An infectious coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is accompanied with elevated risk and adverse outcome among infected patients, even sudden death due to significant multiple organ dysfunction, including dire cardiovascular complications. Myocardial impairment due to invasion of SARS-CoV-2 virus via angiotensin-converting enzyme 2 receptor to cardiovascular cells/tissue leads to endothelial inflammation and dysfunction, de-stabilization of vulnerable atherosclerotic plaques, stent thrombosis and cardiac stress, caused by diminished oxygen supply and cardiac muscle damage and myocardial infarction. A number of promising therapeutic approaches are currently under clinical trials to avoid a massive surge of COVID-19 patients with prevalence of adverse cardiac events.
- cardiovascular diseases
- COVID-19
- treatment strategies
- angiotensin-converting enzyme 2 receptor
- Hypertension
- Myocardial Injury and Heart Failure
- Myocarditis
- Myocardial Arrhythmias
1. Introduction
Coronavirus-19 (COVID-19) is an emerging infectious disease caused by the novel single-stranded RNA enveloped Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2). SARS-CoV-2 is spherical in shape and consists of multiple components, which are essential for their replication and transcription: (1) Spike glycoprotein (S), several club shaped projections on the surface of the envelope called, which helps in anchoring to the host cell and acts as an inducer to neutralize antibodies, (2) A small membrane envelope protein (E), (3) Structural membrane protein (M), which spans the lipid bilayer, (4) Hemagglutinin-esterase glycoprotein (HE), which destroys the sialic acid present on the host cell and help the virus to inject its genetic material, (5) Nucleoprotein (N), and (6) the key component, the positive-sense single-stranded genomic RNA [1][2][3].
COVID-19 virus is predominantly transmitted from human-to-human through respiratory droplets or aerosols (>5-10 μm in diameter) and contact routes. Inhalation of respiratory droplets and aerosols from COVID-19 infected persons is the most potential mode of transmission of the disease [4]. Once in the host system, SARS-CoV-2 multiplies following the classical traditional steps similar to any other virus for its mode of entry into the host cells [5]. The spike protein anchors the virus to the surface of the host cell by binding to the ACE2 receptor [6]. The virus undergoes conformational changes to fuse to the cell membrane of the host cells and engulfs into the cytoplasm of the cell by endosomal pathway. Once inside the cell the virus releases its genomic RNA and multiplies using the host’s molecular machinery. ACE2 is expressed in type II alveolar cells, the predominant portal of entry in the lungs; it is also expressed in the heart, intestine and kidney as well as on the epithelial cells of oral mucosa and the tongue [7][8].
The lungs being a major organ targeted by SARS-CoV-2 infection are severely compromised in delivering their function. In the pulmonary vasculature, SARS-CoV-2 enters through endocytosis and activates ADAM metallopeptidase domain 17 (ADAM17), which in turn cleaves ACE2, which indicates the loss of protection against renin–angiotensin–aldosterone system (RAAS) is mediated by cleaved ACE2 [29][30]. The activation of ADAM17 also triggers acute pulmonary inflammation and infiltration of cytokines and leukocytes in the alveolar space and results in pulmonary edema [9][31]. Overactive systemic inflammation as a response to COVID-19 infection results in cytokine storm and leads to respiratory difficulties and accounts for majority of the deaths during end stage of the treatment [9]. Pulmonary complications associated with COVID-19 infection include diseases such as acute respiratory distress syndrome (ARDS), vascular endothelialitis, sepsis, pulmonary edema and pulmonary embolism [10][11].
Although pulmonary complications are is the dominant clinical manifestation of COVID-19, underlying cardiovascular complications as well as developed acute cardiac injury enhance the vulnerability of the patient. Acute respiratory complication/failure and cytokine storm may cause reduced oxygen supply, which could lead to acute myocardial injury in COVID-19 patients [12].
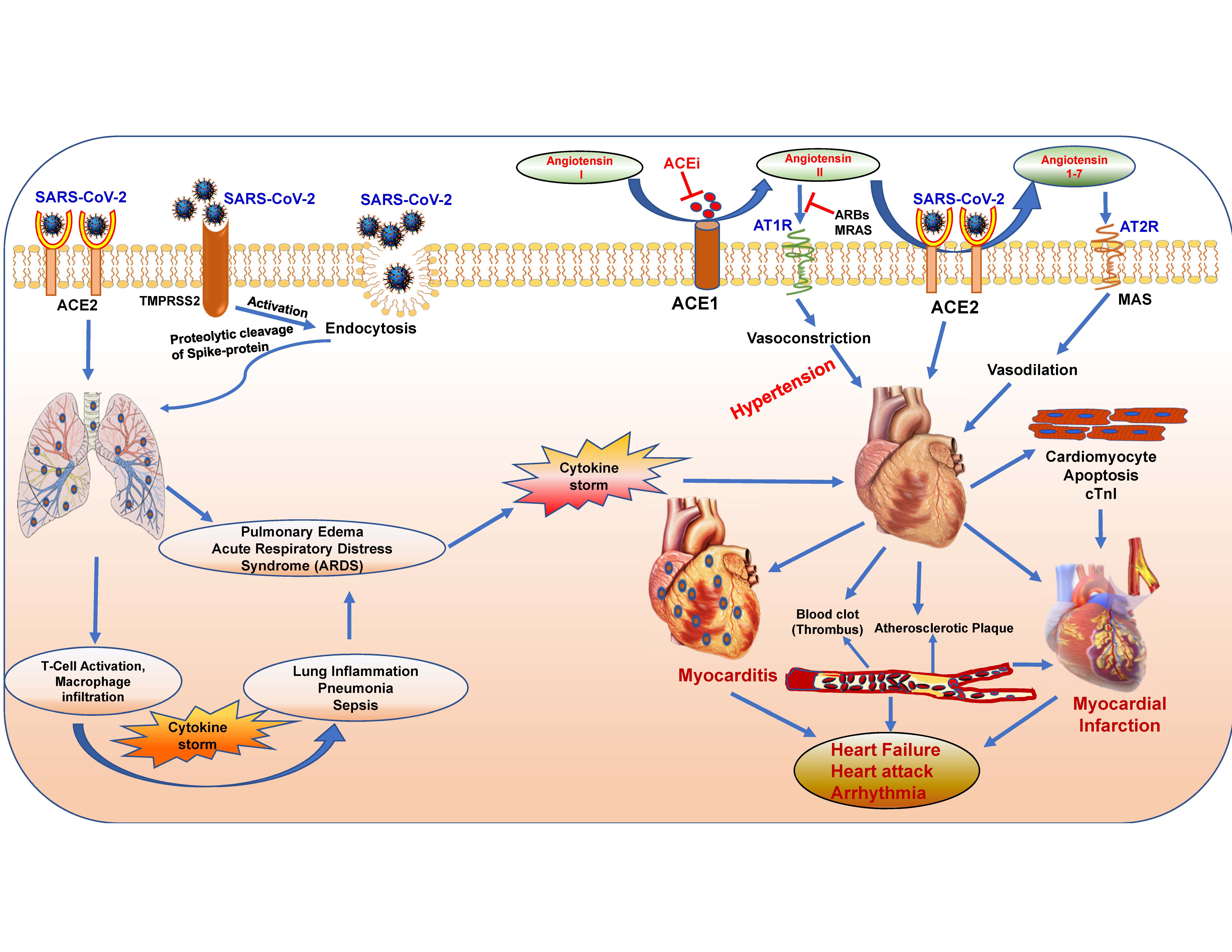
Figure 1. Pulmonary and cardiovascular complications associated with COVID-19 infection. The abundance of SARS-CoV-2 compromises the normal function and leads to complications in lungs (inflammation, hypoxia, cytokine storm, pulmonary edema, acute respiratory distress syndrome) and in heart (myocardial infarction, heart failure, myocarditis and arrhythmia). ACE1, angiotensin I-converting enzyme; ACE2, angiotensin-converting enzyme 2; ACEi, ACE inhibitor; AT1R, angiotensin type 1 receptor; AT2R, angiotensin type 2 receptor; ARBs, angiotensin II type-I receptor blockers; cTnI, cardiac troponin I; MAS, mitochondrial assembly receptor; MRAs, mineralocorticoid receptor antagonists; TMPRSS2, transmembrane serine protease 2.
2. Cardiovascular Complications of COVID-19
Patients with cardiovascular disease (CVD) have an increased risk for severity and mortality with COVID-19 infection, mainly because of the abundance of ACE2 receptor in the cardiovascular system, which serves as a gateway for the entry of virus in lungs and heart [13]. Respiratory illness and acute cardiac injury are major clinical manifestations observed in patients infected with SARS-CoV-2 during the late stage complications of the disease [14]. Reasonably, patients with coronary artery disease or heart failure are vulnerable to developing major cardiac injury, and once such patients are infected with SARS-CoV-2, they are at greatest risk of serious myocardial impairment or cardiac dysfunction, requiring hospitalization due to unexpected deterioration. Eventually, mortality is greater among these patients. A brief view of cardiovascular complications associated with COVID-19 is presented in Figure 1.
Numerous available clinical evidences confirms that the severity of COVID-19 is pronounced in patients with a prevalence of underlying cardiovascular diseases, and in many of these patients, the virus causes severe myocardial injury [15], including myocardial dysfunction, cardiomyopathy, arrhythmias and heart failure during the course of critical illness [16][17][18][19][20][21][22]. According to the mortality data from the CDC (Centers for Disease Control and Prevention), different health conditions contribute to the deaths of COVID-19 patients in United States, which are summarized in Figure 2 [23] (https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm#Comorbidities). Deaths are associated with more than one underlying condition, e.g., deaths involving both diabetes and respiratory arrest or cardiovascular complications and respiratory arrest, etc. Hypertension, diabetes, cardiac arrest, ischemic heart disease and heart failure are the major risk factors and have contributed to the fatalities in COVID-19 cases.
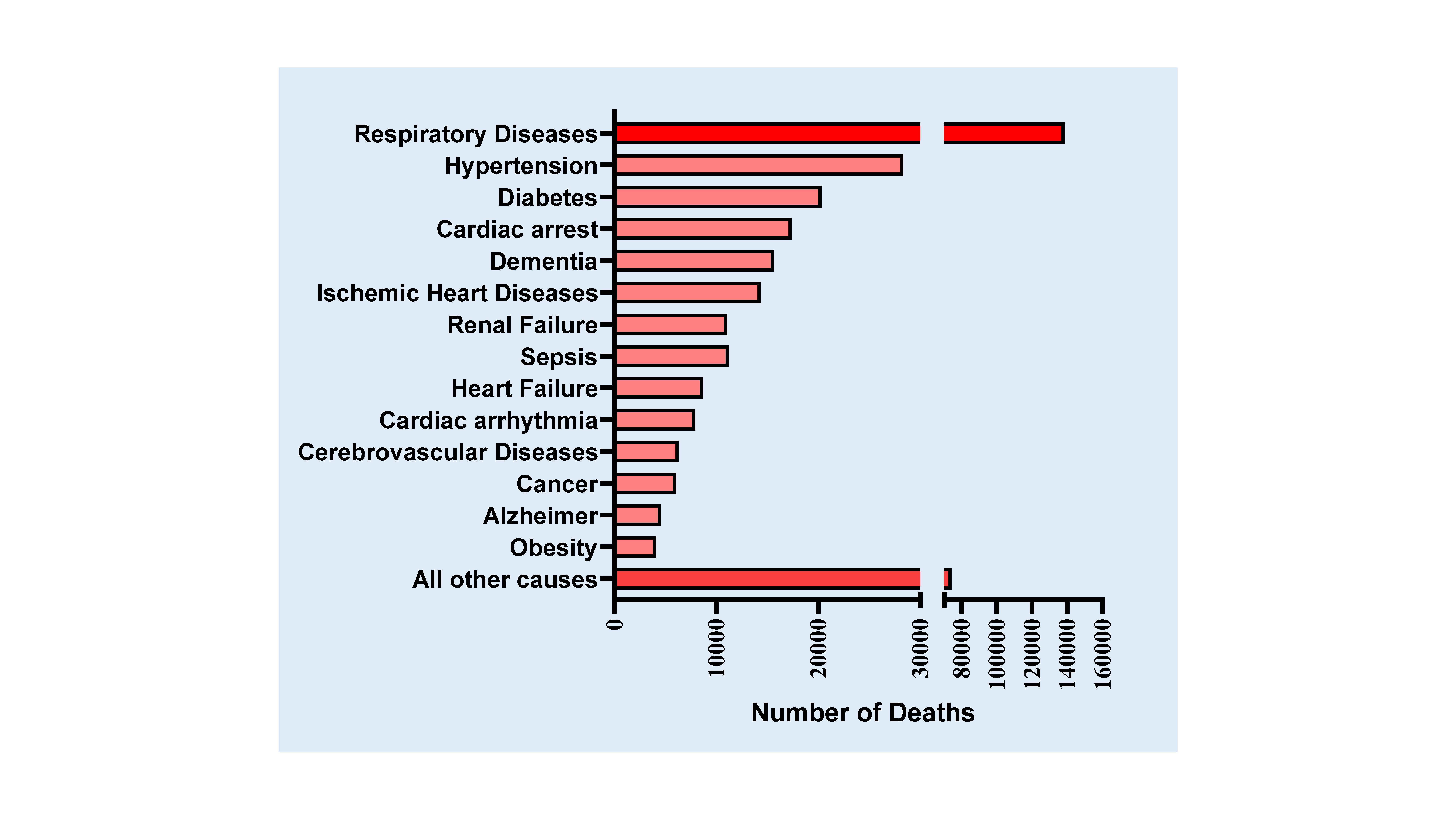
Figure 2. Conditions contributing to the deaths of COVID-19 patients in the United States (according to Death Data of CDC as of July 25, 2020). Deaths involving more than one condition (e.g., deaths involving both diabetes and respiratory arrest) were counted in both totals. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm#Comorbidities.
The renin–angiotensin–aldosterone system (RAAS) consists of an enzymatic cascade that controls blood pressure by regulating circulatory homeostasis, body fluid and systemic vascular resistance, all of which are involved in the regulation of a myriad of cardiovascular system [24]. ACE1 (angiotensin I-converting enzyme) cleaves angiotensin I (Ang-I) to angiotensin II (Ang-II), which binds to and activates Angiotensin Type 1 Receptor (AT1R), and leads to vasoconstriction, inflammation, fibrosis and proliferation [8] (Figure 1). ACE2 converts Ang-II into angiotensin 1-7 (Ang 1-7), which has vasodilating and anti-inflammatory effects by binding to MAS receptor (MAS-R). ACE2 also cleaves Ang-I into angiotensin-1–9, which is further converted into Ang 1–7 by ACE. Therefore, ACE2 regulates abnormal activation of the RAAS, which can prevent the development of hypertension, cardiac hypertrophy, and heart failure [25]. An increase in ACE2/ACE1 ratio protects against endothelial dysfunctions and vascular constriction, and exogenous ACE2 activation attenuates thrombus formation and reduces platelet attachment to vessels [26][27].
The etiology of ACE2 dependent cardiovascular complications with COVID-19 infection is rather complex. SARS-CoV-2 enters cardiovascular cells/tissue by binding to the membrane-bound form of ACE2 (ACE2 receptor). Elevated levels of ACE2 and its activity are the biomarkers of cardiovascular disease including patients with heart failure [28], which indicates that these patients may be more susceptible to COVID-19 infection [29], with worsened prognosis of cardiovascular disease treatment [30]. Measuring plasma angiotensin peptides and plasma ACE2 levels can provide a direct evaluation on the progress of treatment and the state of the RAAS in COVID-19 patients [31]. Nevertheless, earlier clinical studies conveyed that treatment with soluble form of recombinant human ACE2 (rhACE2; APN01 (0.4 mg/kg, IV, BID for 7 days), GSK2586881: 0.4 mg/kg, IV, BID for 3 days) neutralized the excessive SARS-COV virus in the system and preserved the protective cellular effects of ACE2 in ARDS patients [32][33]. Consequently, scientists propose the therapeutic potential of soluble recombinant ACE2, which can overwhelm SARS-CoV-2 to prevent its binding to cellular ACE2 [34]. In addition, ACE inhibitors (ACEi), which upregulate ACE2 expression on the cell surface, have been proven to be successful, and improved the survival rate in patients undergoing COVID-19 treatment [25]. Abundant expression of ACE2 on the cell surface following virus infection may maintain Ang-II degradation, which could reduce AT1R activation and the risk of deleterious outcomes of COVID-19.
While the ACE2 gene is located on the X-chromosome, gender has an impact among COVID-19 patients, where men are at increased risk of susceptibility to COVID-19 infection and CVD complications due to their hemizygous allele for ACE2 compared to heterozygous allele in female [8]. Interestingly, clinical data from 1485 European men and 537 women with heart failure showed elevated level of circulating plasma ACE2 in men than in female [35]. This data complements with an observation of increased prevalence and susceptibility of COVID-19 in males and demonstrates that abundance of ACE2 receptor in cardiovascular cells can lead to severe clinical complications [36][37][38]..
2.1. Hypertension
Considering the importance of ACE2 in the development of hypertension and diabetes mellitus, patients with COVID-19 exhibit severe comorbidities including hypertension and diabetes with poor prognosis. Initial evidence from 44,672 confirmed cases in China showed 4.2% with CVD and 12.8 % with hypertension. However, among the death rate, 6% had hypertension, 7.3% had diabetes and 6.3% suffered from chronic respiratory disease [38]; [39]. In another study involving a small population of 99 patients, 40% had underlying CVD or cerebrovascular disease [40]. Interestingly, data from a small registry of 41 admitted patients showed an alarming 73% were men with a median age of 49 [2]. However, data involving 5700 patients (admitted during March 1 to April 4, 2020) with a median age of 63 from New York City, the epicenter of COVID-19 spread in the United States, showed a slightly different picture [14]. The most common underlying comorbidities were hypertension (57%), obesity (42%), and diabetes (34%). Figure 2 depicts the statistics on distribution of underlying conditions among COVID-19 patients based on the data from Center for Disease Control Department (CDC), United States. The data reveals that hypertension is a major comorbid factor for COVID-19 fatalities.
An increased risk of COVID-19 death was associated with an age greater than 65 years (mortality of 10%), CVDs (coronary artery disease: 10.2%; heart failure: 15.3%; cardiac arrhythmia: 11.5%), chronic obstructive pulmonary disease (14.2%), and current smoking (9.4%). Another detailed observational meta-analysis (49,076 confirmed COVID-19 case) of data available from public domains including databases from MEDLINE, Embase and web of Science showed patients with preexisting condition of CVD, DM and hypertension are significantly associated with a higher risk of developing severe complication with COVID-19 disease. Precisely, the analysis comparing the complications between severe vs non-severe (mild to moderate) COVID-19 cases concluded that CVD was significantly associated with increased illness severity and adverse outcomes among COVID-19 patients [41]. Recently, Center for Disease Control and Prevention (CDC) suggests that children with certain medical conditions, like neurological, genetic and metabolic conditions, or congenital heart disease might be at increased risk of severe illness from COVID-19 compared to other children.
Additional study comprising 191 patients from two hospitals in Wuhan, China reported 48% of patients had underlying comorbidity factors: 30% with hypertension, 19% with diabetes and 8% with coronary heart disease [16]. In another cohort with 1591 confirmed COVID-19 patients (during February 20 and March 18, 2020) with an average median age of 63 from Lombardy, Italy, 68% had at least one underlying comorbidity, hypertension (49%), CVD (21%), hypercholesterolemia (18%), or diabetes (17%) [42]. Moreover, a staggering 82% of the patients were male and the mortality rate was higher in elderly patients aged ≥64 years compared to younger patients (36% vs 15%). Due to the high prevalence of hypertension in the older population, elderly male individuals may be at the highest risk of infection with worse outcomes, and an increased mortality rate with respect to younger patients.
Patients with hypertension are mostly treated with ACE inhibitors (ACEi) and angiotensin II type-I receptor blockers (ARBs), which substantially increases the expression of ACE2, due to negative feedback activation caused by low level of Ang-I in the system [43]. Considering ACE2 as a preferential receptor of SARS-CoV-2, the patient with antihypertensive therapy with ACEi/ARBs may be at higher risk of developing severe COVID-19 with poor prognosis [43]. Remarkably, clinical studies do not support this hypothesis and found no evidence to demonstrate the use of ACEi or ARB as a risk factor in COVID-19 patients [25]. Multiple investigators have demonstrated the beneficial therapeutic effect of ACEi or ARB in prevention of COVID-19 infection [44][45]. Independent studies conducted among hypertensive patients found no association between the use of ACEi or ARB and increased risk of mortality in COVID-19-positive cases [46][47]. A population-based case–control study in the Lombardy region of Italy with a total of 6272 patients with COVID-19 (February 21 and March 11, 2020) reported that 22.2% patients were receiving ARB and 23.9% patients were receiving ACEi [46]. Other antihypertensive drugs were also used more in COVID-19 patients than in controls and they had a more frequent history of hospitalization due to cardiovascular complications. However, this study showed no evidence of association of use of anti-hypertrophic drugs including ACEi or ARBs and susceptibility of COVID-19. Another study with 12,594 patients in the New York University (NYU) Langone Health, in which 5894 patients were positive for COVID-19 (46.8%), reported 4357 patients had a history of hypertension (34.6%)[48]. Among these hypertensive patients, 2573 (59.1%) patients were positive for COVID-19 (59.1%). This study also identified no substantial adverse effect with the use of antihypertensive drugs including ACEi or ARBs in the COVID-19 positive patients.
Therefore, prospective research is warranted to clarify the accuracy of existing contradictory hypotheses regarding the use of ACEi or ARBs to control of blood pressure in hypertensive patients during viral infections. Fundamentally, after entering into the cells via ACE2 receptors and excessive binding of the SARS-CoV-2 result in the downregulation of ACE2 by intracellular degradation and shedding, which could reduce the Ang-II degradation and activation of AT1R with induction of myocardial hyper-inflammatory reaction in response to COVID-19 [25][49]. Due to ACEi or ARB treatment, more ACE2 may be localized in the cell surface after virus binding, which could facilitate Ang-II degradation with reduction of AT1R activation [25].
2.2. Myocardial Injury and Heart Failure
Apart from hypertension and age, acute cardiac injury, chronic heart damage and heart failure have all been observed in patients treated for COVID-19 infection [20][50]. Due to acute inflammation, procoagulant stimulus and endothelial cell dysfunction, various influenza RNA viruses are involved in the development of human atherosclerotic plaques and progression of atherosclerosis. De-stabilization of vulnerable atherosclerotic plaques triggers acute myocardial infarction (MI) or cardiovascular death [51][52]. Myocardial infarction, commonly known as heart attack, is a clinical condition, where oxygen supply to the heart is restricted and results in the irreversible loss of cardiomyocytes due to activation of cardiac apoptosis [53][54][55]. A large population of patients diagnosed with COVID-19 has died due to MI [56]. Data obtained from a laboratory in Lombardy, Italy suggest that 60.7% (17 out of 28 cases) of patients with confirmed COVID-19 and an existing condition of ST-elevation myocardial infarction (STEMI) had to undergo a repeated coronary angiogram and coronary lesion was identified as a major cause of the complication [57]. Myocardial injury was also identified as a major contributor of mortality in COVID-19 patients, as derived using data from hospitals in Wuhan, China [15]. Strikingly, the cardiac Troponin I (cTnI) level, a distinct marker of myocardial injury, was noticeably elevated in 52 patients out of 187 hospitalized patients with COVID-19 (27.8%) and the mortality was nearly 70% in these patients with elevated cTnI. Progressive increased levels of C-reactive protein and N-terminal pro-B-type natriuretic peptide (NT-proBNP) coexisted with elevated cTnI levels in these COVID-19 patients, which enhances the severity of inflammation and ventricular dysfunction.
Among 138 patients treated for COVID-19 (admitted to Zhongnan Hospital of Wuhan University during January 2020), 33 patients had either acute myocardial injury or cardiac arrhythmia, as suggested by their elevated cTnI level of 0.011 ng/ml versus 0.0051 ng/ml, for those who were treated in non-ICU [58]. Several other retrospectives multi-center cohort studies from China have also confirmed the significant elevation of biomarkers of myocardial injury over the course of COVID-19 infection, that were strongly associated with rapid surge of irreversible clinical deterioration and increased mortality [16,17,20,59]. Although limited data are available on the incidence of heart failure in patients with COVID-19, the study with 191 hospitalized patients with confirmed COVID-19 (ranging in age from 18 to 87 years) in Wuhan, China (until January 31, 2020) reported that heart failure was identified in 44 patients (23%), among them, 28 (52%) patients died and 16 (12%) patients recovered [16]. Cardiac injury, as a common complication (19.7%), was associated with an unexpected high risk of mortality during hospitalization of elderly patients with COVID-19 in Wuhan, China [17]. Evidence indicated in another retrospective study that apart from ARDS and sepsis, acute cardiac injury (77%) and heart failure (49%) were the most common critical complications of death in 113 deceased patients with COVID-19 in Wuhan, China [60]. Several other case reports also established that acute or end-stage heart failure was the main pathophysiological manifestation of COVID-19 [37,61,62], which might be associated with hyper-inflammation and oxidative stress [29][63][64].
Interestingly, one recent study indicated a decline in emergency department visits for heart failure during the COVID-19 pandemic, partly due to effective remote clinician-patient interactions [65]. Since patients with CVD are considered to be more vulnerable to SARS-CoV-2 infection, with higher risk of its negative consequences, these patients avoid frequent hospital visits and prefer alternative remote management. However, analyzing the clinical records during the COVID-19 pandemic (between February 20 and April 20, 2020) of emergency department of San Filippo Neri Hospital in Rome, Italy, a study revealed patients with acute heart failure often reported to the emergency department after significant clinical deterioration with high mortality due to failure of routine clinical assessment [66].
Emerging studies indicate that severe COVID-19-related death is associated with coagulopathy, venous thromboembolism ((VTE) and disseminated intravascular coagulation (DIC) [67]. Data obtained from the COVID-19 patient population in Wuhan, China indicate an abnormal coagulation pattern with prolonged prothrombin time [67]. There were 183 patients registered in this study and parameters such as disseminated intravascular coagulation (DIC), antithrombin activity, prothrombin time (PT) and D-dimer, a fibrin degradation product, were measured and compared between survivors and non-survivors. The results showed elevated levels of DIC and D-dimers and prolonged PT in non-survivors and suggest thrombus formation may have contributed to the mortality in these patients. This notion is strongly supported by the observation that treatment of COVID-19 patients with anti-coagulation drug heparin resulted in reduced mortality rate [68]. The 28‐day mortality study between heparin users and nonusers indicated that only selected COVID‐19 patients with markedly higher sepsis‐induced coagulopathy (SIC) score or elevated D‐dimer were benefited from the anticoagulant therapy. Notably, anticoagulant treatment may endanger those patients without significant coagulopathy, because the activation of coagulation with local thrombosis/fibrin deposition could limit the survival and dissemination of microbial pathogens and reduce their invasion [69].
2.3. Myocarditis
Myocarditis is a disease, marked by the inflammation of the heart muscle, most often due to viral infection. This inflammation interferes with the electrical system and compromises the pumping capacity of the heart and results in arrythmia and cardiac arrest [70]. Common diagnosis procedures include electrocardiogram (ECG), MRI (magnetic resonance imaging) and a manifestation of increased cardiac Troponin I (cTnI) level. COVID-19 patients with severe stage of illness manifest systemic hyperinflammation syndrome [71]. This data suggests an effect of adverse inflammatory reaction or cytokine storm in response to COVID-19 treatment and defines a strong role for ACE2 signaling in COVID-19 disease [71]. Several reports have shown that patients with COVID-19 infection are diagnosed with myocarditis [17][20][37][42]. In a case report of a 69-year-old man admitted in Lombardy, Italy with respiratory difficulties and required mechanical ventilator, with worsening heart condition. Transthoracic echocardiography showed mild left ventricle hypertrophy (LVH) with preserved left ventricular ejection fraction and normal wall motion and elevated plasma Troponin level (at 9.0 ng/ml) [59][72] . Cardiovascular MRI was suggestive of myocarditis and the patient tested positive for COVID-19 infection demonstrating SARS-CoV-2 infection was the most likely cause for the incidence of myocarditis [72]. Similarly, 53-year-old healthy woman was diagnosed with acute myopericarditis upon COVID-19 infection. Cardiac MRI showed a severe left ventricular dysfunction (Ejection fraction-35%). The patient also had myocyte necrosis with high-sensitivity cardiac Troponin T (hsTnT) level concentration of 0.24 ng/mL [37]. These reports suggest that patient with COVID-19 infection are prone to myocarditis, and physicians would suspect such conditions along with underlying morbidity factors, like hypertension and other CVD.
2.4. Myocardial Arrhythmias
Emerging clinical and epidemiological evidence suggests that metabolic disarray, hypoxia and accentuated myocardial inflammation due to SARS‐CoV‐2 infection plays a critical role in the pathophysiology of myocardial injury and prevalence of arrhythmic complications [73]. In a clinical cohort with 138 patients with COVID‐19 in Wuhan, China, cardiac arrhythmias were considered a major complication in 23 patients (16.7%) who were transferred to the intensive care unit (ICU) [20]. Specifically, cardiac arrhythmia was more common in ICU patients than in non-ICU patients (44.4% vs. 6.9%). A recent study from New York-Presbyterian/Columbia University Irving Medical Center highlighted the spectrum of life-threatening arrhythmias observed in four patients with COVID-19 infection [74]. Fulminant myocarditis with cardiogenic shock could also coexist with atrial and ventricular arrhythmias, which could increase the severity of COVID-19 patients, including death [75][76]. Therefore, the expected cardiac arrhythmogenic effect of COVID-19 may be an important underlying risk of disease complication, which needs additional precautions and specialized management.
Based on the available clinical data, potential myocardial injury is a relevant challenge among hospitalized patients with COVID-19 with increased risk of mortality; therefore, it is essential for multidisciplinary assessment, including blood pressure control in hypertensive patients as well as cardiovascular evaluation and therapy to reduce the morality for COVID-19 infection. Strikingly, a recent study in Germany involving 100 patients with an average age of 49 years who recently recovered from COVID-19 infection, recognized the cardiovascular sequelae, irrespective of preexisting cardiac conditions [77]. Cardiovascular Magnetic Resonance Imaging (CMR) revealed that 78 patients had abnormal cardiac structural changes, 76 had detectable levels of biomarker of cardiac injury, e.g., elevated level of high-sensitivity cardiac Troponin T (hsTnT), lower left ventricular ejection fraction, higher left ventricle volumes, higher left ventricle mass, and raised native T1 and T2 (quantitative assessments of the myocardium composition), commonly found after a heart attack, and 60 had signs of inflammation.
The exact molecular mechanism by which SARS-COV-2 virus leads to cardiomyocyte injury is not completely understood. However, the abundant expression of ACE-2 receptors in the heart plays an important role in the accumulation of SARS-COV-2 virus in the cardiac tissue, which eventually results in hyperactivation of inflammation and cardiac tissue injury in patients. Recently, autopsy results of 39 patients, who died early in the COVID-19 pandemic in Germany, revealed the most likely localization of SARS-CoV-2 not to be in the cardiomyocytes, but in interstitial cells or macrophages invading the myocardial tissue [78]. However, another emerging study using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) shows SARS-CoV-2 can directly enter and replicate in hiPSC-CMs and induce apoptosis, which results in cessation of cardiomyocyte beating after 72 hours of infection [79].
The majority of the COVID-19 patients suffering from cardiovascular complications show a significant elevation of cTnI, NT-proBNP and interleukin-6 (IL-6) or other cytokines [IL1B, IL1RA, IL7, IL8, IL9, IL10, C-X-C motif chemokine 10 (CXCL10), chemokine (C-C motif) ligand 2 (CCL2), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor-α (TNF-α)] in their blood stream [2][16][80]. Severe hyper-inflammation or cytokine storm due to immunological dysregulation may be the primary contributor to cardiomyocyte injury [81]. Epidemiological studies with other viral RNAs indicated that after entering into the cytoplasm of cardiomyocytes, viral RNA is further transcribed and translated into the viral structural proteins to form the complete infectious virion [82]. Ultimately, infected cardiomyocytes would be lysed, which could lead to activation of the innate immune response with induction of pro-inflammatory cytokines, inflammation-induced destabilization of coronary artery plaques and development of left ventricular dysfunction [83]. Collectively, uncontrolled hyperactivated T-lymphocytes with systemic inflammation appears to be the most common mechanisms of the cardiomyocyte injury in COVID-19 patients with profound CV consequences.
In addition to binding to ACE2 of the host cell, the priming of the transmembrane spike (S) glycoprotein of SARS-CoV-2 by host proteases (furin, a signature protease of highly pathogenic viruses) through cleavage at the S1/S2 and the S2′ sites could enhance its transmissibility and pathogenicity [84]. Multiple evidence has revealed that the Notch signaling plays a major role in maintaining the homeostasis of the cardiovascular system, including atherosclerosis progression and ventricular remodeling after myocardial infarction [85][86][87]. Furin is transcriptionally induced by Notch signaling, but Notch is cleaved at the cell membrane by ADAM10/ADAM17 to enable final cleavage by γ-secretase to form active Notch intracellular domain, which regulates the transcription of target genes in nucleus. Therefore, targeting Notch activation using inhibitor γ-secretase (GSI) could be a promising therapeutic strategy to decline the virus entry into the cardiac cells by reducing furin and increase ADAM17 shedding. The Notch signaling also modulates the activity of innate and adaptive immune responses by inducing macrophage polarization [88]. In microphages, it directly binds to IL-6 promoter in response to interferon (IFN)-γ and promotes IL-6 production, which may cause severe myocardial injury due to triggered “cytokine storm” [89]. Our current understanding on the molecular mechanisms of cardiomyocyte injury for SARS-CoV-2 infection is limited and future in depth rigorous studies are warranted.
3. Treatment Options and Cardiac Complications due to COVID-19 Treatment
Early diagnosis of COVID-19 infection in patients is crucial for the recommendation of appropriate treatment strategy and to address associated CVD complications. Initial symptoms of SARS-COV-2 infection include high fever or chills, cough, shortness of breath, headache, sore throat, new loss of taste or smell, diarrhea and fatigue which appears during 2-14 days after the exposure to the virus. These early indications though similar to regular viral infection should be taken seriously during this pandemic time and diagnosed further for the presence of COVID-19 infection. Currently, the established diagnostic test for the identification of SARS-COV-2 infection is based on Nucleic acid amplification testing (NAAT) or commonly called real-time reverse transcription- polymerase chain reaction (RT-PCR) assay, nucleic acid-based meta-genomic next-generation sequencing (mNGS), reverse transcription loop-mediated isothermal amplification (RT-LAMP) and antigen testing performed with Nasopharyngeal swab specimen [90][91].
In the absence of any pharmaceutical interventions, traditional public health measures are considered to be the mainstay of management tools to curb this worldwide COVID-19 epidemic. Most widely accepted practices are hygienic precautions, isolation and quarantine, social distancing and community containment [20][92]. To minimize cardiovascular complications in highly infectious COVID-19 patients, the patients with COVID-19 infection require routine monitoring of cardiac parameters with echocardiography, telemetry to assess QT interval and electrocardiograph (ECG) to identify the development of cardiomyopathy, arrhythmia, ischemic heart disease and heart failure.
Potential therapeutic options to impede the propagation of COVID-19 and its associated cardiovascular complication are desperately needed during this ongoing severe pandemic. Researchers and clinicians are focusing on developing new drugs against coronavirus as well as repurposing already approved drugs for the treatment of COVID-19 patients. Unapproved antiviral drugs for SARS-CoV-1 and/or Middle East respiratory syndrome coronavirus (MERS-CoV) diseases are also currently being reevaluated as treatment options for COVID-19. However, COVID-19 poses unique problems that were not encountered with the previous known viruses. The major issue was to address the CVD complications, systemic and vascular inflammation, and to deal with comorbid risk like hypertension, diabetes and heart failure. Initial approaches were to emphasis on obstructing the viral replication and inflammation by using antiviral drugs, such as, Remdesivir, liponovir/ritonavir, hydroxy chloroquine (HCQ), corticosteroids and broad-spectrum antibiotics, like Azithromycin, clarithromycin to address inflammation [93][94]. Table 1 summarized the mechanisms of action and beneficial as well as adverse effects of drug treatments for COVID-19.
Table 1. Mechanisms of action and adverse cardiac effects of drug treatments for COVID-19
|
Drug
|
Mechanism of Action |
Drug Interaction |
Side effects |
Cardiac adverse events |
|
Remdesivir (GS-5734) Antiviral drug
|
Inhibits RNA-dependent RNA polymerase. Terminates the viral RNA transcription. |
Hydroxychloroquine or chloroquine |
· Increases levels of liver enzymes, liver inflammation and liver damage.
· Reduces antiviral activity when co-administered with chloroquine or hydroxychloroquine |
Lowers blood pressure and results in cardiopulmonary failure [98][99] |
|
Lopinavir- Ritonavir Antiviral drug |
Inhibits protease enzyme activity in infected cells and reduces virus replication. Strong inhibitors of CYP3A4.
|
Antiarrhythmic Anticoagulants Antiplatelet statins |
Hepatoxicity, dyslipidemia
|
· Inhibits myocardial UPS (ubiquitin proteasome system) and leads to elevated calcineurin and connexin 43 expression that may contribute to cardiac contractile dysfunction [102][103].
· Cardio-metabolic complications.
· Bradycardia, QT and PR interval prolongation due to the interaction with cytochrome P450 enzymes [100,104,105]. |
|
Baricitinib Protein Kinase inhibitor |
Inhibitor of Janus kinase (JAK1 and JAK2) and mainly used treatment of severe rheumatoid arthritis |
Lopinavir or ritonavir and remdesivir |
Unknown |
Unknown |
Tocilizumab/ Sarilumab Humanized monoclonal antibody against IL-6-
|
Binds to membrane-bound (mIL-6R) and soluble interleukin-6 receptor (sIL-6R) and inhibits signal transduction and used as immune-suppressive drug to treat severe rheumatoid arthritis |
Unknown |
Drug interaction may impact inflammation and impairment of drug metabolism.
Hypercholesterolemia |
Hypertension |
|
Siltuximab Chimeric monoclonal antibody anti-IL-6 |
Blocks the activation of IL-6 mediated inflammation and mainly used in neoplastic cancer. |
Unknown |
Unknown |
Unknown |
|
Hydroxychloroquine (HCQ) Antiviral drug
|
· Increases lysosomal pH in antigen-presenting cells and blocks toll-like receptors during inflammation on plasmacytoid dendritic cells (PDCs). · Primarily used as antimalarial drug and in systemic lupus erythematosus and rheumatoid arthritis |
Azithromycin or Lopinavir-Ritonavir or Antiarrhythmic QT-prolonging agents
|
Showed no beneficial effects compared to placebo and could not decrease the mortality rate or hospital stay.
|
· Results in QT prolongation, QRS prolongation, bradycardia and tachycardia with increase the risk of sudden cardiac death [114].
· Ventricular arrhythmia, fibrillation and tachycardia [113][114][115] |
|
Mesenchymal stem cells (MSCs)-derived extracellular vesicles (EVs) |
Compete with virus for cellular uptake. EV could contain the small interfering RNA (siRNA) to interrupt the virus activity |
Unknown |
Unknown |
Unknown |
3.1 RNA-dependent RNA polymerase Inhibitor
Remdesivir
The antiviral drug, Remdesivir (VEKLURY, GS-5734), initially developed for Ebola, inhibits RNA-dependent RNA polymerase and prematurely terminates the viral RNA transcription and shows broad-spectrum antiviral activity against RNA viruses, including SARS-CoV-2 in vitro, and inhibits MERS-CoV, SARS-CoV-1, and SARS-CoV-2 replication in animal models [95]. Remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, as well as a substrate for organic anion transporting polypeptides 1B1 (OATP1B1) and P-glycoprotein (P-gp) transporters. Remdesivir (100 -200 mg/day for 10 days) either treated alone or in combination with anti-inflammatory drugs was effective in curbing the virus and shortening the recovery time of patients undergoing treatment for COVID-19 [96].
A multicenter randomized, double-blind, clinical trial, involving 237 patients with severe COVID-19, conducted in ten hospitals in Wuhan, China, reported that seriously ill patients, receiving Remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) within 10 days of symptom onset, showed a numerically faster time to clinical improvement than those receiving placebo, without any antiviral effect [97]. The study also reported early termination of the treatment due to multiple adverse events (including gastrointestinal symptoms, aminotransferase or bilirubin increases, and worsened cardiopulmonary status) in the Remdesivir-treated patients (66%) (Table 1). In a small pilot study of four critically ill COVID-19 patients with Remdesivir, three patients tested negative for SARS-CoV-2 RNA (swap test) after 3 days of therapy. However, these reports also indicated some adverse side effects including liver injury [98][99].
3.2 Viral Protease Inhibitors
Lopinavir-Ritonavir
Antiviral drugs such as Lopinavir- Ritonavir (Mylan or Kaletra; 400 mg and 100 mg, respectively, twice a day for 14 days), HIV protease inhibitors, used in the clinical trial provided only a moderate benefit of reducing the recovery time by 1 day [100] and in vivo animal study shows that a combination of Remdesivir with Lopinavir-Ritonavir yields better outcome for coronavirus infection [101]. However, the treatment with these protease inhibitors (Lopinavir-Ritonavir) develop cardio-metabolic complications including development of dyslipidemia with an adverse cholesterol profile, which could elicit inflammation with elevated reactive oxygen species (ROS) production, altered myocardial ubiquitin proteasome and calcium-handling pathways together with decreased contractile function [102][103] (Table 1). Lopinavir- Ritonavir treatment inhibits the myocardial UPS (ubiquitin proteasome system) and leads to elevated calcineurin and connexin 43 expression that may contribute to cardiac contractile dysfunction [103]. Without any benefit, Lopinavir-Ritonavir may also cause bradycardia, QT and PR interval prolongation due to the interaction with cytochrome P450 enzymes [100][104][105].
3.3 Attenuating Inflammation
Baricitinib (Olumiant®), an inhibitor of Janus kinase (JAK1 and JAK2) molecule and a drug for the treatment of rheumatoid arthritis was tested (2 mg or 4 mg once daily) in COVID-19 patients [106]. This drug was repurposed in COVID-19 treatment to curb the occurrence of inflammation process due to the use of ACE inhibitors, which moderately reduced the lung inflammation and cytokine [107]. The management of hyperinflammation or cytokine storm has been challenging and accounts for the majority of the mortality associated with adverse cases of COVID-19 patients. Clinical practices to address this complication involves treatment with monoclonal antibody against interleukin-6 receptor (IL-6R) such as Tocilizumab (Actrema®), Siltuximab (Sylvant®) and Sarilumab (Kevzara®) to control the infiltration of macrophages and cytokines in the respiratory system and suppression T-cell activation [108][109]. Tocilizumab specifically binds membrane-bound (mIL-6R) and soluble interleukin-6 receptor (sIL-6R) and inhibits signal transduction. COVID-19 patients treated with Tocilizumab (4 to 8 mg/kg with recommended dose of 400 mg with a maximum dose of 800 mg) in addition to routine therapy showed significant improvement of the clinical outcomes, effectively controlled body temperature with improvement of peripheral oxygen saturation and reduction of inflammatory storm [110].
Considering the emergency to identify a drug that is effective in reducing the complications associated with COVID-19, efforts are also underway to repurpose old drugs that are proven to be clinically safe. Data from RECOVERY trial indicates that dexamethasone, a steroid drug generally used as an anti-inflammatory agent, is effective in reducing the mortality rate by one-third in COVID-19 patients subjected to mechanical ventilation. or who were on ventilators compared to patients receiving standard therapy [111]. Among 6,400 registered COVID-19 patients, 2,100 of them who received 6 mg of dexamethasone for 10 days, had reduced mortality by 20% compared to 4,300 patients who were on standard treatment. More importantly, patients on ventilator support during the critical stage of treatment responded better to dexamethasone compared to patients just receiving oxygen therapy. The outcome of this study is considered a breakthrough in the fight against COVID-19 because dexamethasone is a commonly available drug and cost effective. However, further evidence is required to use dexamethasone in COVID-19 patients.
3.4 Hydroxychloroquine
Another drug that gained much attention for the treatment of COVID-19 is hydroxychloroquine (HCQ, Plaquenil), an anti-malarial compound, which is also widely used for attenuation of systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA) and Sjogren's syndrome [112]. Several clinical studies including trials from NIH (NCT04358068) are testing this drug for COVID-19 treatment, either alone or in combination with Azithromycin [113]. The treatments with hydroxychloroquine alone (400 mg by mouth twice daily for 1 day followed by 200 mg by mouth twice daily for 4 days) or in combination with azithromycin (500 mg by mouth or intravenous daily for 5 days) lead to a prolongation of the QT interval, possibly increasing the risk of sudden cardiac death [113] (Table 1). Another retrospective multicenter cohort study was conducted involving 1438 patients admitted across various hospitals in the city of New York who were diagnosed with COVID-19 (between March 15-28, 2020), those receiving either HCQ alone (dose ranges: 200 mg- 600 mg; once or twice a day) or in combination with Azithromycin (dose ranges: 200 mg to 500 mg; once or twice a day) or Azithromycin alone. The results from the study showed that the probability of death for patients receiving HCQ + Azithromycin was 25.7% (189 out of 735), while patients receiving HCQ alone was 19.9% (54 out of 271) and 10.0% (21 out of 211) in Azithromycin alone group. Cardiac arrest was significantly high in patients receiving HCQ + Azithromycin combination than treatment with placebo or HCQ alone [114]. Another cohort study performed at an academic tertiary care center in Boston, Massachusetts, showed similar high risk of QT prolongation with subsequently developed other ventricular arrhythmias in the HCQ alone (400 mg, twice on day 1, then 400 mg daily on days 2 through 5) or with Azithromycin-treated patients with COVID-19 [115]. An observational study of 1446 admitted patients to the hospital with COVID-19 (between March 7 and April 8, 2020) in New York, revealed that HCQ administration alone was not associated with either a greatly lowered or an increased risk of the composite end point of intubation or death [116]. The treatment regimen of hydroxychloroquine was a loading dose of 600 mg twice on day 1, followed by 400 mg daily for 4 additional days. However, recently the FDA revoked its approval to use HCQ for COVID-19 treatment due to disappointing results [117] (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and). Data from randomized clinical trials suggest that HCQ had no beneficial effects compared to placebo and was not successful in decreasing the mortality rate or in hospital stay (Based on FDA update on July 1, 2020; [118] https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or). Therefore, rigorous, and large-scale studies with careful risk assessment of HCQ should be conducted prior to initiating COVID-19 therapeutics, with close monitoring cardiac manifestations including evaluation of cardiac biomarkers, routine electrocardiograms and electrolyte monitoring.
3.5 Vaccine Development
There is an urgency for the development of a safe and effective vaccine for COVID-19, however, no specific vaccines against SAR-CoV-2 are currently available [16]. Multiple inactivated vaccines candidate for SARS-CoV-2, such as DNA-, RNA-based formulations, recombinant-subunits containing viral epitopes, adenovirus-based vectors and purified inactivated virus are under development [119][120]. Several candidate vaccines are still in the preliminary stage of Phase I clinical trial. The mRNA based vaccine prepared by the USA National Institute of Allergy and Infectious Diseases against SARS-CoV-2 is under Phase I trial [121] (https://www.wsj.com/articles/drugmakers-rush-to-develop-vaccines-against-china-virus-11579813026). INO-4800, a DNA-based vaccine, is also in pipeline and will soon be available for human trial. Preliminary results from pilot studies and clinical trials on new vaccine are encouraging and gives hope for a successful availability of an effective vaccine by end of 2020. Several pharmaceutical companies including Pfizer, Novartis and AstraZeneca and Moderna are testing their candidate vaccine. University of Oxford in collaboration with AstraZeneca are in the development of COVID-19 vaccine and expect to produce 30 million doses in UK by September 2020. Jenner Institute, Oxford, UK is a leader in this effort and launched a Phase III clinical trial of more than 6,000 people in May. However, due to the suspected adverse event in a person receiving the vaccine in the United Kingdom, the clinical trials have been temporarily paused.
Moderna, a USA-based company in collaboration with Switzerland’s Lonza, released positive outcomes from its Phase I clinical trial of their mRNA1273 vaccine for SARS-CoV-2 [122]. Preliminary results are very promising, showing good immune response and due to effectiveness and safety profiles, this vaccine is approved by the U.S. Food and Drug Administration (FDA) for Phase II and Phase III studies [123][124] (https://www.modernatx.com/modernas-work-potential-vaccine-against-covid-19). Novartis announced its plans to initiate a Phase III clinical trial to study effects of canakinumab, an interleukin (IL)-1β blocker, in COVID-19 patients with pneumonia [125]. They aim to rapidly enroll 450 patients at multiple medical centers across France, Germany, Italy, Spain, UK and the USA and randomize them to receive either canakinumab or placebo on top of standard of care (SoC) [126] (https://www.novartis.com/news/novartis-announces-plan-initiate-clinical-trial-canakinumab-patients-covid-19-pneumonia). Pfizer, in partnership with BIONTECH (BNT) has initiated its Phase I/II clinical trial in the USA for its mRNA-based vaccine, the BNT162 prevent COVID-19 [127] (https://www.businesswire.com/news/home/20200505005474/en/). Sinopharm, a Wuhan, China-based pharmaceutical company received approval from the National Medical Products Administration (China) and conducting Phase II clinical trials for its inactivated vaccine BBIBP-CorV. The company already tested 2000 doses of this vaccine and expect to release in the marker by the end of the year 2020. Sinovac is planning to enter its Phase III clinical trial in collaboration with Instituto Butantan in Brazil after observing positive results in its preclinical trail with the vaccine CoronaVac [120]. Ad5-nCoV, an adenovirus type 5 vector-based vaccine developed by Cansiobiologics, China is also in Phase II clinical trial and demonstrated promising effects in the early phase of testing on 108 participants [128]. Inovio pharmaceuticals in collaboration with University of Pennsylvania and Center for Pharmaceutical Research, Kansas City, Missouri, is testing its DNA-based vaccine INO-4800 [129]. Preclinical experiments conducted in guinea pigs showed antibody titer against ACE2 receptor/SARS-Cov2 binding protein.
When countries all over the world are racing to develop their own vaccine against COVID-19, Russia has already approved a vaccine candidate for public use named Sputnik V, that was developed in collaboration with Gamaleya Research Institute of Epidemiology and Microbiology in Moscow [130]. The vaccines comprise either recombinant adenovirus type 26 (rAd26) or recombinant adenovirus type 5 (rAd5) vectors, which contain the gene for SARS-CoV-2 spike glycoprotein (rAd26-S and rAd5-S). Initial results from the ongoing Phase I and II clinical trials are promising, which include total population size of 76 healthy adult volunteers [131]. Among them 38 volunteers were intramuscularly vaccinated with Gam-COVID-Vac Lyo (lyophilized vaccine formulation) and other 38 participants were subjected to Gam-COVID-Vac (frozen vaccine formulation) [131]. Both heterologous recombinant adenoviral (rAd26 and rAd5) vector-based COVID-19 vaccines induced a strong humoral and cellular immune responses with reported safety profiles in participants. However, further investigations with larger scale population (including different underlying medical complications) are needed to demonstrate the effectiveness of this vaccine for prevention of COVID-19. Nevertheless, scientists globally have serious concerns about unforeseen adverse effects of this vaccine without the outcomes of the Phase III trial. Even though, for the development of an efficient vaccine for COVID-19, extensive preclinical studies and clinical trials are essential to carefully evaluate the adverse effect of vaccine, the aforementioned fast-paced preclinical data are encouraging for advancing the preventive strategies against COVID-19.
3.6 Antibody therapy
Several other treatment options such as convalescent plasma therapy (CPT) and monoclonal antibody therapy have been evaluated with some moderate success. CPT is a traditional method where plasma containing the antibody from recovered patients infected with COVID-19 was transfused to the severely ill COVID-19 patients [6][132][133][134]. Studies showed that CP therapy was effective, and the level of neutralizing increased as high as 1:640 times in patients infected with SARS-CoV-2 [6]. Transfusing antibodies from COVID-19 survivors into high-risk patients to neutralized SARS-CoV-2 could provide a quick treatment option until an optimistic vaccine will arrive to prevent this viral infection. Efforts are also underway to design a monoclonal antibody that can target the specific epitope on the spike protein of SARS-CoV-2 and block the virus entry in to the host cell [84][135][136]. Such efforts are still in their preliminary stage [137] and are time consuming, however, they could provide a long-lasting solution for dealing with SARS viruses in general.
3.7 Stem Cell Therapy
Recently, stem cell therapies with secreted extracellular vesicles (EVs) offer a potential therapeutic benefit in COVID-19 patients by attenuating inflammation with regeneration of damaged lung. Mesenchymal stem cells (MSCs)-derived EVs-based therapy could be the most promising reparative strategy in people with COVID-19, because of its high proliferation rate, low invasive nature, and the immunomodulatory, antioxidant and anti-inflammatory properties of MSCs [138]. There are several promising clinical trials with MSC-derived EVs underway (https://clinicaltrials.gov), which could reveal convincing evidence in the encouraging prospect of MSC-based therapies for respiratory complications of COVID-19 patients [139][140].
Despite the above-mentioned beneficial effects of different therapeutics, the safety profiles of these therapies have not been proficiently identified. Specifically, the potential adverse cardiovascular effects of these drugs in COVID-19 patients need urgent attention before rushing the approval of any new drug into clinical application. For most effective treatments for COVID-19, it is important to pay attention to emerging evidence about potential harmful risk of drug interactions.
4. Conclusion
Due to the highly transmissible coronavirus, SARS-CoV-2, new advanced technological tools, like information technology, based on smart phone apps, social media, artificial intelligence (AI), machine learning, etc. accelerates the diagnosis/screening of patients with virus, analysis of available literature, and identification of potential therapeutic targets and other specific clinical features to tackling worldwide COVID-19 pandemic. In the context of disease progression with cardiovascular complications, the researchers are focusing on developing new drugs in parallel to repurposing already clinically approved drugs to avoid a massive surge of COVID-19 patients with a prevalence of cardiovascular complications. Recent therapies under investigation for severe multi-organ failure in COVID-19 patients may have adverse cardiovascular effects, while their clinical efficacy is yet to be established to combating COVID-19. Therefore, urgent understanding of molecular mechanism as well as retrospective and prospective studies with robust diagnosis of cardiovascular impairments will be crucial for development of advanced therapies for the treatment of SARS-CoV-2 virus, which could mitigate the adverse cardiovascular events among COVID-19 patients and save humankind around the globe from this deadly pandemic. Artificial intelligence (AI) particularly plays an important role in predicting the harmful interaction between cardiovascular consequences with the drugs used for COVID-19, by automated interpretation of collected meta-data from various sources.
Abbreviations
|
ACE2 ACEi AI ARBs ARDS AT1R AT2R COVID-19 cTnI CVD hsTnT ORF MERS MODS MRAs RAAS SARS- CoV-2 TMPRSS2 |
Angiotensin-Converting Enzyme 2 ACE inhibitor Artificial Intelligence Angiotensin II type-I receptor blockers Acute respiratory syndrome Angiotensin Type 1 Receptor Angiotensin Type 2 Receptor Coronavirus disease 2019 Cardiac Troponin I Cardiovascular Diseases High sensitive cardiac troponin T Open Reading Frames Middle East respiratory syndrome Multiple organ dysfunction syndrome Mineralocorticoid receptor antagonists Renin-angiotensin-aldosterone system Severe Acute Respiratory Syndrome-Coronavirus-2 Transmembrane Serine Protease 2
|
References
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect 2020, 10.1016/j.jmii.2020.03.022. doi:10.1016/j.jmii.2020.03.022.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; Cheng, Z.; Yu, T.; Xia, J.; Wei, Y.; Wu, W.; Xie, X.; Yin, W.; Li, H.; Liu, M.; Xiao, Y.; Gao, H.; Guo, L.; Xie, J.; Wang, G.; Jiang, R.; Gao, Z.; Jin, Q.; Wang, J.; Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497-506. doi:10.1016/S0140-6736(20)30183-5.
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol 2020, 92, 418-423. doi:10.1002/jmv.25681.
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.Y.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020, 10.1001/jama.2020.3227. doi:10.1001/jama.2020.3227.
- Perlman, S.; Netland, J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol 2009, 7, 439-450. doi:10.1038/nrmicro2147.
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; Peng, C.; Yuan, M.; Huang, J.; Wang, Z.; Yu, J.; Gao, X.; Wang, D.; Yu, X.; Li, L.; Zhang, J.; Wu, X.; Li, B.; Xu, Y.; Chen, W.; Peng, Y.; Hu, Y.; Lin, L.; Liu, X.; Huang, S.; Zhou, Z.; Zhang, L.; Wang, Y.; Zhang, Z.; Deng, K.; Xia, Z.; Gong, Q.; Zhang, W.; Zheng, X.; Liu, Y.; Yang, H.; Zhou, D.; Yu, D.; Hou, J.; Shi, Z.; Chen, S.; Chen, Z.; Zhang, X.; Yang, X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 2020, 117, 9490-9496. doi:10.1073/pnas.2004168117.
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020, 12, 8. doi:10.1038/s41368-020-0074-x.
- Gemmati, D.; Bramanti, B.; Serino, M.L.; Secchiero, P.; Zauli, G.; Tisato, V. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int J Mol Sci 2020, 21. doi:10.3390/ijms21103474.
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; Li, W.W.; Li, V.W.; Mentzer, S.J.; Jonigk, D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020, 383, 120-128. doi:10.1056/NEJMoa2015432.
- Collaborative, C.O. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020, 396, 27-38. doi:10.1016/S0140-6736(20)31182-X.
- Ullah, W.; Saeed, R.; Sarwar, U.; Patel, R.; Fischman, D.L. COVID-19 Complicated by Acute Pulmonary Embolism and Right-Sided Heart Failure. JACC Case Rep 2020, 2, 1379-1382. doi:10.1016/j.jaccas.2020.04.008.
- Guo, J.; Huang, Z.; Lin, L.; Lv, J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc 2020, 9, e016219. doi:10.1161/JAHA.120.016219.
- Turner, A.J.; Hiscox, J.A.; Hooper, N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci 2004, 25, 291-294. doi:10.1016/j.tips.2004.04.001.
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; and the Northwell, C.-R.C.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; Cookingham, J.; Coppa, K.; Diefenbach, M.A.; Dominello, A.J.; Duer-Hefele, J.; Falzon, L.; Gitlin, J.; Hajizadeh, N.; Harvin, T.G.; Hirschwerk, D.A.; Kim, E.J.; Kozel, Z.M.; Marrast, L.M.; Mogavero, J.N.; Osorio, G.A.; Qiu, M.; Zanos, T.P. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 10.1001/jama.2020.6775. doi:10.1001/jama.2020.6775.
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020, 10.1001/jamacardio.2020.1017. doi:10.1001/jamacardio.2020.1017.
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; Guan, L.; Wei, Y.; Li, H.; Wu, X.; Xu, J.; Tu, S.; Zhang, Y.; Chen, H.; Cao, B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395, 1054-1062. doi:10.1016/S0140-6736(20)30566-3.
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; Huang, H.; Yang, B.; Huang, C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020, 10.1001/jamacardio.2020.0950. doi:10.1001/jamacardio.2020.0950.
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020, 46, 846-848. doi:10.1007/s00134-020-05991-x.
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 10.1001/jama.2020.4326. doi:10.1001/jama.2020.4326.
- Wang, C.J.; Ng, C.Y.; Brook, R.H. Response to COVID-19 in Taiwan: Big Data Analytics, New Technology, and Proactive Testing. JAMA 2020, 10.1001/jama.2020.3151. doi:10.1001/jama.2020.3151.
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med 2020, 382, 1653-1659. doi:10.1056/NEJMsr2005760.
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; Du, B.; Li, L.J.; Zeng, G.; Yuen, K.Y.; Chen, R.C.; Tang, C.L.; Wang, T.; Chen, P.Y.; Xiang, J.; Li, S.Y.; Wang, J.L.; Liang, Z.J.; Peng, Y.X.; Wei, L.; Liu, Y.; Hu, Y.H.; Peng, P.; Wang, J.M.; Liu, J.Y.; Chen, Z.; Li, G.; Zheng, Z.J.; Qiu, S.Q.; Luo, J.; Ye, C.J.; Zhu, S.Y.; Zhong, N.S.; China Medical Treatment Expert Group for, C. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020, 382, 1708-1720. doi:10.1056/NEJMoa2002032.
- Centers for Disease Control and Prevention, N.C.f.H.S. Weekly Updates by Select Demographic and Geographic Characteristics. 2020, https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm#Comorbidities.
- Dostal, D.E.; Baker, K.M. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res 1999, 85, 643-650. doi:10.1161/01.res.85.7.643.
- Zores, F.; Rebeaud, M.E. COVID and the Renin-Angiotensin System: Are Hypertension or Its Treatments Deleterious? Front Cardiovasc Med 2020, 7, 71. doi:10.3389/fcvm.2020.00071.
- Olkowicz, M.; Chlopicki, S.; Smolenski, R.T. Perspectives for angiotensin profiling with liquid chromatography/mass spectrometry to evaluate ACE/ACE2 balance in endothelial dysfunction and vascular pathologies. Pharmacol Rep 2015, 67, 778-785. doi:10.1016/j.pharep.2015.03.017.
- Fraga-Silva, R.A.; Sorg, B.S.; Wankhede, M.; Dedeugd, C.; Jun, J.Y.; Baker, M.B.; Li, Y.; Castellano, R.K.; Katovich, M.J.; Raizada, M.K.; Ferreira, A.J. ACE2 activation promotes antithrombotic activity. Mol Med 2010, 16, 210-215. doi:10.2119/molmed.2009.00160.
- Epelman, S.; Tang, W.H.; Chen, S.Y.; Van Lente, F.; Francis, G.S.; Sen, S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol 2008, 52, 750-754. doi:10.1016/j.jacc.2008.02.088.
- Tomasoni, D.; Italia, L.; Adamo, M.; Inciardi, R.M.; Lombardi, C.M.; Solomon, S.D.; Metra, M. COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail 2020, 22, 957-966. doi:10.1002/ejhf.1871.
- Basu, R.; Poglitsch, M.; Yogasundaram, H.; Thomas, J.; Rowe, B.H.; Oudit, G.Y. Roles of Angiotensin Peptides and Recombinant Human ACE2 in Heart Failure. J Am Coll Cardiol 2017, 69, 805-819. doi:10.1016/j.jacc.2016.11.064.
- Oudit, G.Y.; Pfeffer, M.A. Plasma angiotensin-converting enzyme 2: novel biomarker in heart failure with implications for COVID-19. Eur Heart J 2020, 41, 1818-1820. doi:10.1093/eurheartj/ehaa414.
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020, 46, 586-590. doi:10.1007/s00134-020-05985-9.
- Khan, A.; Benthin, C.; Zeno, B.; Albertson, T.E.; Boyd, J.; Christie, J.D.; Hall, R.; Poirier, G.; Ronco, J.J.; Tidswell, M.; Hardes, K.; Powley, W.M.; Wright, T.J.; Siederer, S.K.; Fairman, D.A.; Lipson, D.A.; Bayliffe, A.I.; Lazaar, A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care 2017, 21, 234. doi:10.1186/s13054-017-1823-x.
- Batlle, D.; Wysocki, J.; Satchell, K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 2020, 134, 543-545. doi:10.1042/CS20200163.
- Sama, I.E.; Ravera, A.; Santema, B.T.; van Goor, H.; Ter Maaten, J.M.; Cleland, J.G.F.; Rienstra, M.; Friedrich, A.W.; Samani, N.J.; Ng, L.L.; Dickstein, K.; Lang, C.C.; Filippatos, G.; Anker, S.D.; Ponikowski, P.; Metra, M.; van Veldhuisen, D.J.; Voors, A.A. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 2020, 41, 1810-1817. doi:10.1093/eurheartj/ehaa373.
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y.; Song, J.; Wang, S.; Chao, Y.; Yang, Z.; Xu, J.; Zhou, X.; Chen, D.; Xiong, W.; Xu, L.; Zhou, F.; Jiang, J.; Bai, C.; Zheng, J.; Song, Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020, 10.1001/jamainternmed.2020.0994. doi:10.1001/jamainternmed.2020.0994.
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; Maroldi, R.; Adamo, M.; Ammirati, E.; Sinagra, G.; Lombardi, C.M.; Metra, M. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020, 10.1001/jamacardio.2020.1096. doi:10.1001/jamacardio.2020.1096.
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 10.1001/jama.2020.2648. doi:10.1001/jama.2020.2648.
- Epidemiology Working Group for Ncip Epidemic Response, C.C.f.D.C.; Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020, 41, 145-151. doi:10.3760/cma.j.issn.0254-6450.2020.02.003.
- Li, J.; Wang, X.; Chen, J.; Zhang, H.; Deng, A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol 2020, 10.1001/jamacardio.2020.1624. doi:10.1001/jamacardio.2020.1624.
- Krittanawong, C.; Virk, H.U.H.; Narasimhan, B.; Wang, Z.; Narasimhan, H.; Zhang, H.J.; Sun, T.; Messerli, F.H. Coronavirus disease 2019 (COVID-19) and cardiovascular risk: A meta-analysis. Prog Cardiovasc Dis 2020, 10.1016/j.pcad.2020.05.001. doi:10.1016/j.pcad.2020.05.001.
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; Iotti, G.; Latronico, N.; Lorini, L.; Merler, S.; Natalini, G.; Piatti, A.; Ranieri, M.V.; Scandroglio, A.M.; Storti, E.; Cecconi, M.; Pesenti, A.; Network, C.-L.I.; Nailescu, A.; Corona, A.; Zangrillo, A.; Protti, A.; Albertin, A.; Forastieri Molinari, A.; Lombardo, A.; Pezzi, A.; Benini, A.; Scandroglio, A.M.; Malara, A.; Castelli, A.; Coluccello, A.; Micucci, A.; Pesenti, A.; Sala, A.; Alborghetti, A.; Antonini, B.; Capra, C.; Troiano, C.; Roscitano, C.; Radrizzani, D.; Chiumello, D.; Coppini, D.; Guzzon, D.; Costantini, E.; Malpetti, E.; Zoia, E.; Catena, E.; Agosteo, E.; Barbara, E.; Beretta, E.; Boselli, E.; Storti, E.; Harizay, F.; Della Mura, F.; Lorini, F.L.; Donato Sigurta, F.; Marino, F.; Mojoli, F.; Rasulo, F.; Grasselli, G.; Casella, G.; De Filippi, G.; Castelli, G.; Aldegheri, G.; Gallioli, G.; Lotti, G.; Albano, G.; Landoni, G.; Marino, G.; Vitale, G.; Battista Perego, G.; Evasi, G.; Citerio, G.; Foti, G.; Natalini, G.; Merli, G.; Sforzini, I.; Bianciardi, L.; Carnevale, L.; Grazioli, L.; Cabrini, L.; Guatteri, L.; Salvi, L.; Dei Poli, M.; Galletti, M.; Gemma, M.; Ranucci, M.; Riccio, M.; Borelli, M.; Zambon, M.; Subert, M.; Cecconi, M.; Mazzoni, M.G.; Raimondi, M.; Panigada, M.; Belliato, M.; Bronzini, N.; Latronico, N.; Petrucci, N.; Belgiorno, N.; Tagliabue, P.; Cortellazzi, P.; Gnesin, P.; Grosso, P.; Gritti, P.; Perazzo, P.; Severgnini, P.; Ruggeri, P.; Sebastiano, P.; Covello, R.D.; Fernandez-Olmos, R.; Fumagalli, R.; Keim, R.; Rona, R.; Valsecchi, R.; Cattaneo, S.; Colombo, S.; Cirri, S.; Bonazzi, S.; Greco, S.; Muttini, S.; Langer, T.; Alaimo, V.; Viola, U. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 10.1001/jama.2020.5394. doi:10.1001/jama.2020.5394.
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020, 8, e21. doi:10.1016/S2213-2600(20)30116-8.
- Sun, M.L.; Yang, J.M.; Sun, Y.P.; Su, G.H. [Inhibitors of RAS Might Be a Good Choice for the Therapy of COVID-19 Pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, 219-222. doi:10.3760/cma.j.issn.1001-0939.2020.03.016.
- Furuhashi, M.; Moniwa, N.; Mita, T.; Fuseya, T.; Ishimura, S.; Ohno, K.; Shibata, S.; Tanaka, M.; Watanabe, Y.; Akasaka, H.; Ohnishi, H.; Yoshida, H.; Takizawa, H.; Saitoh, S.; Ura, N.; Shimamoto, K.; Miura, T. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens 2015, 28, 15-21. doi:10.1093/ajh/hpu086.
- Mancia, G.; Rea, F.; Ludergnani, M.; Apolone, G.; Corrao, G. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med 2020, 382, 2431-2440. doi:10.1056/NEJMoa2006923.
- Jarcho, J.A.; Ingelfinger, J.R.; Hamel, M.B.; D'Agostino, R.B., Sr.; Harrington, D.P. Inhibitors of the Renin-Angiotensin-Aldosterone System and Covid-19. N Engl J Med 2020, 382, 2462-2464. doi:10.1056/NEJMe2012924.
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; Katz, S.D.; Fishman, G.I.; Kunichoff, D.; Chen, Y.; Ogedegbe, G.; Hochman, J.S. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N Engl J Med 2020, 382, 2441-2448. doi:10.1056/NEJMoa2008975.
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.M.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009, 39, 618-625. doi:10.1111/j.1365-2362.2009.02153.x.
- Mehra, M.R.; Ruschitzka, F. COVID-19 Illness and Heart Failure: A Missing Link? JACC Heart Fail 2020, 8, 512-514. doi:10.1016/j.jchf.2020.03.004.
- Warren-Gash, C.; Smeeth, L.; Hayward, A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis 2009, 9, 601-610. doi:10.1016/S1473-3099(09)70233-6.
- Nguyen, J.L.; Yang, W.; Ito, K.; Matte, T.D.; Shaman, J.; Kinney, P.L. Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiol 2016, 1, 274-281. doi:10.1001/jamacardio.2016.0433.
- Das, A.; Smolenski, A.; Lohmann, S.M.; Kukreja, R.C. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem 2006, 281, 38644-38652. doi:10.1074/jbc.M606142200.
- Saraste, A.; Pulkki, K.; Kallajoki, M.; Henriksen, K.; Parvinen, M.; Voipio-Pulkki, L.M. Apoptosis in human acute myocardial infarction. Circulation 1997, 95, 320-323. doi:10.1161/01.cir.95.2.320.
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J 2017, 38, 774-784. doi:10.1093/eurheartj/ehw224.
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; Endeman, H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020, 191, 145-147. doi:10.1016/j.thromres.2020.04.013.
- Stefanini, G.G.; Montorfano, M.; Trabattoni, D.; Andreini, D.; Ferrante, G.; Ancona, M.; Metra, M.; Curello, S.; Maffeo, D.; Pero, G.; Cacucci, M.; Assanelli, E.; Bellini, B.; Russo, F.; Ielasi, A.; Tespili, M.; Danzi, G.B.; Vandoni, P.; Bollati, M.; Barbieri, L.; Oreglia, J.; Lettieri, C.; Cremonesi, A.; Carugo, S.; Reimers, B.; Condorelli, G.; Chieffo, A. ST-Elevation Myocardial Infarction in Patients With COVID-19: Clinical and Angiographic Outcomes. Circulation 2020, 141, 2113-2116. doi:10.1161/CIRCULATIONAHA.120.047525.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; Zhao, Y.; Li, Y.; Wang, X.; Peng, Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 10.1001/jama.2020.1585. doi:10.1001/jama.2020.1585.
- Tersalvi, G.; Vicenzi, M.; Calabretta, D.; Biasco, L.; Pedrazzini, G.; Winterton, D. Elevated Troponin in Patients With Coronavirus Disease 2019: Possible Mechanisms. J Card Fail 2020, 26, 470-475. doi:10.1016/j.cardfail.2020.04.009.
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; Wang, T.; Guo, W.; Chen, J.; Ding, C.; Zhang, X.; Huang, J.; Han, M.; Li, S.; Luo, X.; Zhao, J.; Ning, Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020, 368, m1091. doi:10.1136/bmj.m1091.
- Dong, N.; Cai, J.; Zhou, Y.; Liu, J.; Li, F. End-Stage Heart Failure With COVID-19: Strong Evidence of Myocardial Injury by 2019-nCoV. JACC Heart Fail 2020, 8, 515-517. doi:10.1016/j.jchf.2020.04.001.
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; Baldanti, F.; Paolucci, S.; Pelenghi, S.; Iotti, G.A.; Mojoli, F.; Arbustini, E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020, 22, 911-915. doi:10.1002/ejhf.1828.
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Devalaraja, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; van der Harst, P.; Lang, C.C.; Metra, M.; Ng, L.L.; Ponikowski, P.; Samani, N.J.; Zannad, F.; Zwinderman, A.H.; Hillege, H.L.; van Veldhuisen, D.J.; Kakkar, R.; Voors, A.A.; van der Meer, P. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail 2019, 21, 965-973. doi:10.1002/ejhf.1482.
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail 2019, 21, 425-435. doi:10.1002/ejhf.1320.
- Almufleh, A.; Ahluwalia, M.; Givertz, M.M.; Weintraub, J.; Young, M.; Cooper, I.; Shea, E.L.; Mehra, M.R.; Desai, A.S. Short-term Outcomes in Ambulatory Heart Failure during the COVID-19 Pandemic: Insights from Pulmonary Artery Pressure Monitoring. J Card Fail 2020, 26, 633-634. doi:10.1016/j.cardfail.2020.05.021.
- Colivicchi, F.; Di Fusco, S.A.; Magnanti, M.; Cipriani, M.; Imperoli, G. The Impact of the Coronavirus Disease-2019 Pandemic and Italian Lockdown Measures on Clinical Presentation and Management of Acute Heart Failure. J Card Fail 2020, 26, 464-465. doi:10.1016/j.cardfail.2020.05.007.
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020, 18, 844-847. doi:10.1111/jth.14768.
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020, 18, 1094-1099. doi:10.1111/jth.14817.
- Sun, H.; Wang, X.; Degen, J.L.; Ginsburg, D. Reduced thrombin generation increases host susceptibility to group A streptococcal infection. Blood 2009, 113, 1358-1364. doi:10.1182/blood-2008-07-170506.
- Cooper, L.T., Jr. Myocarditis. N Engl J Med 2009, 360, 1526-1538. doi:10.1056/NEJMra0800028.
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant 2020, 39, 405-407. doi:10.1016/j.healun.2020.03.012.
- Doyen, D.; Moceri, P.; Ducreux, D.; Dellamonica, J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet 2020, 395, 1516. doi:10.1016/S0140-6736(20)30912-0.
- Kochi, A.N.; Tagliari, A.P.; Forleo, G.B.; Fassini, G.M.; Tondo, C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol 2020, 31, 1003-1008. doi:10.1111/jce.14479.
- Kochav, S.M.; Coromilas, E.; Nalbandian, A.; Ranard, L.S.; Gupta, A.; Chung, M.K.; Gopinathannair, R.; Biviano, A.B.; Garan, H.; Wan, E.Y. Cardiac Arrhythmias in COVID-19 Infection. Circ Arrhythm Electrophysiol 2020, 13, e008719. doi:10.1161/CIRCEP.120.008719.
- Lakkireddy, D.R.; Chung, M.K.; Gopinathannair, R.; Patton, K.K.; Gluckman, T.J.; Turagam, M.; Cheung, J.; Patel, P.; Sotomonte, J.; Lampert, R.; Han, J.K.; Rajagopalan, B.; Eckhardt, L.; Joglar, J.; Sandau, K.; Olshansky, B.; Wan, E.; Noseworthy, P.A.; Leal, M.; Kaufman, E.; Gutierrez, A.; Marine, J.E.; Wang, P.J.; Russo, A.M. Guidance for Cardiac Electrophysiology During the COVID-19 Pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation 2020, 141, e823-e831. doi:10.1161/CIRCULATIONAHA.120.047063.
- Hu, H.; Ma, F.; Wei, X.; Fang, Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020, 10.1093/eurheartj/ehaa190. doi:10.1093/eurheartj/ehaa190.
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; Vehreschild, M.; Nagel, E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020, 10.1001/jamacardio.2020.3557. doi:10.1001/jamacardio.2020.3557.
- Lindner, D.; Fitzek, A.; Brauninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P.; Blankenberg, S.; Puschel, K.; Westermann, D. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol 2020, 10.1001/jamacardio.2020.3551. doi:10.1001/jamacardio.2020.3551.
- Sharma, A.; Garcia, G.; Arumugaswami, V.; Svendsen, C.N. Human iPSC-Derived Cardiomyocytes are Susceptible to SARS-CoV-2 Infection. bioRxiv 2020, 10.1101/2020.04.21.051912. doi:10.1101/2020.04.21.051912.
- Chen, C.; Zhou, Y.; Wang, D.W. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 2020, 45, 230-232. doi:10.1007/s00059-020-04909-z.
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020, 17, 259-260. doi:10.1038/s41569-020-0360-5.
- Blyszczuk, P. Myocarditis in Humans and in Experimental Animal Models. Front Cardiovasc Med 2019, 6, 64. doi:10.3389/fcvm.2019.00064.
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D'Acquisto, F.; Nicklin, S.A.; Marian, A.J.; Nosalski, R.; Murray, E.C.; Guzik, B.; Berry, C.; Touyz, R.M.; Kreutz, R.; Wang, D.W.; Bhella, D.; Sagliocco, O.; Crea, F.; Thomson, E.C.; McInnes, I.B. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020, 116, 1666-1687. doi:10.1093/cvr/cvaa106.
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281-292 e286. doi:10.1016/j.cell.2020.02.058.
- Fortini, F.; Vieceli Dalla Sega, F.; Caliceti, C.; Lambertini, E.; Pannuti, A.; Peiffer, D.S.; Balla, C.; Rizzo, P. Estrogen-mediated protection against coronary heart disease: The role of the Notch pathway. J Steroid Biochem Mol Biol 2019, 189, 87-100. doi:10.1016/j.jsbmb.2019.02.008.
- Ferrari, R.; Rizzo, P. The Notch pathway: a novel target for myocardial remodelling therapy? Eur Heart J 2014, 35, 2140-2145. doi:10.1093/eurheartj/ehu244.
- Gude, N.; Joyo, E.; Toko, H.; Quijada, P.; Villanueva, M.; Hariharan, N.; Sacchi, V.; Truffa, S.; Joyo, A.; Voelkers, M.; Alvarez, R.; Sussman, M.A. Notch activation enhances lineage commitment and protective signaling in cardiac progenitor cells. Basic Res Cardiol 2015, 110, 29. doi:10.1007/s00395-015-0488-3.
- Wongchana, W.; Palaga, T. Direct regulation of interleukin-6 expression by Notch signaling in macrophages. Cell Mol Immunol 2012, 9, 155-162. doi:10.1038/cmi.2011.36.
- Rizzo, P.; Vieceli Dalla Sega, F.; Fortini, F.; Marracino, L.; Rapezzi, C.; Ferrari, R. COVID-19 in the heart and the lungs: could we "Notch" the inflammatory storm? Basic Res Cardiol 2020, 115, 31. doi:10.1007/s00395-020-0791-5.
- Guo, G.; Ye, L.; Pan, K.; Chen, Y.; Xing, D.; Yan, K.; Chen, Z.; Ding, N.; Li, W.; Huang, H.; Zhang, L.; Li, X.; Xue, X. New Insights of Emerging SARS-CoV-2: Epidemiology, Etiology, Clinical Features, Clinical Treatment, and Prevention. Front Cell Dev Biol 2020, 8, 410. doi:10.3389/fcell.2020.00410.
- Patel, A.; Jernigan, D.B.; nCo, V.C.D.C.R.T. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak - United States, December 31, 2019-February 4, 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 140-146. doi:10.15585/mmwr.mm6905e1.
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; Brodie, D.; Beckman, J.A.; Kirtane, A.J.; Stone, G.W.; Krumholz, H.M.; Parikh, S.A. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol 2020, 75, 2352-2371. doi:10.1016/j.jacc.2020.03.031.
- Ng, C.S.; Kasumba, D.M.; Fujita, T.; Luo, H. Spatio-temporal characterization of the antiviral activity of the XRN1-DCP1/2 aggregation against cytoplasmic RNA viruses to prevent cell death. Cell Death Differ 2020, 27, 2363-2382. doi:10.1038/s41418-020-0509-0.
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020, 30, 269-271. doi:10.1038/s41422-020-0282-0.
- Gordon, C.J.; Tchesnokov, E.P.; Woolner, E.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; Gotte, M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 2020, 295, 6785-6797. doi:10.1074/jbc.RA120.013679.
- Ledford, H. Hopes rise for coronavirus drug remdesivir. Nature 2020, 10.1038/d41586-020-01295-8. doi:10.1038/d41586-020-01295-8.
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; Hu, Y.; Luo, G.; Wang, K.; Lu, Y.; Li, H.; Wang, S.; Ruan, S.; Yang, C.; Mei, C.; Wang, Y.; Ding, D.; Wu, F.; Tang, X.; Ye, X.; Ye, Y.; Liu, B.; Yang, J.; Yin, W.; Wang, A.; Fan, G.; Zhou, F.; Liu, Z.; Gu, X.; Xu, J.; Shang, L.; Zhang, Y.; Cao, L.; Guo, T.; Wan, Y.; Qin, H.; Jiang, Y.; Jaki, T.; Hayden, F.G.; Horby, P.W.; Cao, B.; Wang, C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569-1578. doi:10.1016/S0140-6736(20)31022-9.
- Durante-Mangoni, E.; Andini, R.; Bertolino, L.; Mele, F.; Florio, L.L.; Murino, P.; Corcione, A.; Zampino, R. Early experience with remdesivir in SARS-CoV-2 pneumonia. Infection 2020, 10.1007/s15010-020-01448-x. doi:10.1007/s15010-020-01448-x.
- Ferner, R.E.; Aronson, J.K. Remdesivir in covid-19. BMJ 2020, 369, m1610. doi:10.1136/bmj.m1610.
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; Li, X.; Xia, J.; Chen, N.; Xiang, J.; Yu, T.; Bai, T.; Xie, X.; Zhang, L.; Li, C.; Yuan, Y.; Chen, H.; Li, H.; Huang, H.; Tu, S.; Gong, F.; Liu, Y.; Wei, Y.; Dong, C.; Zhou, F.; Gu, X.; Xu, J.; Liu, Z.; Zhang, Y.; Li, H.; Shang, L.; Wang, K.; Li, K.; Zhou, X.; Dong, X.; Qu, Z.; Lu, S.; Hu, X.; Ruan, S.; Luo, S.; Wu, J.; Peng, L.; Cheng, F.; Pan, L.; Zou, J.; Jia, C.; Wang, J.; Liu, X.; Wang, S.; Wu, X.; Ge, Q.; He, J.; Zhan, H.; Qiu, F.; Guo, L.; Huang, C.; Jaki, T.; Hayden, F.G.; Horby, P.W.; Zhang, D.; Wang, C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med 2020, 382, 1787-1799. doi:10.1056/NEJMoa2001282.
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schafer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; Spahn, J.E.; Bauer, L.; Sellers, S.; Porter, D.; Feng, J.Y.; Cihlar, T.; Jordan, R.; Denison, M.R.; Baric, R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 2020, 11, 222. doi:10.1038/s41467-019-13940-6.
- Gan, S.K.; Samaras, K.; Thompson, C.H.; Kraegen, E.W.; Carr, A.; Cooper, D.A.; Chisholm, D.J. Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes 2002, 51, 3163-3169. doi:10.2337/diabetes.51.11.3163.
- Reyskens, K.M.; Fisher, T.L.; Schisler, J.C.; O'Connor, W.G.; Rogers, A.B.; Willis, M.S.; Planesse, C.; Boyer, F.; Rondeau, P.; Bourdon, E.; Essop, M.F. Cardio-metabolic effectsof HIV protease inhibitors (lopinavir/ritonavir). PLoS One 2013, 8, e73347. doi:10.1371/journal.pone.0073347.
- Giudicessi, J.R.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Urgent Guidance for Navigating and Circumventing the QTc-Prolonging and Torsadogenic Potential of Possible Pharmacotherapies for Coronavirus Disease 19 (COVID-19). Mayo Clin Proc 2020, 95, 1213-1221. doi:10.1016/j.mayocp.2020.03.024.
- Chu, C.M.; Cheng, V.C.; Hung, I.F.; Wong, M.M.; Chan, K.H.; Chan, K.S.; Kao, R.Y.; Poon, L.L.; Wong, C.L.; Guan, Y.; Peiris, J.S.; Yuen, K.Y.; Group, H.U.S.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004, 59, 252-256. doi:10.1136/thorax.2003.012658.
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30-e31. doi:10.1016/S0140-6736(20)30304-4.
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020, 20, 400-402. doi:10.1016/S1473-3099(20)30132-8.
- Solis-Garcia Del Pozo, J.; Galindo, M.F.; Nava, E.; Jordan, J. A systematic review on the efficacy and safety of IL-6 modulatory drugs in the treatment of COVID-19 patients. Eur Rev Med Pharmacol Sci 2020, 24, 7475-7484. doi:10.26355/eurrev_202007_21916.
- Della-Torre, E.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Napolitano, A.; La Marca, S.; Boffini, N.; Da Prat, V.; Di Terlizzi, G.; Lanzillotta, M.; Rovere Querini, P.; Ruggeri, A.; Landoni, G.; Tresoldi, M.; Ciceri, F.; Zangrillo, A.; De Cobelli, F.; Dagna, L.; Group, S.-R.S.; members, S.-R.S.G. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis 2020, 10.1136/annrheumdis-2020-218122. doi:10.1136/annrheumdis-2020-218122.
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; Zhang, X.; Pan, A.; Wei, H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020, 117, 10970-10975. doi:10.1073/pnas.2005615117.
- Ledford, H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature 2020, 582, 469. doi:10.1038/d41586-020-01824-5.
- Ben-Zvi, I.; Kivity, S.; Langevitz, P.; Shoenfeld, Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol 2012, 42, 145-153. doi:10.1007/s12016-010-8243-x.
- Saleh, M.; Gabriels, J.; Chang, D.; Soo Kim, B.; Mansoor, A.; Mahmood, E.; Makker, P.; Ismail, H.; Goldner, B.; Willner, J.; Beldner, S.; Mitra, R.; John, R.; Chinitz, J.; Skipitaris, N.; Mountantonakis, S.; Epstein, L.M. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection. Circ Arrhythm Electrophysiol 2020, 13, e008662. doi:10.1161/CIRCEP.120.008662.
- Rosenberg, E.S.; Dufort, E.M.; Udo, T.; Wilberschied, L.A.; Kumar, J.; Tesoriero, J.; Weinberg, P.; Kirkwood, J.; Muse, A.; DeHovitz, J.; Blog, D.S.; Hutton, B.; Holtgrave, D.R.; Zucker, H.A. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA 2020, 10.1001/jama.2020.8630. doi:10.1001/jama.2020.8630.
- Mercuro, N.J.; Yen, C.F.; Shim, D.J.; Maher, T.R.; McCoy, C.M.; Zimetbaum, P.J.; Gold, H.S. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020, 10.1001/jamacardio.2020.1834. doi:10.1001/jamacardio.2020.1834.
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.K.; Kubin, C.; Barr, R.G.; Sobieszczyk, M.E.; Schluger, N.W. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020, 382, 2411-2418. doi:10.1056/NEJMoa2012410.
- (FDA), U.S.F.a.D.A. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. 2020, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and.
- (FDA), U.S.F.a.D.A. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems.
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med 2020, 382, 1969-1973. doi:10.1056/NEJMp2005630.
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; Gao, H.; Ge, X.; Kan, B.; Hu, Y.; Liu, J.; Cai, F.; Jiang, D.; Yin, Y.; Qin, C.; Li, J.; Gong, X.; Lou, X.; Shi, W.; Wu, D.; Zhang, H.; Zhu, L.; Deng, W.; Li, Y.; Lu, J.; Li, C.; Wang, X.; Yin, W.; Zhang, Y.; Qin, C. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77-81. doi:10.1126/science.abc1932.
- McKay, P.L.a.B. Drugmakers Rush to Develop Vaccines Against China Virus. The Wall Street Journal 2020, https://www.wsj.com/articles/drugmakers-rush-to-develop-vaccines-against-china-virus-11579813026.
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gomez Roman, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov 2020, 19, 305-306. doi:10.1038/d41573-020-00073-5.
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; McDermott, A.; Flach, B.; Doria-Rose, N.A.; Corbett, K.S.; Morabito, K.M.; O'Dell, S.; Schmidt, S.D.; Swanson, P.A., 2nd; Padilla, M.; Mascola, J.R.; Neuzil, K.M.; Bennett, H.; Sun, W.; Peters, E.; Makowski, M.; Albert, J.; Cross, K.; Buchanan, W.; Pikaart-Tautges, R.; Ledgerwood, J.E.; Graham, B.S.; Beigel, J.H.; m, R.N.A.S.G. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med 2020, 10.1056/NEJMoa2022483. doi:10.1056/NEJMoa2022483.
- Moderna, I. Moderna Announces Phase 3 COVE Study of mRNA Vaccine Against COVID-19 (mRNA-1273) Begins. 2020, https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-phase-3-cove-study-mrna-vaccine-against-covid.
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; Hlh Across Speciality Collaboration, U.K. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033-1034. doi:10.1016/S0140-6736(20)30628-0.
- AG, N. Novartis announces plan to initiate clinical trial of canakinumab for patients with COVID-19 pneumonia. 2020, https://www.novartis.com/news/novartis-announces-plan-initiate-clinical-trial-canakinumab-patients-covid-19-pneumonia.
- SE, P.I.a.B. Pfizer and BioNTech Dose First Participants in the U.S. as Part of Global COVID-19 mRNA Vaccine Development Program BUSINESS WIRE 2020, https://www.businesswire.com/news/home/20200505005474/en/.
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; Jia, S.Y.; Jiang, H.D.; Wang, L.; Jiang, T.; Hu, Y.; Gou, J.B.; Xu, S.B.; Xu, J.J.; Wang, X.W.; Wang, W.; Chen, W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845-1854. doi:10.1016/S0140-6736(20)31208-3.
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; Xu, Z.; Walters, J.; Bhojnagarwala, P.; Yang, M.; Chokkalingam, N.; Pezzoli, P.; Parzych, E.; Reuschel, E.L.; Doan, A.; Tursi, N.; Vasquez, M.; Choi, J.; Tello-Ruiz, E.; Maricic, I.; Bah, M.A.; Wu, Y.; Amante, D.; Park, D.H.; Dia, Y.; Ali, A.R.; Zaidi, F.I.; Generotti, A.; Kim, K.Y.; Herring, T.A.; Reeder, S.; Andrade, V.M.; Buttigieg, K.; Zhao, G.; Wu, J.M.; Li, D.; Bao, L.; Liu, J.; Deng, W.; Qin, C.; Brown, A.S.; Khoshnejad, M.; Wang, N.; Chu, J.; Wrapp, D.; McLellan, J.S.; Muthumani, K.; Wang, B.; Carroll, M.W.; Kim, J.J.; Boyer, J.; Kulp, D.W.; Humeau, L.; Weiner, D.B.; Broderick, K.E. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun 2020, 11, 2601. doi:10.1038/s41467-020-16505-0.
- Mahase, E. Covid-19: Russia approves vaccine without large scale testing or published results. BMJ 2020, 370, m3205. doi:10.1136/bmj.m3205.
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; Izhaeva, F.M.; Popova, O.; Ozharovskaya, T.A.; Esmagambetov, I.B.; Favorskaya, I.A.; Zrelkin, D.I.; Voronina, D.V.; Shcherbinin, D.N.; Semikhin, A.S.; Simakova, Y.V.; Tokarskaya, E.A.; Lubenets, N.L.; Egorova, D.A.; Shmarov, M.M.; Nikitenko, N.A.; Morozova, L.F.; Smolyarchuk, E.A.; Kryukov, E.V.; Babira, V.F.; Borisevich, S.V.; Naroditsky, B.S.; Gintsburg, A.L. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. The Lancet 2020, 10.1016/s0140-6736(20)31866-3. doi:10.1016/s0140-6736(20)31866-3.
- Zhang, B.; Liu, S.; Tan, T.; Huang, W.; Dong, Y.; Chen, L.; Chen, Q.; Zhang, L.; Zhong, Q.; Zhang, X.; Zou, Y.; Zhang, S. Treatment With Convalescent Plasma for Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Chest 2020, 158, e9-e13. doi:10.1016/j.chest.2020.03.039.
- Bloch, E.M.; Shoham, S.; Casadevall, A.; Sachais, B.S.; Shaz, B.; Winters, J.L.; van Buskirk, C.; Grossman, B.J.; Joyner, M.; Henderson, J.P.; Pekosz, A.; Lau, B.; Wesolowski, A.; Katz, L.; Shan, H.; Auwaerter, P.G.; Thomas, D.; Sullivan, D.J.; Paneth, N.; Gehrie, E.; Spitalnik, S.; Hod, E.A.; Pollack, L.; Nicholson, W.T.; Pirofski, L.A.; Bailey, J.A.; Tobian, A.A. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 2020, 130, 2757-2765. doi:10.1172/JCI138745.
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 2020, 20, 398-400. doi:10.1016/S1473-3099(20)30141-9.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; Muller, M.A.; Drosten, C.; Pohlmann, S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271-280 e278. doi:10.1016/j.cell.2020.02.052.
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 2020, 17, 613-620. doi:10.1038/s41423-020-0400-4.
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; van Haperen, R.; Osterhaus, A.; van Kuppeveld, F.J.M.; Haagmans, B.L.; Grosveld, F.; Bosch, B.J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 2020, 11, 2251. doi:10.1038/s41467-020-16256-y.
- Fatima, F.; Ekstrom, K.; Nazarenko, I.; Maugeri, M.; Valadi, H.; Hill, A.F.; Camussi, G.; Nawaz, M. Non-coding RNAs in Mesenchymal Stem Cell-Derived Extracellular Vesicles: Deciphering Regulatory Roles in Stem Cell Potency, Inflammatory Resolve, and Tissue Regeneration. Front Genet 2017, 8, 161. doi:10.3389/fgene.2017.00161.
- Rajarshi, K.; Chatterjee, A.; Ray, S. Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol Rep (Amst) 2020, 26, e00467. doi:10.1016/j.btre.2020.e00467.
- Chrzanowski, W.; Kim, S.Y.; McClements, L. Can Stem Cells Beat COVID-19: Advancing Stem Cells and Extracellular Vesicles Toward Mainstream Medicine for Lung Injuries Associated With SARS-CoV-2 Infections. Front Bioeng Biotechnol 2020, 8, 554. doi:10.3389/fbioe.2020.00554.
