Electrically conductive hydrogels (ECHs), an emerging class of biomaterials, have garnered tremendous attention due to their potential for a wide variety of biomedical applications, from tissue-engineered scaffolds to smart bioelectronics. Along with the development of new hydrogel systems, 3D printing of such ECHs is one of the most advanced approaches towards rapid fabrication of future biomedical implants and devices with versatile designs and tuneable functionalities.
- 3D Printing
- ECHs
1. Introduction
Hydrogels are a three-dimensional (3D) network of crosslinked hydrophilic polymers with high water sorption properties. Electrically conductive scaffolds (ECSs), including electrically conductive hydrogels (ECHs) are potential candidates for biomedical applications, such as bioelectronics, drug delivery and tissue engineering of skin, muscle, cardiac, nerve and bone tissues [1]. ECHs have been at the frontline of “smart conductive biomaterials” development due to their resemblance to biological tissues in terms of mechanical properties, water retention, bioactivity and other extracellular matrix-like properties [2]. ECHs have made it possible to minimize the property mismatch at bioelectronic interfaces, providing wet and ion-rich physiological environments in a 3D nanostructured conductive network, offering an extremely high surface area for seamless bio-integration that is difficult to accomplish on a conventional electronic interface [3]. Moreover, ECHs as electrodes can promote signal transductions between biological and electrical circuits by accurately controlling/allowing localized amplification and/or filtering of bio-derived signals [4], and an improved cell adhesion, proliferation and differentiation can be achieved with electrical stimulation [5]. However, achieving desired conductivity and mechanical properties (e.g., toughness and stretchability) is the key obstacle in formulating ECHs for bioelectronics while retaining biocompatibility. In addition, the integration of other features, such as wet adhesion along with self-healing and shape memory features, into ECHs is also crucial to many functional applications of hydrogel bioelectronics and implantable devices [6]. Although metallic electrodes such as gold, platinum, glassy carbon etc. are used as implantable devices, their usage often leads to poor long-term stimulation and recording performances. Thus, significant challenges exist for developing conducting polymer gels for neural interfaces, which require intimate contact between the excitable tissue and the electrode for stimulation of cells, which is often limited by the presence of extracellular fluid through which the signal transmission occurs. ECH offers the potential to support the intimate contact between the tissue and the electrode and combine the best of both worlds of biology and electronics (
).
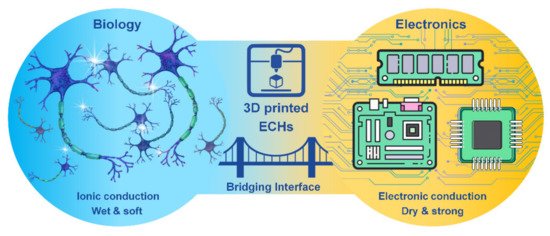
3D printed electrically conductive hydrogels (ECHs) as the bridging interface of biology and electronics.
A wide variety of ECHs have been synthesised to date by mixing various types of conventional insulating polymer matrices (providing structural support and water sorption properties) with conductive polymers or filler materials (providing electrical conductivity) [5]. Among electrically conductive polymers, poly(3,4-ethylenedioxythiophene) (PEDOT), polyaniline (PANI) and polypyrrole (PPy) are attractive materials for biomedical applications due to their biocompatibility (including cell viability, adhesion, proliferation and differentiation), and tuneable electrical conductivity (by doping). However, their tissue engineering applications are often limited by their poor processability and mechanical brittleness, which has led to the development of several conductive polymer-based hybrid ECHs [7]. On the other hand, electrically conductive fillers, such as carbon-based materials, transition metal carbides/nitrides and liquid metals provide highly efficient electron transport channels across polymer matrix (having covalent/or non-covalent interactions with polymer chains) to achieve high conductivity [8]. Over the last decade, graphene-based materials have drawn exceptional attention as conductive fillers for ECH composites due to their natural abundance, outstanding electrical conductivity, mechanical properties, and cell adhesion, proliferation and differentiation support qualities [9]. In recent years, MXenes and liquid metals have gained increasing research interest in the field of biomedical engineering due to their unique combination of properties, including hydrophilicity or high fluidity, metallic conductivity and good biocompatibility [10][11]. Such conductive filler materials not only offer the flexibility of tuning desired structure and physicochemical properties of ECHs but also influence rheological properties of inks during 3D scaffold fabrication.
Several methods for fabrication of 3D scaffolds, such as solvent casting, moulding, electrospinning and 3D printing have been reported in the literature [12]. In particular, 3D printing of hydrogels has gained increasing research attention in recent years as rational design strategy for emerging biomedical applications, where the technology involves layer-by-layer fabrication of digitally derived 3D model objects through progressive addition of materials as inks [13]. Moreover, hydrogel-based 3D bioprinting offers the advantage of constructing living structures from bioinks containing live cells, growth factors and other biocompatible materials [14]. In combination with 3D bioprinting, fabrication of 3D ECHs might be one of the most advanced approaches towards next-level bioelectronics regarding potential functionalities and design possibilities. However, the scientific community still faces numerous challenges in synthesis, 3D printing and crosslinking of electrically conductive materials (inks). For more than a decade, ECHs themselves have been studied for potential tissue engineering and biosensor applications [5], while the 3D printing of such functional materials has been investigated only recently [15]. Moreover, in the past five years, there has been a significant rise in the number of publications (
) on “3D printing hydrogels” and “conductive hydrogels”. However, the number of publications on 3D printing of conductive hydrogels has been much lower than the overall number of publications in the general field of “3D printing hydrogels”. In this review, recent achievements, challenges and future perspective in 3D printing of ECHs comprising electrically conductive biocompatible polymers (PEDOT, PANI and PPy) or fillers (graphene, MXenes and liquid metals) that have potential applications in the field of biomedicine, including tissue engineering, bioelectronics and medical devices, are summarized.
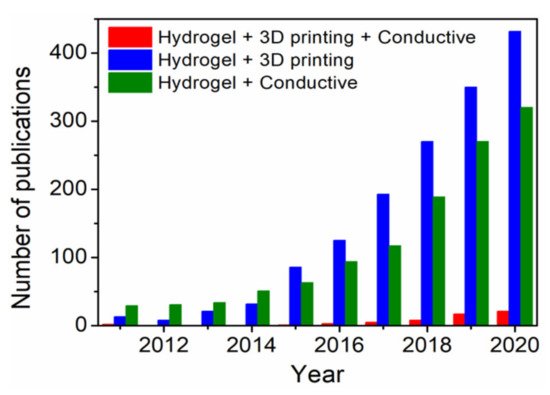
Publication trends over the last ten years obtained with keywords “Hydrogel”, “3D printing”, “Conductive” and their combination using Web of Science.
2. Mechanism of Electrical Conductivity in ECHs
Electrical conductivity (or its inverse, electrical resistivity), is a fundamental material property that quantifies how strongly it conducts or resists electric current. The overall electrical conductivity of ECHs swollen in biological fluids (electrolytes) comes from the electronic functionality of conductive polymers and the ionic contribution and depends on the inter- and intra-chain charge transfer along polymer chains throughout the matrix/networks [16]. The electronic conductivity of ECHs relies on the conjugated backbone, formed by a series of π-bonds and strongly localized σ-bonds, of the conductive polymer (e.g., PEDOT, PANI and PPy) chains. During polymerisation, the p-orbitals in the series of π-bonds overlap each other, triggering electron redistribution. The delocalized electrons are allowed to move freely within the polymeric backbone, inducing intrinsically electronic conductance into the conductive polymers [16]. Similar to the ionic conductivity, the electronic conductivity is often improved by ionically conductive dopants. Their purpose is to introduce a charge carrier into the conductive polymer networks, disrupting the stable crystal lattice backbone and allowing charge to travel along the polymer chain in the form of polarons or bipolarons [17]. For instance, while PANI has a low conductivity of ~10
S cm
in its emaraldine form, its protonated salts with polaron and bipolaron states achieve much higher conductivity of ~10
S cm
[18]. A schematic of electrical conductivity of conductive polymers is shown in
A. In ECHs comprising insulating polymer matrix with 3D interpenetrating networks of fillers (e.g., graphene and MXenes) with inherent electrical conductivity, the mechanism of electron transfer can be described by percolation theory [3]. The percolation behaviour and change in electronic conductance of the system as a function of conductive filler concentration can be conceptualized as shown in
B. In the insulation region, almost no conduction occurs due to insufficient volume fraction of fillers to create an effective electron transfer pathway. whereas when percolation threshold is reached with the increase in the concentration of fillers, conductivity increases (several orders in magnitude) and finally reaches stable conduction region with further increase in concentration of fillers forming a stable interpenetrating network structure [3].
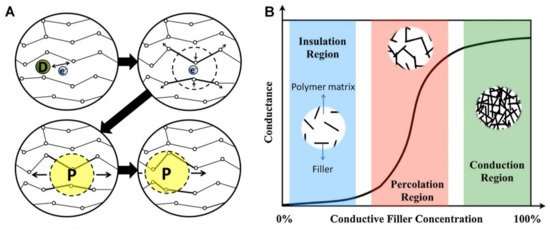
(
) Schematic of electrical conductivity of conductive polymers; where, the dopant D creates a delocalized charge (by adding or removing an electron to/from the polymer chain), which is then localized (energetically favourable) and surrounded by a local distortion of the crystal lattice, creating polaron P (a radical ion associated with a lattice distortion), which then travels along the polymer chain to conduct electricity. Adapted with permission from Ref. [16]. Copyright 2014, Elsevier. (
) Schematic of percolation behaviour and electrical conductance as a function of conductive filler concentration in an insulting matrix. Adapted with permission from Ref. [3]. Copyright 2019, Elsevier.
3. 3D Printing of ECHs
3D printing, also referred to as additive manufacturing, is an umbrella term that covers several digital model-based layer-by-layer deposition techniques. Among them, fused deposition modelling (FDM), direct ink writing (DIW), inkjet printing and stereolithography (SLA) have been successfully applied for fabrication of complex 3D structures of ECHs [19]. An overview of the basic operating principles of these techniques is illustrated in
, and their respective advantages and limitations are discussed in the following sub-sections.
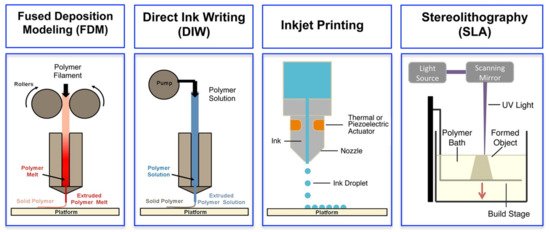
Schematics of different 3D printing methods: extrusion-based methods, such as fused deposition modeling (FDM) and direct ink writing (DIW), inkjet printing and light-based stereolithography (SLA). Adapted with permission from Ref. [19]. Copyright 2016, American Chemical Society.
3.1. Fused Deposition Modelling (FDM)
FDM is an extrusion-based printing method, where a filament made of thermoplastics is heated to reach a semi-liquid state and extruded through a nozzle, followed by self-solidification upon cooling on the printed platform, to form a solid layer on top of another [19]. The advantages of this method include low cost and simplicity, whereas the main disadvantage is the relatively low printing resolution. Moreover, FDM is not suitable for bioprinting, thereby making it the least favourable method for printing of ECHs.
3.2. Direct Ink Writing (DIW)
DIW is also an extrusion-based printing method, where a shear-thinning viscous liquid or paste is extruded (under pressure) through the nozzle, followed by solidification via evaporation of solvent, rapid cryogenisation, sol-gel transition, crosslinking or post-treatment on the printed platform, to form a solid layer on top of another [19]. DIW is more suitable and widely applied method for printing of soft hydrogels, especially for biomedical applications, due to the broad choices of materials, provided that the deposited ink can be rapidly solidified. A variety of materials can be used for fabrication of ECHs using this method, ranging from pristine polymers [20] to composite materials [21]. Moreover, DIW is the only printing technology that has been successfully applied for 3D bioprinting of ECHs [22]. The rheological properties of the inks play a crucial role in the DIW process. A 3D printable ink usually requires shear-thinning characteristics, wherein the ink pastes exhibit low elastic shear modulus under high shear stress to flow through the nozzle and high static elastic modulus to maintain its shape after deposition for printing of multiple layers [23]. DIW is the most popular technique for 3D printing of ECHs; however, its main drawback is also the low printing resolution.
3.3. Inkjet Printing
In inkjet printing, the ink droplets are propelled through a nozzle by a thermal/piezoelectric actuator and selectively deposited on demand to the desired location of a substrate. Precise deposition of small droplets helps to print high-resolution structures, followed by solidification of the printed droplets by chemical or thermal process such as curing or sintering [19]. Inkjet 3D printed ECHs can be fabricated by sequential deposition or spray-coating (on a substrate covered by a patterned mask) of two distinct solutions consisting of (i) monomer precursor and (ii) oxidising agent in a layer-by-layer fashion, with masks placed on previous layer [24]. However, inkjet printing of ECHs is challenging due to the strict requirements for ink formulation regarding its viscosity, surface tension and the evaporation rate.
3.4. Stereolithography (SLA)
In SLA, photocurable polymer solution (that is contained in a reservoir) is converted into photopolymerized solid in a layer-by-layer fashion using light energy (e.g., UV or laser beam) [19]. Currently, SLA is used to achieve the best resolution printing of ECHs, and it can work with a wide variety of materials [25]. However, its drawbacks include conducting polymer’s intense absorption (due to the presence of long chains of conjugated π bonds) in the UV and visible region, and their complex redox chemistries, which may be incompatible with the reactive species formed during some photoinitiator decomposition [19].
References
- Sikorski, P. Electroconductive scaffolds for tissue engineering applications. Biomater. Sci. 2020, 8, 5583–5588.
- Lu, H.; Zhang, N.; Ma, M. Electroconductive hydrogels for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1568.
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240.
- Vo, R.; Hsu, H.-H.; Jiang, X. Hydrogel facilitated bioelectronic integration. Biomater. Sci. 2021, 9, 23–37.
- Min, J.H.; Patel, M.; Koh, W.G. Incorporation of conductive materials into hydrogels for tissue engineering applications. Polymers 2018, 10, 1078.
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional conductive hydrogels for bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301.
- Guo, B.; Ma, P.X. Conducting polymers for tissue engineering. Biomacromolecules 2018, 19, 1764–1782.
- Wang, B.; Sun, Y.; Ding, H.; Zhao, X.; Zhang, L.; Bai, J.; Liu, K. Bioelectronics-related 2D materials beyond graphene: Fundamentals, properties, and applications. Adv. Funct. Mater. 2020, 30, 2003732.
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274.
- Yan, J.; Lu, Y.; Chen, G.; Yang, M.; Gu, Z. Advances in liquid metals for biomedical applications. Chem. Soc. Rev. 2018, 47, 2518–2533.
- Zhang, Y.-Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. Mxene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251.
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L.-s. Fabrication of scaffolds in tissue engineering: A review. Front. Mech. Eng. 2018, 13, 107–119.
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543.
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479.
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors—A review. Acta Biomater. 2020, 101, 1–13.
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353.
- Bredas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315.
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J. Control of polyaniline conductivity and contact angles by partial protonation. Polym. Int. 2008, 57, 66–69.
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693.
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604.
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648.
- Spencer, A.R.; Shirzaei Sani, E.; Soucy, J.R.; Corbet, C.C.; Primbetova, A.; Koppes, R.A.; Annabi, N. Bioprinting of a cell-laden conductive hydrogel composite. ACS Appl. Mater. Interfaces 2019, 11, 30518–30533.
- Tran, T.S.; Dutta, N.K.; Choudhury, N.R. Poly(ionic liquid)-stabilized graphene nanoinks for scalable 3D printing of graphene aerogels. ACS Appl. Nano Mater. 2020, 3, 11608–11619.
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.-K.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292.
- Wu, Y.; Chen, Y.X.; Yan, J.; Quinn, D.; Dong, P.; Sawyer, S.W.; Soman, P. Fabrication of conductive gelatin methacrylate–polyaniline hydrogels. Acta Biomater. 2016, 33, 122–130.
