Glasses are solid amorphous materials which transform into liquids upon heating through the glass transition.
- Glasses
Glasses are solid amorphous materials which transform into liquids upon heating through the glass transition. The International Commission on Glass defines glass as a state of matter, usually produced when a viscous molten material is cooled rapidly to below its glass transition temperature, with insufficient time for a regular crystal lattice to form [1]. The solid-like behaviour of glasses is separated from the liquid-like behaviour at higher temperatures by the glass transition temperature, Tg [2]. The IUPAC Compendium on Chemical Terminology defines glass transition as a second order transition in which a supercooled melt yields, on cooling, a glassy structure[3]. It states that below the glass-transition temperature the physical properties of glasses vary in a manner similar to those of the crystalline phase. Moreover, it is deemed that the bonding structure of glasses has the same symmetry signature in terms of Hausdorff-Besikovitch dimensionality of chemical bonds as for the crystalline materials[4].
Introduction
Glass is one of the most ancient of all materials known and used by mankind. The natural glass, obsidian, was first used by man thousands of years ago to form knives, arrow tips and jewellery. Manmade glass objects from Mesopotamia have been dated as early as 4,500 BC and from Egypt from 3,000 BC. The high chemical resistance of glass allows it to remain stable in corrosive environments for many thousands and even millions of years. Several glasses are found in nature such as obsidians (volcanic glasses), fulgarites (formed by lightning strikes), tektites found on land in Australasia and associated microtektites from the bottom of the Indian Ocean, moldavites from central Europe, and Libyan Desert glass from western Egypt. Some of these glasses have been in the natural environment for about 300 million years with low alteration rates of less than a millimetre per one million years. For example, the natural glass obsidian is formed when lava erupts from volcanoes and cools rapidly without sufficient time for crystal growth. The composition of a typical California obsidian is (wt%) 75SiO2 13.5Al2O 1.6FeO/Fe2O3 1.4CaO 4.3Na2O 4.5K2O 0.7MnO. Obsidian glass edges can be extremely sharp reaching almost molecular thinness and was known for its ancient use as knives and projectile tips. Tektites are other natural glasses, typically up to a few centimetres in size, which have most probably been formed by the impact of large meteorites on Earth's surface which melted the Earth's surface material resulting on cooling in glass. The age of tektites found in Czech Republic, moldavites of typical composition (75-80)SiO2 (9-12)Al2O (1-3)FeO/Fe2O3 (2-3)CaO 0.3Na2O 3.5K2O, is assessed to be ~15 million years[5].
Glasses are irreplaceable in the day-by-day life with important technological, medical and scientific applications including physics, chemistry, biology, geology as well as artistic and decorative uses. Glasses are typically formed on enough rapid cooling of molten materials therefore, following Michael Faraday, the glass can be defined as a solid solution of different substances one in another. Glasses are also formed and occur naturally for example volcanic glasses such as obsidians.
Glass formation (vitrification)
Glasses are most frequently produced by a melt cooling below its glass transition temperature sufficiently fast to avoid formation of crystalline phases. Glass-forming materials such as dioxides do not require very fast cooling whereas materials prone to crystallization such as metals require a very fast cooling (quenching), for example the first metallic glasses had to be cooled extremely rapidly with rates of the order of 106 K/s to avoid crystallization. Glasses can be formed by several methods[6]:
- Melt quenching,
- Physical vapour deposition,
- Solid state reactions (thermo- and mechanochemical methods),
- Liquid state reactions (sol-gel method),
- Irradiation of crystalline solids (radiation amorphization),
- Under action of high pressures (pressure amorphization).
Glass formation from melts (vitrification) is a matter of bypassing crystallization and formation of glass is easier in more complex systems[7]. Most glasses used in commerce are oxide glasses (Table 1).
Table 1. Commercial oxide glass compositions [ACMP].
|
Glass family |
Oxide, mass % |
|||||||||
|
SiO2 |
Na2O |
CaO |
Al2O3 |
MgO |
B2O3 |
BaO |
PbO |
K2O |
ZnO |
|
|
Vitreous silica (Furnace tubes, Si melting crucibles) |
|
|
|
|
|
|
|
|
|
|
|
Soda-Lime Silicate: |
|
|
|
|
|
|
|
|
|
|
|
Window |
72.0 |
14.2 |
10.0 |
0.6 |
2.5 |
|
trace |
|
0.6 |
|
|
Container |
74.0 |
15.3 |
5.4 |
1.0 |
3.7 |
|
|
|
0.6 |
|
|
Bulb and tube |
73.3 |
16.0 |
5.2 |
1.3 |
3.5 |
|
|
|
|
|
|
Tableware |
74.0 |
18.0 |
7.5 |
0.5 |
|
|
|
|
|
|
|
Sodium borosilicate: |
|
|
|
|
|
|
|
|
|
|
|
Chemical glassware |
81.0 |
4.5 |
|
2.0 |
|
12.0 |
|
|
|
|
|
Nuclear waste immobilisation |
43-53 |
6-24 |
0-14 |
3-19 |
0-5.3 |
8-17 |
misc. |
misc. |
misc. |
misc. |
|
Lead-alkali silicate: |
|
|
|
|
|
|
|
|
|
|
|
Lead “crystal” |
59.0 |
2.0 |
|
0.4 |
|
|
|
25.0 |
12.0 |
1.5 |
|
Television funnel |
54.0 |
6.0 |
3.0 |
2.0 |
2.0 |
|
|
23.0 |
8.0 |
|
|
Aluminosilicate: |
|
|
|
|
|
|
|
|
|
|
|
Halogen lamp |
57.0 |
0.01 |
10.0 |
16.0 |
7.0 |
4.0 |
6.0 |
|
trace |
|
|
Fibreglass “E” |
52.9 |
|
17.4 |
14.5 |
4.4 |
9.2 |
|
|
1.0 |
|
|
Optical (Crown) |
68.9 |
8.8 |
|
|
|
10.1 |
2.8 |
|
8.4 |
1.0 |
Glass transition temperature
Crystalline materials melt at well defined melting temperatures Tm whereas amorphous materials transform from the glassy (solid) state to the molten (liquid) state at the glass transition temperatures Tg which play the role of melting temperatures of non-crystalline solids. The liquid-glass transition is accompanied by significant changes in physical properties, e.g. glasses are brittle, thus changes should occur at the molecular level although the material at molecular scale is topologically disordered both in liquid and glassy states. Namely rearrangements that occur in an amorphous material at the glass transition temperature cause characteristic discontinuities of derivative thermodynamic parameters such as the coefficient of thermal expansion or the specific heat[8]. Figure 1 shows the main characteristics of glass–liquid transition that demonstrate the thermodynamic origin and structural changes behind transformation:
(a) The temperature (T) dependences of the isobaric heat capacity (Cp) by differential scanning calorimetry (DSC) during the heating of a glass at a typical rate of 10 K/min. The glass−liquid transition during upscan is always featured by a Cp jump, whereas the vitrification (liquid−glass transition) on cooling during downscan is reflected by a gradual Cp drop[9].
(b) The temperature dependence of the first sharp diffraction minimum (FSDM) value of pair-distribution function (PDFmin) on the scattering of incident neutron or X-rays indicating that at the Tg there is a substantial change of slope due to structural changes at the glass transition[10].
(c) The temperature dependence of the first temperature differential of FSDM value d(PDFmin)/dT indicating that at the Tg, there is a stepwise change of slope due to structural changes at the glass transition[10].
(d) The temperature dependence of the Hausdorff–Besicovitch dimen-sion D=dimH(broken bonds) of the set of broken chemical bonds at the glass transition changes suddenly as change the material properties. The set of broken bonds termed configurons has D = 0 below the Tg, and D = 2.55 ± 0.05 above it[11].
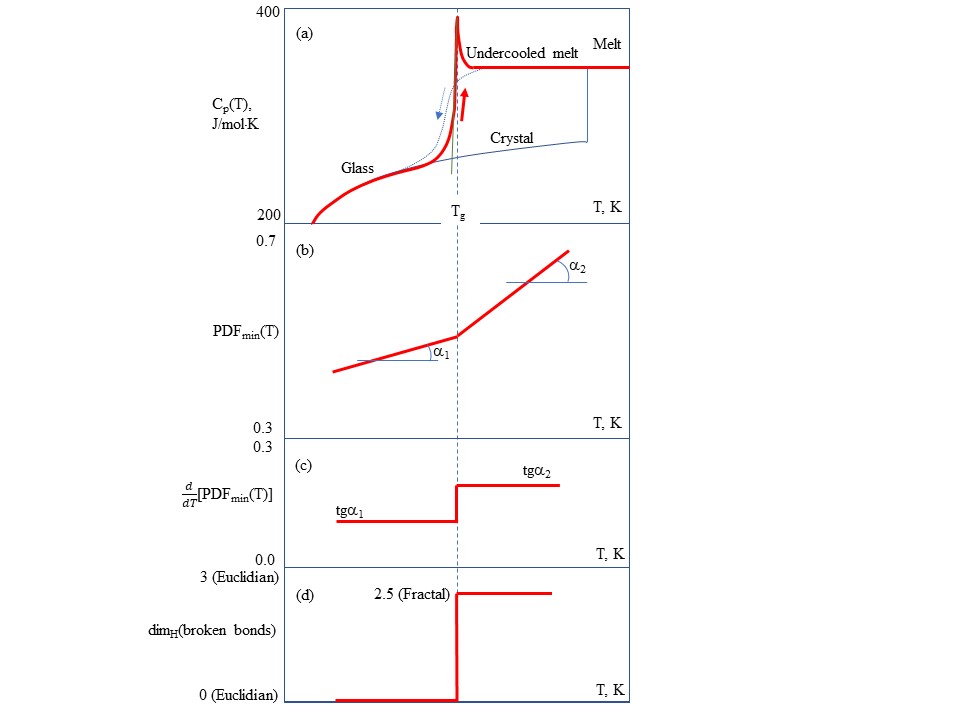
Fig. 1. The main characteristics of glass–liquid transition that demonstrate the thermodynamic origin and structural changes behind transformation. (a) The temperature (T) dependences of the isobaric heat capacity (Cp) by differential scanning calorimetry (DSC). (b) The temperature dependence of the first sharp diffraction minimum (FSDM) value of pair-distribution function (PDFmin) on the scattering of incident neutron or X-rays. (c) The temperature dependence of the first temperature differential of FSDM value d(PDFmin)/dT. (d) The temperature dependence of the Hausdorff–Besicovitch dimension D=dimH(broken bonds) of the set of broken chemical bonds.
The discontinuities allow to detect the Tg or, accounting for cooling rate dependences, - the glass transition interval where a supercooled liquid transforms to a glass[12]. Vitrification manifests itself as a second-order phase transition however its description in terms of the Landau theory is difficult as there is no clarity about the order parameter describing this transition. Although similar to a second order phase transformation the glass-liquid transition is a kinetically-controlled phenomenon which exhibits a range of Tg depending on the cooling rate with maximal Tg at highest rates of cooling.
References
- The International Commission on Glass. . The International Commission on Glass.. Retrieved 2021-4-17
- Richet, P., Conradt, R., Takada, A., Dyon, J. . Encyclopedia of Glass Science, Technology, History, and Culture. ; Richet, P., Conradt, R., Takada, A., Dyon, J. , Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 1568 p..
- A.D. McNaught, A. Wilkinson. The IUPAC Compendium on Chemical Terminology.; A.D. McNaught, A. Wilkinson, Eds.; Royal Society of Chemistry: Cambridge, 1997; pp. p..
- Michael I Ojovan; William E Lee; Topologically disordered systems at the glass transition. Journal of Physics: Condensed Matter 2006, 18, 11507-11520, 10.1088/0953-8984/18/50/007.
- Varshneya A.K. . Fundamentals of inorganic glasses.; Society of Glass Technology: Sheffield, 2006; pp. p..
- Michael I. Ojovan; Viscosity and Glass Transition in Amorphous Oxides. Advances in Condensed Matter Physics 2008, 2008, 1-23, 10.1155/2008/817829.
- Michael I. Ojovan; Glass Formation. Encyclopedia of Glass Science, Technology, History, and Culture 2021, 1, 249-259, 10.1002/9781118801017.ch3.1.
- Michael Ojovan; The Modified Random Network (MRN) Model within the Configuron Percolation Theory (CPT) of Glass Transition. Ceramics 2021, 4, 121-134, 10.3390/ceramics4020011.
- Yuan-Zheng Yue; Characteristic temperatures of enthalpy relaxation in glass. Journal of Non-Crystalline Solids 2008, 354, 1112-1118, 10.1016/j.jnoncrysol.2006.11.027.
- Michael I. Ojovan; Dmitri V. Louzguine-Luzgin; Revealing Structural Changes at Glass Transition via Radial Distribution Functions. The Journal of Physical Chemistry B 2020, 124, 3186-3194, 10.1021/acs.jpcb.0c00214.
- Michael I. Ojovan; Configurons: Thermodynamic Parameters and Symmetry Changes at Glass Transition. Entropy 2008, 10, 334-364, 10.3390/e10030334.
- Damba S. Sanditov; Michael I. Ojovan; Relaxation aspects of the liquid—glass transition. Uspekhi Fizicheskih Nauk 2018, 189, 113-133, 10.3367/ufnr.2018.04.038319.
