- phytochemicals
- cancer treatment
1. Introduction
Cancer is caused by the rapid and uncontrolled growth of cells that eventually lead to tumor formation. Failure of early diagnosis and treatment can lead to metastasis or death. Any event that increases the expression of oncogenes or decreases the expression of tumor suppressor genes can result in cancer [1]. Genetic and epigenetic alterations are among the most critical factors in the onset of carcinoma, due to the altered expression of gene-coding and non-coding RNAs (ncRNA) that regulate apoptosis, proliferation, and differentiation [2][3]. Nevertheless, several lifestyle factors, such as physical inactivity, alcohol consumption, smoking, poor nutrition, and exposure to ultraviolet radiation or X-rays, can contribute to healthy cells’ transition into malignant cells [4]. Surgery, chemotherapy, and radiotherapy are standard and conventional methods of cancer treatment. However, due to chemotherapy and radiotherapy’s side effects and resistance to treatment in some patients, many researchers have turned their attention to alternate approaches, including natural compounds [5].
Cancer is caused by the rapid and uncontrolled growth of cells that eventually lead to tumor formation. Failure of early diagnosis and treatment can lead to metastasis or death. Any event that increases the expression of oncogenes or decreases the expression of tumor suppressor genes can result in cancer [1]. Genetic and epigenetic alterations are among the most critical factors in the onset of carcinoma, due to the altered expression of gene-coding and non-coding RNAs (ncRNA) that regulate apoptosis, proliferation, and differentiation [2,3]. Nevertheless, several lifestyle factors, such as physical inactivity, alcohol consumption, smoking, poor nutrition, and exposure to ultraviolet radiation or X-rays, can contribute to healthy cells’ transition into malignant cells [4]. Surgery, chemotherapy, and radiotherapy are standard and conventional methods of cancer treatment. However, due to chemotherapy and radiotherapy’s side effects and resistance to treatment in some patients, many researchers have turned their attention to alternate approaches, including natural compounds [5].
Precision medicine is a suitable and individualized treatment method for each patient, followed by molecular diagnostic tests of disease at the molecular level based on the genetic content, phenotypic, biomarker, or psychosocial characteristics of each patient [6]. This method allows the medicine to be prescribed only for patients who benefit from that and reduce the failure rate of pharmaceutical clinical trials, also saves costs and avoids side effects in individuals who do not benefit from that treatment [7]. Precision medicine is an approach for preventing and treating various diseases, including cancer, that considers the patient’s lifestyle, environment, and genetic diversity for treatment. Therefore, treating a patient with a particular cancer is different from other people who have the same type and stage of cancer [8].
Of the total human genome, only 2% is transcribed into proteins, with the remaining regions encoded as non-coding RNAs [3]. Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs containing more than 200 nucleotides in length, which play an essential role in cell biological activities, such as differentiation, proliferation, apoptosis, and growth. Dysregulation of lncRNAs plays a fundamental role in developing various diseases, such as cancer [9]. Most lncRNAs are localized and function within the nucleus, but some only operate in the cytoplasm. lncRNAs can stimulate or suppress transcription by various mechanisms, including epigenetic changes (such as chromatin remodeling or histone modification), transcription factor decay, microRNA sponge, a scaffold for various proteins, and splicing regulation [10]. Altered expression of lncRNAs, such as PTENP1, linc-PINT, and GAS5, plays an essential role in regulating apoptosis in cancer cells [11][12][13]. In human embryonic and adult life, angiogenesis plays a vital role in several physiological processes. Nevertheless, ectopic angiogenic processes have also been associated with the pathogenesis of cancer. Since tumor cells need more nutrients, oxygen, and the removal of metabolites than normal cells, this leads to angiogenesis activation [14]. Moreover, one of the preconditions for cancer metastasis is epithelial-to-mesenchymal transition (EMT) [15]. lncRNAs are essential regulators of tumor angiogenesis and EMT in various cancers such as gastrointestinal, lung, breast, and brain tumors by regulating oncogenic pathways, angiogenic factors, and epigenetic alterations [16]. Therefore, lncRNAs, such as MALAT1, UBE2CP3, HULC, UCA1, and PCA3 play an essential role in cancer metastasis by altering gene expression [17][18][19].
Of the total human genome, only 2% is transcribed into proteins, with the remaining regions encoded as non-coding RNAs [3]. Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs containing more than 200 nucleotides in length, which play an essential role in cell biological activities, such as differentiation, proliferation, apoptosis, and growth. Dysregulation of lncRNAs plays a fundamental role in developing various diseases, such as cancer [9]. Most lncRNAs are localized and function within the nucleus, but some only operate in the cytoplasm. lncRNAs can stimulate or suppress transcription by various mechanisms, including epigenetic changes (such as chromatin remodeling or histone modification), transcription factor decay, microRNA sponge, a scaffold for various proteins, and splicing regulation [10]. Altered expression of lncRNAs, such as PTENP1, linc-PINT, and GAS5, plays an essential role in regulating apoptosis in cancer cells [11,12,13]. In human embryonic and adult life, angiogenesis plays a vital role in several physiological processes. Nevertheless, ectopic angiogenic processes have also been associated with the pathogenesis of cancer. Since tumor cells need more nutrients, oxygen, and the removal of metabolites than normal cells, this leads to angiogenesis activation [14]. Moreover, one of the preconditions for cancer metastasis is epithelial-to-mesenchymal transition (EMT) [15]. lncRNAs are essential regulators of tumor angiogenesis and EMT in various cancers such as gastrointestinal, lung, breast, and brain tumors by regulating oncogenic pathways, angiogenic factors, and epigenetic alterations [16]. Therefore, lncRNAs, such as MALAT1, UBE2CP3, HULC, UCA1, and PCA3 play an essential role in cancer metastasis by altering gene expression [17,18,19].
Phytochemicals are a group of natural compounds extracted from fruits, vegetables, and other plants that can play an essential role in cancer prevention and treatment due to their anti-inflammatory, antioxidant, and anticancer properties [20]. One reason for the failure of cancer treatment is the resistance of cancer cells to chemotherapy, and the other is that anticancer drugs are unable to differentiate normal proliferating cells from malignant cells [21]. Genetic and epigenetic modifications in cancer cells cause the expression of surface and intracellular proteins to be different from normal cells [22]. From ancient times, natural compounds could improve many diseases, including cancer, without any side effects. Previous studies have shown that lncRNAs are potential therapeutic targets of bioactive phytochemicals. These compounds could regulate the expression of lncRNA directly and indirectly without any side effects [23][24]. In vivo and in vitro studies reveal that phytochemicals could inhibit proliferation, invasion, migration, EMT, metastasis and induce chemosensitization and radiosensitization of cancer cells by downregulation of oncogenic lncRNAs or upregulation of tumor suppressor lncRNAs [25][26][27].
Phytochemicals are a group of natural compounds extracted from fruits, vegetables, and other plants that can play an essential role in cancer prevention and treatment due to their anti-inflammatory, antioxidant, and anticancer properties [20]. One reason for the failure of cancer treatment is the resistance of cancer cells to chemotherapy, and the other is that anticancer drugs are unable to differentiate normal proliferating cells from malignant cells [21]. Genetic and epigenetic modifications in cancer cells cause the expression of surface and intracellular proteins to be different from normal cells [22]. From ancient times, natural compounds could improve many diseases, including cancer, without any side effects. Previous studies have shown that lncRNAs are potential therapeutic targets of bioactive phytochemicals. These compounds could regulate the expression of lncRNA directly and indirectly without any side effects [23,24]. In vivo and in vitro studies reveal that phytochemicals could inhibit proliferation, invasion, migration, EMT, metastasis and induce chemosensitization and radiosensitization of cancer cells by downregulation of oncogenic lncRNAs or upregulation of tumor suppressor lncRNAs [25,26,27].
2. Non-Coding RNAs
Non-coding RNAs (ncRNAs) constitute a group of RNAs that are not converted by ribosomes into proteins. The ncRNAs are divided into two classes: regulatory ncRNAs and structural ncRNAs [19]. The regulatory ncRNAs include small ncRNAs (20–50 nucleotides), medium ncRNAs (50–200 nucleotides), and long non-coding RNAs (lncRNAs) (longer than 200 nucleotides). Among essential structural ncRNAs are ribosomal RNAs (rRNAs) and transfer RNAs (tRNAs) [28].
Non-coding RNAs (ncRNAs) constitute a group of RNAs that are not converted by ribosomes into proteins. The ncRNAs are divided into two classes: regulatory ncRNAs and structural ncRNAs [19]. The regulatory ncRNAs include small ncRNAs (20–50 nucleotides), medium ncRNAs (50–200 nucleotides), and long non-coding RNAs (lncRNAs) (longer than 200 nucleotides). Among essential structural ncRNAs are ribosomal RNAs (rRNAs) and transfer RNAs (tRNAs) [33].
2.1. Categories of lncRNAs
Previous studies have confirmed that lncRNAs are the most significant ncRNAs, playing an essential role in the advancement and development of malignant and non-malignant human diseases. Cell stages and/or tissue types contribute to lncRNAs function and biogenesis mechanisms. They are also subdivided into several groups depending on their biogenesis, function, and structure [28][29]. lncRNAs are referred to as antisense, bidirectional, enhancer, intergenic, and intronic based on their origins [30][31]. Similar to messenger RNAs (mRNAs), some lncRNAs appear to be capped at their 5’ end and polyadenylated at their 3’ end, respectively, following RNA-polymerase II-mediated transcription. Biogenesis of lncRNAs can be provided by several agents, including ribonuclease P (RNase P)-mediated cleavage for the generation of mature ends, the formation of small nucleolar RNA (snoRNA), and small nucleolar ribonucleoprotein (snoRNP) complex caps at their ends, and formation of linear and circular lncRNAs [28][32].
Previous studies have confirmed that lncRNAs are the most significant ncRNAs, playing an essential role in the advancement and development of malignant and non-malignant human diseases. Cell stages and/or tissue types contribute to lncRNAs function and biogenesis mechanisms. They are also subdivided into several groups depending on their biogenesis, function, and structure [33,34]. lncRNAs are referred to as antisense, bidirectional, enhancer, intergenic, and intronic based on their origins [35,36]. Similar to messenger RNAs (mRNAs), some lncRNAs appear to be capped at their 5’ end and polyadenylated at their 3’ end, respectively, following RNA-polymerase II-mediated transcription. Biogenesis of lncRNAs can be provided by several agents, including ribonuclease P (RNase P)-mediated cleavage for the generation of mature ends, the formation of small nucleolar RNA (snoRNA), and small nucleolar ribonucleoprotein (snoRNP) complex caps at their ends, and formation of linear and circular lncRNAs [33,37].
2.2. Functions of lncRNAs
In humans, the lncRNAs are functionally involved in all processes of normal and malignant cell development and differentiation [33]. In multiple-factor cross-linking, lncRNAs have been shown to alter gene expression by inducing epigenetic modifications, such as chromosome remodeling, histone modification, and nucleosome positioning changes. Another principal mechanism of lncRNAs is chromatin remodeling regulation through interaction with switching defective/sucrose nonfermenting (SWI/SNF) and chromatin remodeling complex [34][35]. As a chromosome reconstitution complex, SWI/SNF comprises multiple subunits and plays a crucial role in nucleosome positioning changes [36]. lncRNAs also contribute to gene expression regulation by adjusting histone modifications, including acetylation, de-methylation, and histone methylation. lncRNAs mostly target S-adenosyl-L-homocysteine hydrolase, DNA damage-inducible protein α (GADD45A), Sirtuin6 (SIRT6), and polycomb repressive complex 2 (PRC2) [37][38][39]. They also have a regulatory effect on the expression of nearby coding genes, including transcription factors such as tumor protein 53 (TP53 or p 53), nuclear factor-κB (NF-κB), octamer-binding transcription factor 4 (Oct4), sex-determining region Y-box2 (Sox2), and c-Myc proto-oncogene protein [40][41]. Many of these factors mediate several signaling pathways and play a key role in migration, apoptosis, differentiation, and proliferation. The Tumor-suppressor p53 gene helps regulate various genes involved in apoptosis, cell cycle, and DNA repair. The expression of p53 protein is typically balanced by translation, post-translational modifications (PTMs), and protein stability [42]. Numerous studies have described the significance of lncRNAs in the p53 gene regulatory network. Furthermore, lncRNAs are also known to mediate the NF-κB signaling pathway. lncRNAs can also regulate the Akt (also referred to as protein kinase B) pathway, Notch, and canonical Wnt pathway via a cross-linking network [43][44].
In humans, the lncRNAs are functionally involved in all processes of normal and malignant cell development and differentiation [38]. In multiple-factor cross-linking, lncRNAs have been shown to alter gene expression by inducing epigenetic modifications, such as chromosome remodeling, histone modification, and nucleosome positioning changes. Another principal mechanism of lncRNAs is chromatin remodeling regulation through interaction with switching defective/sucrose nonfermenting (SWI/SNF) and chromatin remodeling complex [39,40]. As a chromosome reconstitution complex, SWI/SNF comprises multiple subunits and plays a crucial role in nucleosome positioning changes [41]. lncRNAs also contribute to gene expression regulation by adjusting histone modifications, including acetylation, de-methylation, and histone methylation. lncRNAs mostly target S-adenosyl-L-homocysteine hydrolase, DNA damage-inducible protein α (GADD45A), Sirtuin6 (SIRT6), and polycomb repressive complex 2 (PRC2) [42,43,44]. They also have a regulatory effect on the expression of nearby coding genes, including transcription factors such as tumor protein 53 (TP53 or p 53), nuclear factor-κB (NF-κB), octamer-binding transcription factor 4 (Oct4), sex-determining region Y-box2 (Sox2), and c-Myc proto-oncogene protein [45,46]. Many of these factors mediate several signaling pathways and play a key role in migration, apoptosis, differentiation, and proliferation. The Tumor-suppressor p53 gene helps regulate various genes involved in apoptosis, cell cycle, and DNA repair. The expression of p53 protein is typically balanced by translation, post-translational modifications (PTMs), and protein stability [47]. Numerous studies have described the significance of lncRNAs in the p53 gene regulatory network. Furthermore, lncRNAs are also known to mediate the NF-κB signaling pathway. lncRNAs can also regulate the Akt (also referred to as protein kinase B) pathway, Notch, and canonical Wnt pathway via a cross-linking network [48,49].
3. Role of lncRNAs in Human Cancers
Carcinogenesis (tumorigenesis or oncogenesis) is the process that leads to the formation of cancer cells [45]. As such, carcinogenesis involves the following steps: (1) the emergence of mutations in single or multiple DNA repair and tumor-suppressor genes and (2) the formation of oncogenes mediated by a mutation in proto-oncogenes. These eventually develop specific transformed malignant cells with some unique characteristics, known as hallmarks of cancer [46]. Eight hallmarks of cancer have been identified, categorized into three distinct classes: abnormal growth, increased motility, and altered mode of energy metabolism [46]. The first class includes growth suppressor evasion, immortality, immune destruction evasion, and proliferative signaling sustainability. The second class includes angiogenesis induction, invasion initiation, and metastasis. Finally, the third class includes energy metabolism reprogramming [46]. Many studies have demonstrated the significance of lncRNAs in carcinogenesis and their specific effect on cancer hallmarks (
Carcinogenesis (tumorigenesis or oncogenesis) is the process that leads to the formation of cancer cells [50]. As such, carcinogenesis involves the following steps: (1) the emergence of mutations in single or multiple DNA repair and tumor-suppressor genes and (2) the formation of oncogenes mediated by a mutation in proto-oncogenes. These eventually develop specific transformed malignant cells with some unique characteristics, known as hallmarks of cancer [51]. Eight hallmarks of cancer have been identified, categorized into three distinct classes: abnormal growth, increased motility, and altered mode of energy metabolism [51]. The first class includes growth suppressor evasion, immortality, immune destruction evasion, and proliferative signaling sustainability. The second class includes angiogenesis induction, invasion initiation, and metastasis. Finally, the third class includes energy metabolism reprogramming [51]. Many studies have demonstrated the significance of lncRNAs in carcinogenesis and their specific effect on cancer hallmarks (
).
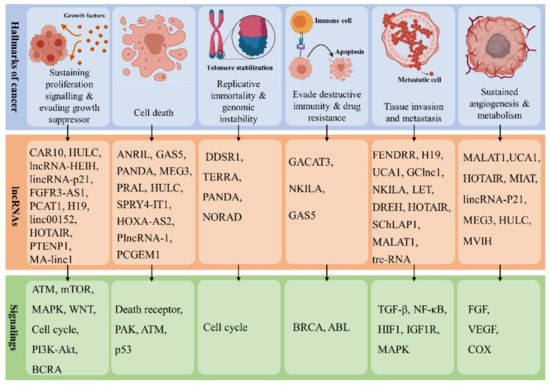
Figure 2.
A schematic view of controlling cancer hallmarks and associated signaling pathway by lncRNAs.
In cancer, lncRNAs can serve as a tumor-suppressive agent, an oncogenic agent, or both [17]. A large number of lncRNAs are linked with each hallmark of cancer. Among the well-known lncRNAs involved in tumor progression in humans, those associated with proliferation, survival and migration of cancer cells are presented in
. This table attempts to describe detailed information of each lncRNA, including oncogenic or tumor-suppressive activity, associated cancer, molecular function, and outcome.
Table 1.
Summary of various well-known cancer-related lncRNAs, their molecular functions, and cellular outcomes.
| lncRNAs | Type | Related Cancer | Molecular Function | Outcome | Reference | |
|---|---|---|---|---|---|---|
| ANRIL | Oncogenic | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Positive correlation MMP3, caspse-9, caspase-3, Bcl-2, E2F1, c-Myc, and miR-122-5p; Negative correlation p15, p16, TIMP2, Bax, miR-99a, and miR-449a |
Induction of cell proliferation, cell cycle, migration, metastasis, and EMT; Inhibition of apoptosis and autophagy cell death |
[47][48][49][50] | [52,53,54,55] |
| CCAT1/2 | Oncogenic | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Positive correlation GAC | Induction of reprogramming of energy metabolism | [51][52] | [56,57] |
| CRNDE | Oncogenic | Bladder, breast, colon, gastric, leukemia, liver, lung, ovarian, pancreas, renal, SNC, and uterine | Positive correlation GLUT4; Negative correlation Insulin, IGF-I, and IGF-II |
Induction of cell proliferation, migration, metastasis, and reprogramming of energy metabolism | [53][54][55] | [58,59,60] |
| GAS5 | Tumor suppressor | Bladder, breast, colon, gastric, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Positive correlation PTEN and p21; Negative correlation ERK1/2, NF-κB, CDK6, E2F1, cyclinD1, Vimentin, and MMP2 |
Induction of autophagy cell death; Inhibition of cell survival, proliferation, migration, metastasis, and cell cycle |
[12][13][17][56] | [12,13,17,61] |
| H19 | Oncogenic | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Positive correlation c-Myc, cyclinA2, CDK4, cyclinB1, cyclin D1, and cyclin E1; Negative correlation RB, EGFR, p21, and IGF-II |
Induction of cell proliferation, cell cycle, migration, metastasis, EMT, tumor angiogenesis, and immune escape; Inhibition of apoptosis and autophagic cell death |
[17][57][58][59] | [17,62,63,64] |
| HOTAIR | Oncogenic | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Positive correlation cyclin D1, cyclin E1, CDK4, CDK2, E2F1, P38, Bcl-2, NOTCH1, β-catenin, N-cadherin, Vimentin, Snail, Twist, MMP-9, MMP-2, MMP-3, FGF1, VEGFA, Ang2, and GLUT1; Negative correlation p53, p21, p16, PIK3R3, caspase-9, and caspase-3 |
Induction of cell proliferation, cell cycle, invasion, metastasis, EMT, tumor angiogenesis, and immune escape; Inhibition of apoptotic and autophagic cell death |
[17][60][61] | [17,65,66] |
| HULC | Oncogenic | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, SNC, and uterine | Positive correlation ZEB1, ZO-1, LC3-II/LC3-I, pmTOR, E2F1, and Snail; Negative correlation E-cadherin |
Induction of cell proliferation, migration, and metastasis; Inhibition of apoptotic cell death |
[17][18] | [17,18] |
| MALAT1 | Oncogenic | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Positive correlation CDK4, ZEB2, slug, β-catenin, N-cadherin, vimentin, Twist, MMP1, MMP-9, VEGF, TGFA, and TGF-β Negative correlation BAX, E-cadherin, MMP19, TIMP-3, and miR-200s |
Induction of cell cycle, cell proliferation, EMT, differentiation, migration, metastasis, chemoresistance and tumor angiogenesis; Inhibition of DNA damage, apoptosis and autophagy cell death |
[62][63][64][65][66] | [67,68,69,70,71] |
| MEG3 | Tumor suppressor | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Positive correlation p53, caspase-3, procaspase-9, cyt. c, and Bax; Negative correlation PI3K, Akt, mTOR cyclin D1, cyclin B1, CDK1, and Bcl-2 |
Induction of apoptotic and autophagic cell death; Inhibition of cell proliferation, invasion, metastasis, and cell cycle |
[17][67][68][69] | [17,72,73,74] |
| PVT1 | Oncogenic | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Positive correlation EZH2, c-Myc, CD115, FGF2, and HIF-1α; Negative correlation miR-31, miR-152, miR-186, and miR-195 |
Induction of cell proliferation, migration, metastasis, cell cycle, and angiogenesis; Inhibition of apoptotic cell death |
[17][70] | [17,75] |
| TERRA | Tumor suppressor | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Negative correlation TRF2 |
Induction of apoptotic cell death; Inhibition of cell proliferation, invasion, metastasis, and cell cycle |
[71][72][73] | [76,77,78] |
| UCA1 | Oncogenic | Bladder, breast, colon, gastric, head and neck, leukemia, liver, lung, osteosarcoma, ovarian, pancreas, prostate, renal, SNC, and uterine | Positive correlation PI3K, AMPK, cyclinD1, ZEB1, ZEB2, N-cadherin, Vimentin, Snail, β-catenin, MMP-7, and FGFR1; Negative correlation p27, E-cadherin, and MMP-14 |
Induction of cell proliferation, migration, metastasis, cell cycle, EMT, and reprogramming of energy metabolism; Inhibition of cellular apoptosis |
[19][74][75] | [19,79,80] |
3.1. Role in EMT
It has been shown that these ncRNAs mainly target some of the essential signaling pathways and transcription factors in cancer cells. Additionally, lncRNAs can influence the metabolism of cancer cells [52]. Shi et al. [64] identified that MALAT1, a prominent oncogenic lncRNA in human cancers, induces EMT. Induction of EMT destroys the polarity of epithelial cells and reduces intercellular adhesion. The MALAT1/miR-124/calpain small subunit 1 (capn4) axis has been demonstrated to be one of the key factors contributing in EMT, invasion, and proliferation of nasopharyngeal carcinoma (NPC) cells [64]. Furthermore, MALAT1 regulates tumor angiogenesis and progression by activating phosphatidylinositol 3-kinase (PI3K)/Akt, extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK), and Wnt/β-catenin signaling pathways [66]. Like MALAT1, ANRIL also contributes to the induction of EMT. The ANRIL, as an oncogenic lncRNA, can facilitate the survival and proliferation of malignant cells. ANRIL has been shown to inhibit apoptosis through the silencing of Kruppel-like factor 2 (KLF2) and p21 genes [49]. It can also facilitate cancer metastasis and cell invasion by introducing matrix metalloproteinase-3 (MMP-3 or stromelysin-1) protein expression [48][50]. Moreover, lncRNAs, such as CASC9, CCAT1, ZEB2-AS1, and lncTCF7, are involved in EMT with different molecular mechanisms, and regulation of their expression can play a therapeutic role for cancer [76][77].
It has been shown that these ncRNAs mainly target some of the essential signaling pathways and transcription factors in cancer cells. Additionally, lncRNAs can influence the metabolism of cancer cells [57]. Shi et al. [69] identified that MALAT1, a prominent oncogenic lncRNA in human cancers, induces EMT. Induction of EMT destroys the polarity of epithelial cells and reduces intercellular adhesion. The MALAT1/miR-124/calpain small subunit 1 (capn4) axis has been demonstrated to be one of the key factors contributing in EMT, invasion, and proliferation of nasopharyngeal carcinoma (NPC) cells [69]. Furthermore, MALAT1 regulates tumor angiogenesis and progression by activating phosphatidylinositol 3-kinase (PI3K)/Akt, extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK), and Wnt/β-catenin signaling pathways [71]. Like MALAT1, ANRIL also contributes to the induction of EMT. The ANRIL, as an oncogenic lncRNA, can facilitate the survival and proliferation of malignant cells. ANRIL has been shown to inhibit apoptosis through the silencing of Kruppel-like factor 2 (KLF2) and p21 genes [54]. It can also facilitate cancer metastasis and cell invasion by introducing matrix metalloproteinase-3 (MMP-3 or stromelysin-1) protein expression [53,55]. Moreover, lncRNAs, such as CASC9, CCAT1, ZEB2-AS1, and lncTCF7, are involved in EMT with different molecular mechanisms, and regulation of their expression can play a therapeutic role for cancer [81,82].
3.2. Role in NF-κB Signaling and Telomerase Activity
Nuclear factor-κB (NF-κB) helps active cancer cells’ responses to the inflammatory microenvironment and regulates the migration, proliferation, and survival of neoplastic cells [78]. GAS5, a lncRNA tumor suppressor, targets NF-κB. GAS5 expression can prevent the activation of NF-κB and ERK1/2 pathways. It can also control the cell cycle by arresting cells in the G0 phase [13]. HULC is a lncRNA that is upregulated in lung squamous cell carcinoma. This lncRNA has a negative effect on protein tyrosine phosphatase receptor type O (PTPRO) via phosphorylation and activation of NF-κB and enhances the proliferation of LSCC cells [79]. NF-κB interacting lncRNA (NKILA) works as a tumor suppressor lncRNA, and its low expression in laryngeal cancer is related to shorter overall survival. Overexpression of NKILA can promote apoptosis and sensitize laryngeal cancer cells to radiation via repression of IκB phosphorylation and NF-κB activation [80]. Additionally, HOTAIR by negative regulation of miR-218 in CRC cells can activate NF-κB signaling and promotes 5-FU resistance and proliferation [81].
Nuclear factor-κB (NF-κB) helps active cancer cells’ responses to the inflammatory microenvironment and regulates the migration, proliferation, and survival of neoplastic cells [83]. GAS5, a lncRNA tumor suppressor, targets NF-κB. GAS5 expression can prevent the activation of NF-κB and ERK1/2 pathways. It can also control the cell cycle by arresting cells in the G0 phase [13]. HULC is a lncRNA that is upregulated in lung squamous cell carcinoma. This lncRNA has a negative effect on protein tyrosine phosphatase receptor type O (PTPRO) via phosphorylation and activation of NF-κB and enhances the proliferation of LSCC cells [84]. NF-κB interacting lncRNA (NKILA) works as a tumor suppressor lncRNA, and its low expression in laryngeal cancer is related to shorter overall survival. Overexpression of NKILA can promote apoptosis and sensitize laryngeal cancer cells to radiation via repression of IκB phosphorylation and NF-κB activation [85]. Additionally, HOTAIR by negative regulation of miR-218 in CRC cells can activate NF-κB signaling and promotes 5-FU resistance and proliferation [86].
Additionally, lncRNAs play an essential role in maintaining cancer cell immortality by regulating telomerase function. Malignant cells have been shown to avoid apoptosis by using telomerase to increase telomeres’ size by inserting repeats onto the edge 3’ of chromosomes [82]. Telomeric repeat-containing RNA (TERRA) includes a heterogeneous class of telomeric region-transcribed lncRNAs [71]. As a lncRNA tumor suppressor, the expression of TERRA contributes to the negative regulation of telomerase activity, whose downregulation plays a key role in carcinogenesis. RB and TP53 genes have been found to regulate TERRA expression [71][73]. Tan et al. [83] show that MALAT1 by regulation of TERT can increase the telomerase activity in bone marrow mesenchymal stem cells. lncRNA MEG3 has been downregulated in non-small cell lung cancer and has a close relation with poor prognosis in patients. Its overexpression in A549 cells could inhibit invasion, migration, proliferation, and telomerase activity by downregulating Dyskeratosis congenita 1 (DKC1) [84]. DKC1 is an essential subunit of ribonucleoprotein telomerase which plays an important role in the stabilization and activity of telomerase [85].
Additionally, lncRNAs play an essential role in maintaining cancer cell immortality by regulating telomerase function. Malignant cells have been shown to avoid apoptosis by using telomerase to increase telomeres’ size by inserting repeats onto the edge 3’ of chromosomes [87]. Telomeric repeat-containing RNA (TERRA) includes a heterogeneous class of telomeric region-transcribed lncRNAs [76]. As a lncRNA tumor suppressor, the expression of TERRA contributes to the negative regulation of telomerase activity, whose downregulation plays a key role in carcinogenesis. RB and TP53 genes have been found to regulate TERRA expression [76,78]. Tan et al. [88] show that MALAT1 by regulation of TERT can increase the telomerase activity in bone marrow mesenchymal stem cells. lncRNA MEG3 has been downregulated in non-small cell lung cancer and has a close relation with poor prognosis in patients. Its overexpression in A549 cells could inhibit invasion, migration, proliferation, and telomerase activity by downregulating Dyskeratosis congenita 1 (DKC1) [89]. DKC1 is an essential subunit of ribonucleoprotein telomerase which plays an important role in the stabilization and activity of telomerase [90].
3.3. Role in Energy Metabolism
Unbalanced metabolic homeostasis by high energy production induces tumor growth, cancer progression, and metastasis. Glucose metabolism is involved in glucose uptake, lactate production, and oxidative phosphorylation. Uncontrolled growth, survival and drug resistance of cancer cells are linked to elevated glucose metabolism [86]. In cancer cells, a transformed mode of energy metabolism occurs in response to tumor oxygenation, which may affect other important aspects of malignancies regulated by lncRNAs. Hypoxia-inducible factor-1α (HIF-1α) has proved effective in facilitating the expression of specific lncRNAs, such as in UCA1, under hypoxic conditions [87]. Xu et al. [88] showed that UCA1, as an oncogenic lncRNA, assists in the regulation of the proliferation, invasion, and metastasis of cancer cells by targeting the KLF832 transcription factor and activating MMP14, fibroblast growth factor receptor-1 (FGFR-1)/ERK, and zinc finger E-box binding homeobox 1 and 2 (ZEB1/2)-fascin homolog 1 (FSCN1) pathways [19][74][75]. Furthermore, LINK-A lncRNA expression has been identified as an essential factor in facilitating the glycolytic reprogramming and tumorigenesis of cancer cells by triggering signaling pathways dependent on LINK-A, e.g., Akt, leading to carcinogenesis and the development of resistance to Akt inhibitors [43]. Previous studies reveal that lncRNA-p23154 and MACC1-AS1 are upregulated in human cancers, and these lncRNAs could promote cellular glycolysis and glucose metabolism via modulation of glucose transporter 1 (GLUT1) [89][90].
Unbalanced metabolic homeostasis by high energy production induces tumor growth, cancer progression, and metastasis. Glucose metabolism is involved in glucose uptake, lactate production, and oxidative phosphorylation. Uncontrolled growth, survival and drug resistance of cancer cells are linked to elevated glucose metabolism [91]. In cancer cells, a transformed mode of energy metabolism occurs in response to tumor oxygenation, which may affect other important aspects of malignancies regulated by lncRNAs. Hypoxia-inducible factor-1α (HIF-1α) has proved effective in facilitating the expression of specific lncRNAs, such as in UCA1, under hypoxic conditions [92]. Xu et al. [93] showed that UCA1, as an oncogenic lncRNA, assists in the regulation of the proliferation, invasion, and metastasis of cancer cells by targeting the KLF832 transcription factor and activating MMP14, fibroblast growth factor receptor-1 (FGFR-1)/ERK, and zinc finger E-box binding homeobox 1 and 2 (ZEB1/2)-fascin homolog 1 (FSCN1) pathways [19,79,80]. Furthermore, LINK-A lncRNA expression has been identified as an essential factor in facilitating the glycolytic reprogramming and tumorigenesis of cancer cells by triggering signaling pathways dependent on LINK-A, e.g., Akt, leading to carcinogenesis and the development of resistance to Akt inhibitors [48]. Previous studies reveal that lncRNA-p23154 and MACC1-AS1 are upregulated in human cancers, and these lncRNAs could promote cellular glycolysis and glucose metabolism via modulation of glucose transporter 1 (GLUT1) [94,95].
3.4. Role in Drug Resistance
Recently, remarkable evidence has been obtained regarding the regulatory roles of lncRNAs in therapeutic responses of cancer cells to chemotherapy drugs. The term of drug resistance is reoffered to an inherent character of malignant cells to administrated antitumor agents, ending in poor overall survival of cancer patients [91]. In
Recently, remarkable evidence has been obtained regarding the regulatory roles of lncRNAs in therapeutic responses of cancer cells to chemotherapy drugs. The term of drug resistance is reoffered to an inherent character of malignant cells to administrated antitumor agents, ending in poor overall survival of cancer patients [96]. In
Table 1, some of the well-known lncRNAs involved in the event of cancer drug resistance have been summarized. The principal mechanisms of drug resistance in cancer cells are: (1) overexpression of drug efflux transporters like ATP-binding cassette (ABC) superfamily pumps [92]; (2) enhanced repair of chemotherapy induced-genome damages [93]; (3) alternation signaling pathways controlling drug resistance [94]; (4) enhanced modulation of drug metabolism; (5) increasing resistance to cell death mechanisms [95]; and (6) EMT [94].
, some of the well-known lncRNAs involved in the event of cancer drug resistance have been summarized. The principal mechanisms of drug resistance in cancer cells are: (1) overexpression of drug efflux transporters like ATP-binding cassette (ABC) superfamily pumps [97]; (2) enhanced repair of chemotherapy induced-genome damages [98]; (3) alternation signaling pathways controlling drug resistance [99]; (4) enhanced modulation of drug metabolism; (5) increasing resistance to cell death mechanisms [100]; and (6) EMT [99].
ANRIL is one of the well-studied lncRNAs inducing chemotherapy resistances in cancer cells. Through an experimental study, Li and Zhu, (2019) have reported a tight correlation of ANRIL with cisplatin resistance in osteosarcoma cells. In ANRIL-depleted cancer cells, they have observed a significant improvement of cisplatin sensitivity via miR-125a-5p/STAT3 pathway [96]. Furthermore, ANRIL has a profound impact on paclitaxel sole resistance. It has been demonstrated that ANRIL overexpression could powerfully inhibit the apoptotic death of lung adenocarcinoma cells following the paclitaxel sole treatment [97]. Likewise, the expression of lncRNA HOTAIR in lung tumors has been linked to chemoresistance. Within the downregulation of p21 and upregulation of Kruppel-like factor 4 (KLF4), it has been found that HOTAIR overexpression could influence the cisplatin resistance in the lung tumors [98][99]. Through an experimental study, Guo et al. (2018) observed a significant improvement of cisplatin sensitivity in HOTAIR-depleted lung cancer cells following the inhibition of multidrug resistance-associated protein-1 (MRP-1) and Wnt signaling pathway [100]. Docetaxel, doxorubicin, gemcitabine, gefitinib, methotrexate, oxaliplatin, sunitinib, tamoxifen, trastuzumab, and 5-FU are the other types of anticancer agents with antitumor efficacies negatively regulated by lncRNAs [101]. The evidence of current studies has clearly shown the interfering effects of lncRNAs on the outcomes of conventional treatments of cancer, such as chemotherapy. Simultaneous administration of complementary agents targeting lncRNAs in tumors could be an effective approach for improving the efficiency of chemotherapies.
ANRIL is one of the well-studied lncRNAs inducing chemotherapy resistances in cancer cells. Through an experimental study, Li and Zhu, (2019) have reported a tight correlation of ANRIL with cisplatin resistance in osteosarcoma cells. In ANRIL-depleted cancer cells, they have observed a significant improvement of cisplatin sensitivity via miR-125a-5p/STAT3 pathway [101]. Furthermore, ANRIL has a profound impact on paclitaxel sole resistance. It has been demonstrated that ANRIL overexpression could powerfully inhibit the apoptotic death of lung adenocarcinoma cells following the paclitaxel sole treatment [102]. Likewise, the expression of lncRNA HOTAIR in lung tumors has been linked to chemoresistance. Within the downregulation of p21 and upregulation of Kruppel-like factor 4 (KLF4), it has been found that HOTAIR overexpression could influence the cisplatin resistance in the lung tumors [103,104]. Through an experimental study, Guo et al. (2018) observed a significant improvement of cisplatin sensitivity in HOTAIR-depleted lung cancer cells following the inhibition of multidrug resistance-associated protein-1 (MRP-1) and Wnt signaling pathway [105]. Docetaxel, doxorubicin, gemcitabine, gefitinib, methotrexate, oxaliplatin, sunitinib, tamoxifen, trastuzumab, and 5-FU are the other types of anticancer agents with antitumor efficacies negatively regulated by lncRNAs [106]. The evidence of current studies has clearly shown the interfering effects of lncRNAs on the outcomes of conventional treatments of cancer, such as chemotherapy. Simultaneous administration of complementary agents targeting lncRNAs in tumors could be an effective approach for improving the efficiency of chemotherapies.
