The c-Jun N-terminal kinase (JNK) signalling pathway is a conserved response to a wide range of internal and external cellular stress signals.
- MAP kinase
- cell signalling
- glioblastoma
- neurodegenerative disorders
- CNS injuries
1. Introduction
The CNS is exposed to stress stimuli during development and adulthood, and under pathological aggressions. These events trigger the Jun N-terminal kinase signalling pathway (JNK) as a mechanism to coordinate cellular responses to stress, and maintain tissue homeostasis. The JNK pathway includes a conserved mitogen-activated protein kinase (MAPK), which belongs to the stress-activated protein kinase (SAPK) group, a group of kinases that can be activated by any internal or external stimuli that cause cell stress. Furthermore, the JNK pathway in the CNS and other tissues can be activated by UV irradiation, glucose deprivation, DNA damage, heat stress, bacterial and viral infection, oxidative stress, inflammatory cytokines and growth factors [1,2,3,4,5,6,7,8,9,10][1][2][3][4][5][6][7][8][9][10].
The molecular MAPK cascade presents high homology from Drosophila to mammals (Figure 1) [11]. In Drosophila, JNK pathway-signalling is initiated by the interaction of the ligand protein Eiger (Egr), the unique TNF superfamily member of ligands [12,13][12][13], with TNF receptors (TNFRs) Grindewal (Grnd) [14], or Wengen (Wng) [15]. Ligand–receptor interaction initiates a cascade of phosphorylations that mediates the JNK signalling pathway [11]. In Drosophila, JNK is encoded by a single gene: basket (Bsk); this simplifies the genetic studies that have contributed to decipher the role of JNK under physiological and pathological stressful scenarios [16,17][16][17]. In mammals, the JNK cascade involves four kinases, and mitogens or cytokines induce MAP3K family activation [11]. MAPK cascade triggers cytosolic JNK dual phosphorylation and initiates the phosphorylation of cytoplasmic and nuclear proteins [18], including cytoskeletal and mitochondrial proteins, nuclear transcription factors, membrane proteins or nuclear hormone receptors [19]. Thus, gene expression derived from JNK activation leads to a variety of responses, depending on the cell type and the scenario [11]. In mammals, the JNK pathway is encoded by jnk1, jnk2 and jnk3 [20]. JNK1 and JNK2 are found ubiquitously, whereas JNK3 is restricted to the brain, cardiac smooth muscle and testis [20,21][20][21]. In particular, the JNK pathway is highly active in the CNS as compared with other tissues. Therefore, it emerges as a critical regulator of CNS cells under physiological and pathological conditions [22,23][22][23]. It has been demonstrated in mouse that different JNK isoforms undergo compensatory mechanisms during early brain development. Although a single deficiency of each JNK isoform is viable, the mutants still present different phenotypes, highlighting the importance of each individual enzyme (reviewed in [23]). Besides, each isoform has different temporal and regional expression patterns (reviewed in [21]).
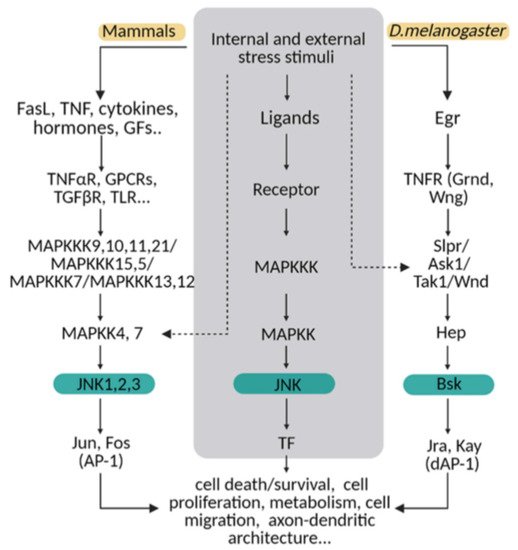
Figure 1. The JNK pathway is conserved between mammals and flies. Upon internal and external stress stimuli, ligands (ex. TNF/Egr) activate transmembrane receptors (ex. TNFαR/ Grnd) and initiate a cellular response based on a MAPK cascade phosphorylation. In mammals, different ligands can trigger the signal path; while in Drosophila, Eiger (Egr) is the TNF ligand that predominantly initiates the stress response via TNF receptors (Grindewal, Grnd; or Wengen, Wng). In mammals, there is MAPK path gene redundancy that is shown in this diagram compared with flies. MAPKs are encoded by multiple genes in mammals, whereas in Drosophila, one single gene is described for each enzyme. The cellular response converges in JNK/Bsk phosphorylation, which triggers the expression of transcription factors as Jun/Jra and Fos/Jay, known as an AP-1/dAP-1 complex. Finally, AP1/dAP-1 modulates the transcriptional program of genes involved in a variety of biological activities. This pathway can be activated at other steps indicated with dotted lines in the diagram.
The JNK signalling pathway mediates embryonic development; metabolism and growth; lifespan; programmed cell death; cell migration, repair and proliferation; immunity; and axonal transport [24,25,26,27,28][24][25][26][27][28]. In addition, the literature reveals a dual role of the JNK pathway in cell death and cell survival, depending on the cell type and the context [11]. This two-faded role is particularly important in CNS pathologies such as neurodegeneration and tumorigenesis, in which the cellular stress-associated signals are increased [29,30][29][30]. Likewise, JNK signalling is involved in neurogenesis, axonal growth, axonal transport, brain metabolism, animal behavior, neurulation, migration and axon–dendritic architecture in different species [21,22,23,31,32][21][22][23][31][32].
2. JNK in Regeneration/Repair after an Injury to the CNS
CNS damage produced by brain stroke, spinal-cord injury or neurodegenerative diseases often results in permanent disabilities due to the limited regenerative capacity of this tissue. Several studies have supported the importance of JNK signalling in response to nerve injuries in both degeneration and repair. In the injured axons, retrograde transport of JNK can alter somal transcription of the injury-response molecules ATF3 and Hsp27, important for axonal outgrowth [72,73][33][34]. On the other hand, genetic or pharmacological inhibition of JNK signalling in multiple models of axonal injury delayed axonal degeneration [74[35][36],75], indicating that JNK is also required for axonal degeneration. Moreover, upon nerve injury of a rat model, JNK3 inhibited axonal growth through the interaction with the Kluppel-like transcription factor 9 (KLF9) [76][37]. JNK signalling also activates members of the Bcl-2 family, triggering apoptosis after injury or stress [77,78,79][38][39][40].
MAP3K is one of the first members of the JNK pathway. It is a dual-leucine zipper kinase (DLK), a conserved kinase with orthologues in mammals (MAP3K DLK), Drosophila (DLK/Wallenda) and C. elegans (DLK-1) [80][41]. MAP3K is an important axon-injury sensor [81][42]. DLK–JNK signalling contributes in multiple ways to axonal injury response and axonal regeneration. The DLK/Wallenda protein is present in axons, and protein levels are increased in response to axonal injury in Drosophila [82,83][43][44]. JNK-dependent phosphorylation of DLK is required for the stabilization of DLK levels [84][45]. DLK regulates microtubule stability [81[42][46][47],85,86], essential for axonal regeneration in spinal-cord injury [87][48]. DLK/Wallenda overexpression has also been shown to be protective in Drosophila motor neuron axons [83][44]. DLK is also an essential molecule for the injury-dependent activation of the retrograde transport of p-STAT3 to the cell body, necessary for the activation of the neuronal regenerative program in mice [88][49]. Moreover, it has been demonstrated that in C. elegans, DLK-1 is both necessary and sufficient for injury-induced autophagy activation; DLK-1 limits the levels of LIN-12 and NOTCH proteins, suggested to promote axon regeneration [89][50].
2.1. JNK Signalling in Glial Cells upon Injury
All the evidence summarized above demonstrates the importance of JNK signalling in injured axons; however, JNK-signalling activation also occurs in glial cells in response to injury. Different injury paradigms in Drosophila showed a consistent activation of AP-1 transcription in glial cells upon injury, downstream of JNK. Unknown molecules from axonal debris activate the engulfment receptor Draper, which in turn regulates draper transcriptional upregulation via STAT92E [90][51] and the JNK pathway [74][35]. Draper is a conserved receptor with orthologues in mammals (MEGF10) and C. elegans (CED-1), and it is required for engulfment in several cell types, including germ line, epithelial cells, microglia and astrocytes [91,92,93,94][52][53][54][55].
In Drosophila and C. elegans, downregulation of JNK reduces axon regeneration and provokes axon debris accumulation following injury. In the injury context, JNK also activates MMP-1 expression [95][56], which is required for glial cells to infiltrate in the injured tissue and remove cell debris via Draper. Glial-specific overexpression of draper is sufficient to rescue engulfment defects associated with the loss of JNK signalling [74][35]. In addition, knockdown of the JNK pathway components blocks CED-1 mediated axon regeneration and axon debris removal [96][57], which suggests a role for JNK in Drpr/CED-1-mediated axon regrowth.
2.2. JNK in Neurogenesis and Regeneration
Finally, JNK could also trigger CNS regeneration by promoting neuronal differentiation from neural stem cells (NSCs). So far, neurogenesis in mammals is restricted to two major regions, the subependymal zone (SEZ) and the subgranular zone (SGZ) of the dentate gyrus, where adult NSCs are present, but the neuronal differentiation rate of NSCs is limited [97][58]. Therefore, a promising strategy to boost CNS regeneration is to find candidates to promote neural differentiation from NCSs. Noncanonical Wnt signalling such as the Wnt/JNK pathway has a positive effect on neuronal differentiation in cell culture and during development [98,99,100][59][60][61]. Moreover, it has been recently shown that Wnt5a upregulates miRNA200b-3p expression through MAPK/JNK signalling, and miRNA200b-3p suppresses the RhoA/Rock signalling required for neuronal differentiation. Thus, Wnt5a promotes NSC differentiation into neurons, and more remarkably, transplantation of NSCs overexpressing Wnt5a results in tissue repair and locomotor functional recovery in rats after spinal-cord injury [97][58]. However, other studies have revealed that different JNK isoforms are involved in the adult neurogenesis control in a different manner. For instance, inhibition of JNK1 or mice lacking JNK1 show an increased number of neural progenitors in the SGZ of the hippocampus, whereas the absence of JNK3 reduces it [101,102][62][63]. These results again point out the importance of finding drugs with high specificity for the different JNK isoforms to treat CNS disorders.
(References would be added automatically after the entry is online)
References
- Anfinogenova, N.D.; Quinn, M.T.; Schepetkin, I.A.; Atochin, D.N. Alarmins and c-Jun N-Terminal Kinase (JNK) Signaling in Neuroinflammation. Cells 2020, 9, 2350.
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247.
- Hamdi, M.; Kool, J.; Cornelissen-Steijger, P.; Carlotti, F.; Popeijus, H.E.; Van Der Burgt, C.; Janssen, J.M.; Yasui, A.; Hoeben, R.C.; Terleth, C.; et al. DNA damage in transcribed genes induces apoptosis via the JNK pathway and the JNK-phosphatase MKP-1. Oncogene 2005, 24, 7135–7144.
- Kamata, H.; Honda, S.I.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005, 120, 649–661.
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869.
- López-Camarillo, C.; Ocampo, E.A.; Casamichana, M.L.; Pérez-Plasencia, C.; Álvarez-Sánchez, E.; Marchat, L.A. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int. J. Mol. Sci. 2012, 13, 142–172.
- Nikolic, I.; Leiva, M.; Sabio, G. The role of stress kinases in metabolic disease. Nat. Rev. Endocrinol. 2020, 16, 697–716.
- Rosette, C.; Karin, M. Ultraviolet light and osmotic stress: Activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 1996, 274, 1194–1197.
- Song, J.J.; Lee, Y.J. Differential activation of the JNK signal pathway by UV irradiation and glucose deprivation. Cell. Signal. 2007, 19, 563–572.
- Zanke, B.W.; Boudreau, K.; Rubie, E.; Winnett, E.; Tibbles, L.A.; Zon, L.; Kyriakis, J.; Liu, F.F.; Woodgett, J.R. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr. Biol. 1996, 6, 606–613.
- La Marca, J.E.; Richardson, H.E. Two-Faced: Roles of JNK Signalling During Tumourigenesis in the Drosophila Model. Front. Cell Dev. Biol. 2020, 8, 42.
- Igaki, T.; Kanda, H.; Yamamoto-Goto, Y.; Kanuka, H.; Kuranaga, E.; Aigaki, T.; Miura, M. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 2002, 21, 3009–3018.
- Moreno, E.; Yan, M.; Basler, K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol. 2002, 12, 1263–1268.
- Sanchez, J.A.; Mesquita, D.; Ingaramo, M.C.; Ariel, F.; Milan, M.; Dekanty, A. Eiger/TNFα-mediated Dilp8 and ROS production coordinate intra-organ growth in drosophila. PLoS Genet. 2019, 15, e1008133.
- Igaki, T.; Pastor-Pareja, J.C.; Aonuma, H.; Miura, M.; Xu, T. Intrinsic Tumor Suppression and Epithelial Maintenance by Endocytic Activation of Eiger/TNF Signaling in Drosophila. Dev. Cell 2009, 16, 458–465.
- Stronach, B.E.; Perrimon, N. Stress signaling in Drosophila. Oncogene 1999, 18, 6172–6182.
- Yang, D.; Thomas, J.M.; Li, T.; Lee, Y.; Liu, Z.; Smith, W.W. The Drosophila hep pathway mediates Lrrk2-induced neurodegeneration. Biochem. Cell Biol. 2018, 96, 441–449.
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40.
- Bogoyevitch, M.A.; Kobe, B. Uses for JNK: The Many and Varied Substrates of the c-Jun N-Terminal Kinases. Microbiol. Mol. Biol. Rev. 2006, 70, 1061–1095.
- Bogoyevitch, M.A. The isoform-specific functions of the c-Jun N-terminal kinases (JNKs): Differences revealed by gene targeting. BioEssays 2006, 28, 923–934.
- Coffey, E.T. Nuclear and cytosolic JNK signalling in neurons. Nat. Rev. Neurosci. 2014, 15, 285–299.
- Antoniou, X.; Borsello, T. The JNK signalling transduction pathway in the brain. Front. Biosci. 2012, 4, 2110–2120.
- Yamasaki, T.; Kawasaki, H.; Nishina, H. Diverse Roles of JNK and MKK Pathways in the Brain. J. Signal Transduct. 2012, 2012, 1–9.
- Agnès, F.; Suzanne, M.; Noselli, S. The Drosophila JNK pathway controls the morphogenesis of imaginal discs during metamorphosis. Development 1999, 126, 5453–5462.
- Horiuchi, D.; Collins, C.A.; Bhat, P.; Barkus, R.V.; DiAntonio, A.; Saxton, W.M. Control of a Kinesin-Cargo Linkage Mechanism by JNK Pathway Kinases. Curr. Biol. 2007, 17, 1313–1317.
- Igaki, T. Correcting developmental errors by apoptosis: Lessons from Drosophila JNK signaling. Apoptosis 2009, 14, 1021–1028.
- Nikoloudaki, G.; Brooks, S.; Peidl, A.P.; Tinney, D.; Hamilton, D.W. JNK Signaling as a Key Modulator of Soft Connective Tissue Physiology, Pathology, and Healing. Int. J. Mol. Sci. 2020, 21, 1015.
- Tafesh-Edwards, G.; Eleftherianos, I. JNK signaling in Drosophila immunity and homeostasis. Immunol. Lett. 2020, 226, 7–11.
- Musi, C.A.; Agrò, G.; Santarella, F.; Iervasi, E.; Borsello, T. JNK3 as Therapeutic Target and Biomarker in Neurodegenerative and Neurodevelopmental Brain Diseases. Cells 2020, 9, 2190.
- Portela, M.; Venkataramani, V.; Fahey-Lozano, N.; Seco, E.; Losada-Perez, M.; Winkler, F.; Casas-Tintó, S. Glioblastoma cells vampirize WNT from neurons and trigger a JNK/MMP signaling loop that enhances glioblastoma progression and neurodegeneration. PLoS Biol. 2019, 17, e3000545.
- Curran, B.P.; Murray, H.J.; O’Connor, J.J. A role for c-Jun N-terminal kinase in the inhibition of long-term potentiation by interleukin-1β and long-term depression in the rat dentate gyrus in vitro. Neuroscience 2003, 118, 347–357.
- Schellino, R.; Boido, M.; Vercelli, A. JNK Signaling Pathway Involvement in Spinal Cord Neuron Development and Death. Cells 2019, 8, 1576.
- Lindwall, C.; Kanje, M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol. Cell. Neurosci. 2005, 29, 269–282.
- Pathak, A.; Clark, S.; Bronfman, F.C.; Deppmann, C.D.; Carter, B.D. Long-distance regressive signaling in neural development and disease. Wiley Interdiscip. Rev. Dev. Biol. 2021, 10, e382.
- Macdonald, J.M.; Doherty, J.; Hackett, R.; Freeman, M.R. The c-Jun kinase signaling cascade promotes glial engulfment activity through activation of draper and phagocytic function. Cell Death Differ. 2013, 20, 1140–1148.
- Syc-Mazurek, S.B.; Libby, R.T. Axon injury signaling and compartmentalized injury response in glaucoma. Prog. Retin. Eye Res. 2019, 73, 100769.
- Apara, A.; Galvao, J.; Wang, Y.; Blackmore, M.; Trillo, A.; Iwao, K.; Brown, D.P.; Fernandes, K.A.; Huang, A.; Nguyen, T.; et al. KLF9 and JNK3 Interact to Suppress Axon Regeneration in the Adult CNS. J. Neurosci. 2017, 37, 9632–9644.
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 396–405.
- Puthalakath, H.; O’Reilly, L.A.; Gunn, P.; Lee, L.; Kelly, P.N.; Huntington, N.D.; Hughes, P.D.; Michalak, E.M.; McKimm-Breschkin, J.; Motoyama, N.; et al. ER Stress Triggers Apoptosis by Activating BH3-Only Protein Bim. Cell 2007, 129, 1337–1349.
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190.
- Jin, Y.; Zheng, B. Multitasking: Dual Leucine Zipper-Bearing Kinases in Neuronal Development and Stress Management Shall we add “MAPK” or “MAP3K”? Or both? Annu. Rev. Cell Dev. Biol. 2019, 35, 501–521.
- Valakh, V.; Frey, E.; Babetto, E.; Walker, L.J.; DiAntonio, A. Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol. Dis. 2015, 77, 13–25.
- Xiong, X.; Wang, X.; Ewanek, R.; Bhat, P.; DiAntonio, A.; Collins, C.A. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J. Cell Biol. 2010, 191, 211–223.
- Xiong, X.; Collins, C.A. A Conditioning Lesion Protects Axons from Degeneration via the Wallenda/DLK MAP Kinase Signaling Cascade. J. Neurosci. 2012, 32, 610–615.
- Huntwork-Rodriguez, S.; Wang, B.; Watkins, T.; Ghosh, A.S.; Pozniak, C.D.; Bustos, D.; Newton, K.; Kirkpatrick, D.S.; Lewcock, J.W. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J. Cell Biol. 2013, 202, 747–763.
- Hirai, S.-I.; Banba, Y.; Satake, T.; Ohno, S. Axon Formation in Neocortical Neurons Depends on Stage-Specific Regulation of Microtubule Stability by the Dual Leucine Zipper Kinase-c-Jun N-Terminal Kinase Pathway. J. Neurosci. 2011, 31, 6468–6480.
- Simard-Bisson, C.; Bidoggia, J.; Larouche, D.; Guérin, S.L.; Blouin, R.; Hirai, S.-I.; Germain, L. A Role for DLK in Microtubule Reorganization to the Cell Periphery and in the Maintenance of Desmosomal and Tight Junction Integrity. J. Investig. Dermatol. 2017, 137, 132–141.
- Hellal, F.; Hurtado, A.; Ruschel, J.; Flynn, K.C.; Laskowski, C.J.; Umlauf, M.; Kapitein, L.C.; Strikis, D.; Lemmon, V.; Bixby, J.; et al. Microtubule Stabilization Reduces Scarring and Causes Axon Regeneration after Spinal Cord Injury. Science 2011, 331, 928–931.
- Shin, J.E.; Cho, Y.; Beirowski, B.; Milbrandt, J.; Cavalli, V.; DiAntonio, A. Dual Leucine Zipper Kinase is Required for Retrograde Injury Signaling and Axonal Regeneration. Neuron 2012, 74, 1015–1022.
- Ko, S.-H.; Apple, E.C.; Liu, Z.; Chen, L. Age-dependent autophagy induction after injury promotes axon regeneration by limiting NOTCH. Autophagy 2020, 16, 2052–2068.
- Doherty, J.; Sheehan, A.E.; Bradshaw, R.; Fox, A.N.; Lu, T.-Y.; Freeman, M.R. PI3K Signaling and Stat92E Converge to Modulate Glial Responsiveness to Axonal Injury. PLoS Biol. 2014, 12, e1001985.
- Casas-Tintó, S.; Lolo, F.-N.; Moreno, E. Active JNK-dependent secretion of Drosophila Tyrosyl-tRNA synthetase by loser cells recruits haemocytes during cell competition. Nat. Commun. 2015, 6, 10022.
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468.
- Etchegaray, J.I.; Timmons, A.K.; Klein, A.P.; Pritchett, T.L.; Welch, E.; Meehan, T.L.; Li, C.; McCall, K. Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells. Development 2012, 139, 4029–4039.
- Morizawa, Y.M.; Hirayama, Y.; Ohno, N.; Shibata, S.; Shigetomi, E.; Sui, Y.; Nabekura, J.; Sato, K.; Okajima, F.; Takebayashi, H.; et al. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 2017, 8, 28.
- Purice, M.D.; Ray, A.; Münzel, E.J.; Pope, B.J.; Park, D.J.; Speese, S.D.; Logan, M.A. A novel Drosophila injury model reveals severed axons are cleared through a Draper/MMP-1 signaling cascade. eLife 2017, 6, e23611.
- Chiu, H.; Zou, Y.; Suzuki, N.; Hsieh, Y.-W.; Chuang, C.-F.; Wu, Y.-C.; Chang, C. Engulfing cells promote neuronal regeneration and remove neuronal debris through distinct biochemical functions of CED-1. Nat. Commun. 2018, 9, 4842.
- Li, X.; Peng, Z.; Long, L.; Lu, X.; Zhu, K.; Tuo, Y.; Chen, N.; Zhao, X.; Wang, L.; Wan, Y. Transplantation of Wnt5a-modified NSCs promotes tissue repair and locomotor functional recovery after spinal cord injury. Exp. Mol. Med. 2020, 52, 2020–2033.
- Blakely, B.D.; Bye, C.R.; Fernando, C.V.; Prasad, A.A.; Pasterkamp, R.J.; Macheda, M.L.; Stacker, S.A.; Parish, C.L. Ryk, a Receptor Regulating Wnt5a-Mediated Neurogenesis and Axon Morphogenesis of Ventral Midbrain Dopaminergic Neurons. Stem Cells Dev. 2013, 22, 2132–2144.
- Jang, S.; Park, J.-S.; Jeong, H.-S. Neural Differentiation of Human Adipose Tissue-Derived Stem Cells Involves Activation of the Wnt5a/JNK Signalling. Stem Cells Int. 2015, 2015, 1–7.
- Park, S.-Y.; Kang, M.-J.; Han, J.-S. Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells. Mol. Brain 2018, 11, 39.
- Castro-Torres, R.D.; Landa, J.; Rabaza, M.; Busquets, O.; Olloquequi, J.; Ettcheto, M.; Beas-Zarate, C.; Folch, J.; Camins, A.; Auladell, C.; et al. JNK Isoforms Are Involved in the Control of Adult Hippocampal Neurogenesis in Mice, Both in Physiological Conditions and in an Experimental Model of Temporal Lobe Epilepsy. Mol. Neurobiol. 2019, 56, 5856–5865.
- Mohammad, H.; Marchisella, F.; Ortega-Martinez, S.; Hollos, P.; Eerola, K.; Komulainen, E.; Kulesskaya, N.; Freemantle, E.; Fagerholm, V.; Savontous, E.; et al. JNK1 controls adult hippocampal neurogenesis and imposes cell-autonomous control of anxiety behaviour from the neurogenic niche. Mol. Psychiatry 2018, 23, 362–374.
