Cells exert, sense, and respond to the different physical forces through diverse mechanisms and translating them into biochemical signals. The adhesion of cells is crucial in various developmental functions, such as to maintain tissue morphogenesis and homeostasis and activate critical signaling pathways regulating survival, migration, gene expression, and differentiation. More importantly, any mutations of adhesion receptors can lead to developmental disorders and diseases.
- single-cell adhesion
- mechanotransduction
- microbial cell adhesion
- single-molecule adhesion
1. Introduction
Within a living organism, cells gather information about their surroundings and process them by chemical, electrical and mechanical signals. Chemical and electrical signals are well understood; however, there is much need to learn about mechanical signaling. Along with the traditional knowledge of chemical and electric signaling as the primary mechanism, now mechanical signaling is also known to play an essential role in a vast range of biological activities. Many cells such as immune cells [1], neurons [2], endothelial cells [3], muscle cells [4] and osteocytes [5] are mechanically sensitive, and thus, they generate force. The process known as mechanotransduction is the force between the cell and its surrounding that transmits mechanical signals which are converted into biochemical signals. Mechanotransduction and mechanical activities play a central role in various cell processes such as cell growth [6], differentiation [7], meiosis and mitosis [8], apoptosis [9] and homeostasis [10]. Thus, the malfunction of mechanical stimuli sensed by the cell can have severe consequences which lead to diseases, such as vascular diseases [11], kidney diseases [12], dystrophy [13] and cancer [14]. Thus, it is imperative to characterize and understand mechanical signaling to the same extent as chemical and electrical signaling.
The study of single-cell adhesion is one of the most important and complicated aspects to understand in life sciences. Different cells adhere to their surrounding surfaces which help them to survive. It also helps in other vital cellular processes such as embryogenesis, cell orientation, morphogenesis, cell motility, immune responses, development, and reorganization. The process involves a multitude of factors present both intrinsic and extrinsic to the cell membrane, such as the cytoskeleton [15], membrane-bound adhesion proteins [16] and glycocalyx elements [17]. The transmembrane proteins, e.g., integrins, form adhesion sites to anchor between the cell and matrix or the other cell’s adhesion molecule. These adhesion molecules are attached to the cytoskeleton, the actin filament through the focal adhesion (FA) complex, which is highly organized with a cluster of molecules (Figure 1).
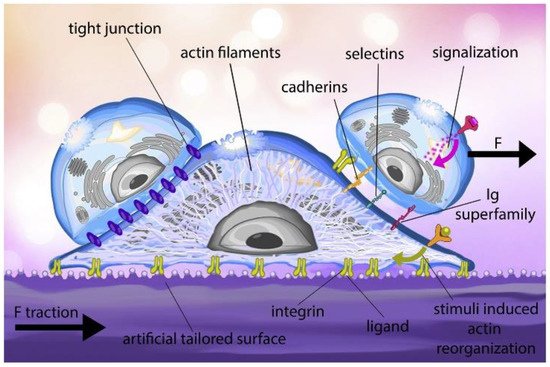
Figure 1. Cell adhesion process: the ligand on the artificially tailored surface binds to the integrin receptors found on the cell membrane. Throughout the adhesion process, the actin filament structure of the cell is reorganized, and a traction force is generated in the substrate. External stimuli also regulate the reorganization of cytoskeletal. After surface adhesion, the cell can interact with other cells through membrane proteins such as cadherins, selectins and the Immunoglobulin (Ig) superfamily. In tissues, cell junctions, a variety of multiprotein complexes (e.g., tight junctions), can form between cells to promote intercellular communication and mechanical stability. (F—Force applied by cell on its surrounding) (Reproduced with permission from [18]).
The passive cell adhesion process is an in vitro process in static medium culture (e.g., culture flasks or petri dishes), where cells undergo morphologic alterations driven by passive deformation and dynamic reorganization of the cytoskeleton. In in vitro conditions, cell adhesion progresses using passive adsorption to the surface, where the initial contact is made by the cell glycocalyx coat, followed by attachment, the spreading of the cell and the formation of focal adhesions. A recent study by Kanyo et al. discovered that the components of the glycocalyx could regulate the speed and magnitude of adhesion [19]. Moreover, an increase and decrease in adhesion can be achieved by using their technique. This is further modulated by the signalization process [20], flow circulation [21] or the cell-extracellular matrix (ECM) under blood flow in in vivo conditions, known to be a dynamic process. The in vivo dynamic cell adhesion is mediated through molecular bonding along with the non-covalent interactions between cell-surface receptors and ligands ECM. The in vivo cell adhesion cascade and signaling events involve two main phases—the docking phase (occurs between the rolling of cells to endothelial cells and to cell arrest) and the locking phase (consists of firm adhesion to the transmigration of the cell) [22]. A wide range of receptors is expressed on the surface of the cells, which helps to bind different ligands with varying affinity. The longer the cell adhesion time to the substrate, the stronger the adhesion strength, which is directly proportional to the number of integrin-ligand pairs, thus increasing the overall contact time. The lateral spacing of ligands, substrate rigidity, ligand tether length, and other factors are responsible for the adhesion strength [23]. The glycocalyx, consisting of glycolipids, proteoglycans, glycoproteins, and polysaccharides, is also involved in the cell adhesion process in addition to integrins [24]. The understanding of cell-surface interaction will help develop any devices or materials for applications in biology and biomedical purposes, as it is necessary to have the medical device in the body compatible with the surrounding tissue. Thus, it will benefit scaffold-based tissue engineering and medical fields.
2. Single-Cell Mechanical Properties for Mechanotransduction Studies
Cell-ECM mechanical interactions influence cell behavior and function. Tremendous interest has been created in developing various methods for measuring cellular mechanical properties in physiology and diseases. Numerous techniques have been designed for cell adhesion studies at the single-cell as well as population level. Here, we have categorized cell adhesion into cell attachment (cell generated forces) and detachment events (ECM mechanical forces), particularly for single-cell studies. Figure 2 shows that single-cell adhesion attachment events are focused on the cell-substrate attachment mechanism via the formation of molecular bonds. In contrast, the detachment events involve the application of force and breakage of molecular bonds to detach the adhered cell from the substrate. Table 1 summarizes the advantages and limitations of techniques used to study single-cell adhesion.
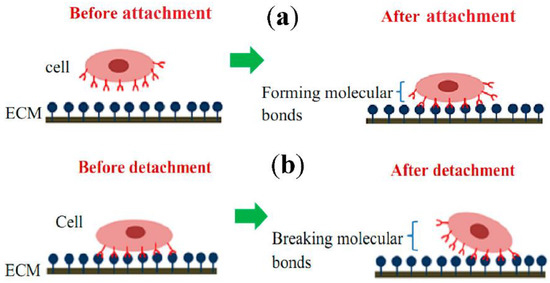
Figure 2. Schematic diagram of single-cell adhesion (a) attachment event via the formation of molecular bonds, (b) detachment event via breakage of molecular bonds. (ECM—Extracellular matrix) (Reproduced with permission from [25] under Creative Commons Attribution 4.0 International License).
Table 1. Comparison of advantages and limitations in the techniques used for cell adhesion studies.
Methods | Strength | Weaknesses | References | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Polyacrylamide—Traction Force Microscopy | (PA-TFM) | Real-time observation; | Mechanical and chemical adjustment flexibility; | No expensive equipment needed; | Fabrication is inexpensive; | Adaptable to a variety of cells | Needs to record both unstressed and stressed state of the substrate; | Suffers from uncertainties in tracking beads’ position | ||||||||||||||||||
Three Dimensional Traction Force Quantification (3D-TFM) | Real-time observation; Flexible to chemical and mechanical adjustment; | Adaptable to a large variety of cells; | Flexible to chemical and mechanical adjustment; | Adaptable to a large variety of cells | It needs a high-end confocal microscope; | Requires high computational processing; | Suffers from uncertainties in tracking beads’ position; | Limited penetration depth in scattering media; | Complications caused by phototoxicity and photobleaching; | Long measurement time required to acquire larger volumes | ||||||||||||||||
Micropatterning | (Micropillar/Micropost) | Real-time observation; Force quantification easier; | The micropillar stiffness is manipulated by its geometry; | Gives good precision over surface chemical properties on the micrometer scale | The substrate can alter a cell’s behavior; | Requires sophisticated equipment to fabricate; | Needs a skilled operator; | The sensitivity of the microposts to the particular cell type needs to be optimized | ||||||||||||||||||
Micropipette Aspiration | Real-time observation and measurement; | Common lab equipment | Alignment of probe and cell; High skilled (experienced) operator; | Operator variable; | Cell damage (hard contact) | |||||||||||||||||||||
Computer Controlled Micropipette | Higher sensitivity; | Fewer side effects; | Measured in a relatively short time period | Alignment of probe and cell requires micromanipulator; | Expensive equipment | |||||||||||||||||||||
Atomic Force Microscopy | Real-time observation; Precise data for short term adhesion studies | Alignment of probe and cell requires micromanipulator; | Time-consuming; | Need a skilled operator; | Operator variable; | Cell damage (hard contact); | Expensive equipment; | Not real-time measurement | ||||||||||||||||||
Biomembrane Force Probe | Real-time observation; | Precise data for short term adhesion studies | The low maximum force (pN); Restricted to short term adhesion; | High skilled (experienced) operator; | Operator variable; | Probe variable (fluctuation of probe due to thermal excitation) | ||||||||||||||||||||
Optical Tweezers | Real-time observation; | Precise data for short term adhesion studies; | Compatible with a microfluidic device | The low maximum force (pN); Restricted to short term adhesion; | High skilled (experienced) operator; | Operator variable; | Cell damage | |||||||||||||||||||
Microfluidics | Straightforward construction and operation; | Real-time observation and measurement; | Convenience in size (compatible with cell sizes); | Fast and simple to operate; | Non-invasive to cell | Low detachment force; | Restricted to short-term adhesion |
3. Adhesion Force Measurement for Single-Cell Microorganisms
The pathogenicity of the microorganism strongly depends on the multicellular generation assemblies known as a biofilm. The biofilm grows due to the bacterial attachment that occurs on all wet surfaces and is undesirable to be formed on human-made surfaces because of infections in the biomaterials, food contamination, plugging or corrosion of the pipeline, etc. Bacterial cell colonization is responsible for environmental change such as chemical composition [44][123], compliance [45][124], wettability [46][125] and nano topography [47][126]. Biofilm formation consists of the critical step of the surface attachment of a single bacterial cell, which is mediated by adhesins and extracellular polymeric substances (EPS) such as DNA, proteins, lipids, oligopeptides, and lipopolysaccharides. As shown in Figure 3a, the host-bacteria adaptation mechanism often depends on the extracellular components, which facilitate adhesion between approaching surfaces by reducing their energy barrier [48][127]. The EPS secretion accumulates eventually after the initial cell attachment, leading to changes in biofilm elasticity and stiffness. Initially, the proliferation of bacteria within the monolayer depends upon the level of confinement. Then, just after a few division cycles, they form microcolonies and become 3D. The physics behind the morphogenesis of microcolonies involves complex mechanical couplings between adhesion, friction, cell elongation, cell rearrangement and steric interactions (Figure 3b,c) [49][50][128,129]. The bacteria adhere to the surface during monolayer expansion; after that, bacteria build up turgor pressure during the cell elongation. This pressure is massive compared to the substrate-bacteria adhesion force, resulting in the detachment of neighboring cells and even their ruptured adhesion [51][130]. Hence, it is still unclear how they dynamically and spatially coordinate between cell elongation and adhesion forces to maintain surface-attached communities. However, biofilm formation is also essential in bioremediation, waste-water treatment, and symbiosis to form the internal microbiome of an organism. Thus, it is vital to probe EPS-mediated adhesion and quantify the single-cell-surface interaction mechanism, which may help to lead new strategies to gain insights into the biofilm such as in removal strategies, antifouling surfaces or in agriculture and health care [52][131].
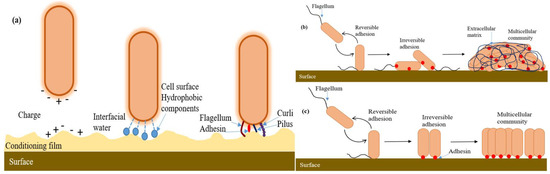
Figure 3. Biofilm formation of the microorganism. (a) First interactions between the surface and the bacterium. Once the bacterium is close to the surface, the heterogeneity of both the surface and the bacterium at the microscale level affects the adhesion process. Although the bacterial cell is usually negatively charged, the cell surface is highly heterogeneous, exhibiting different charges around the cell body. In addition, the presence of the conditioning film (orange) can modify the physicochemical properties of the solid surface (by altering charge, potential and surface tension) and thus affect local adhesion. At the nanoscale level, the thin layer of water (interfacial water, light blue) present on the surface can potentially be a barrier to cell adhesion. Hydrophobic components on the cell surface (dark blue), such as proteins, the polymeric brush layer and extracellular polysaccharides, can displace interfacial water between the bacterium and the surface and enhance hydrophobic interactions, thereby promoting close contact between the bacterium and the surface. Once the bacterium is sufficiently close to the surface (<1 nm), adhesins (red) and bacterial cell appendages (such as flagella (brown), pili (blue) and curli (purple)) can interact with the solid surface and have direct or indirect roles in adhesion. (b) For some bacteria, such as Pseudomonas fluorescens or Escherichia coli, initial surface contact is mediated by flagella and pili, which leads to polar adhesion. These bacteria transition from reversible to irreversible adhesion by repositioning the cell body to a longitudinal position. The adhesion is enhanced by the synthesis of protein or polysaccharide adhesins (LapA for P. fluorescens (red) or Pel for E. coli). Irreversible adhesion leads to the formation of a multicellular community embedded in an extracellular matrix composed of polysaccharides, proteins and DNA. (c) Bacteria, such as Caulobacter crescentus or Agrobacterium tumefaciens, initiate surface attachment through the polar flagellum. Cells stay attached via their pole and achieve permanent adhesion through the secretion of a polar adhesin (red dots: holdfast for C. crescentus and UPP for A. tumefaciens). Consequently, the incipient multicellular community is composed of cells mainly oriented polarly and is devoid of an extracellular matrix. For simplicity, the presence of a sinusoidal flagellum illustrates the motility of free-swimming bacteria, and the flagellum is the only external appendage shown. (Figure redrawn from [50][129]).
Eukaryotic cell or bacteria (single-body) detection is essential and required in a large number of sectors, such as environment monitoring, food safety, biomedicine, and pharmaceutical industries. While keeping minimum disruption of the measuring system, the risk of contamination should be kept low by using contactless detection techniques. The single-body detection of bacteria in food, drinking water, and bio-fluids (e.g., blood and urine) would help us in the early diagnosis of various infections and, hence, early-stage treatments with increased success. Moreover, single-cell detection would be of great help in the diagnosis of candidiasis (systemic fungal infection caused by Candida albicans) or to detect the presence of CTCs in the bloodstream.
4. Single-Molecule Adhesion Force
While studying mechanical forces, it is necessary to classify the interactions at either a cellular or molecular level to determine the approach and methods that can be used in obtaining information on a sensitive mechanical system. Cellular interactions involve nN range forces resulting from the application of force by whole-cell on the environment. This helps us to understand the interactions between cells and valuable information obtained to study the cell behavior and morphology affected by the forces. At molecular level interactions, the information is gained regarding conformational changes due to forces and how this mechanotransduction pathway is triggered. In a study reported by Chowdhury et al., the power of molecular forces shows notch receptor activation dependent on the energy required between 4 and 12 pN [53][146]. Thus, a considerable variation in force magnitude is being studied when compared between cellular and molecular forces.
The macromolecules that cause structural changes of the cell under the influence of the mechanical force can be directly probed using the single-molecule force spectroscopy (SMFS) technique. The insights from the SMFS experiments can make us understand the fundamentally important molecular mechanisms with the help of a single-cell, which can sense, transduce, and generate mechanical forces in vivo. SMFS can be probed into both mechanical and non-mechanical proteins on cell membranes to provide insights into their functionality. Researchers have taken an interest in a concept called free energy landscape (theoretical space, where a protein molecule diffuses and samples different conformations) [54][147] by applying mechanical forces, where the molecule is forced to sample conformations in an accelerated manner along a specific reaction coordinate. This allows us to observe and quantify discrete states of a molecule that may be biologically relevant but transient in the absence of force. The experimental apparatus commonly used for the SMFS experiments include centrifuge force microscopy, acoustic force microscopy, AFM, optical or magnetic tweezers, and BFP [55][148]. SMFS can study various biological systems where mechanical forces play an essential role. For example, cell adhesion [56][57][149,150], ECM [58][59][151,152], blood coagulation [60][61][153,154], muscles [62][63][155,156], hearing [64][65][157,158], DNA/RNA molecular motors [66][67][159,160], protein folding at the exit tunnel of the ribosome [68][69][161,162], protein unfolding and proteolysis by the proteasome [70][71][163,164], and many more. Single-molecule AFM (smAFM) and steered molecular dynamics (SMD) were used to elucidate the response of contactin-4 (CNTN-4; cell adhesion molecules—CAM localized at the neuronal membrane, a key role in maintaining mechanical integrity and signaling properties of the synapse) protein to mechanical stress. The robot-enhanced AFM technique help us to understand the existence of weak interactions stabilized between domains and provides insights into the nanomechanics of a multidomain protein [72][165]. Zhang et al. employed the in vitro SMFS technique along with confocal microscopy to study the modulation effect of the cAMP-PKA-dependent pathway on intracellular adhesion molecule-4 (ICAM-4; mediator of abnormal adhesion between RBCs and endothelial cells, appears on the RBC membrane and binds to receptor αvβ3) activation [73][166]. The study showed that the unbinding force between ICAMP-4 and αvβ3 for normal and sickle cell RBCs remained the same. In a study by Burgos-Bravo et al., OT was used as a single-molecule force transducer along with the Dudko–Hummer–Szabo model to calculate the kinetics of Thy-1/αvβ3 (integrin mediated bidirectional cell-cell communication between neurons and astrocytes) dissociation [74][167]. The OT with fluorescence imaging was used to monitor the conformational changes of individual protein in protein-DNA coupling. This technique provides 40 times higher coupling yield as compared to the existing ones [75][97].
SMFS has also enabled the functional analysis and imaging of individual receptors on the surface of the pathogen. SMFS helps to capture the binding force and dynamics of single adhesins. In the past few years, the most striking discovery is the Staphylococcal adhesins, i.e., SdrG, ClfA, and ClfB, bind to their protein-ligand with ~2 nN strong forces, equivalent to the covalent bonds. Thus, pathogens have developed much stronger bonds as compared to the known prototypical streptavidin-biotin bond (strongest in nature, ~0.2 nN) to sustain pathogenicity [76][168]. Herman et al. used both SCFS and SMFS and found that SdrG adhesin binds Fg, reflecting the DLL (dock, lock and latch) mechanism with a 2 nN force [77][169]. Harimawan and Ting used the SMFS-mediated AFM technique to probe the adhesive nature of EPS produced by B. subtilis and P. aeruginosa. The comparison indicated that the presence of polysaccharides promoted EPS adhesion strength, while a minimum adherence effect was observed in the case of proteins. Thus, enhanced cell adhesion leads to the growth of biofilm [78][170]. Another study of interest has shown the role of the adhesion forces of bacterial appendages, e.g., pili, which is incriminated in the virulence of the pathogen, as they can resist mechanical stress applied by the mucus or the blood flow in the human body. In a study by Becke et al., the binding mechanism of pili adhesions, such as RrgA and RrgB of S. pneumoniae to fibronectin (Fn) and collagen (Cn), respectively, was demonstrated [79][171]. In another study by Rivas-Pardo et al., a high mechanostability was linked to many adhesins to the Spy0128 pilus mediated adhesion of S. pyogenes [80][172]. Still, the techniques used currently remain niche even though there is a high-potential for SMFS; however, it has not been widely adopted by the molecular biosciences community.
