Osteoporosis (OP) and vascular calcification (VC) represent relevant health problems that frequently coexist in the elderly population. Traditionally, they have been considered independent processes, and mainly age-related. However, an increasing number of studies have reported their possible direct correlation, commonly defined as “bone-vascular crosstalk”. Vitamin K2 (VitK2), a family of several natural isoforms also known as menaquinones (MK), has recently received particular attention for its role in maintaining calcium homeostasis.
- vitamin K2
- menaquinone
- calcium paradox
- osteoporosis
- vascular calcification
1. Introduction
Osteoporosis (OP) is the most common bone disease that affects elderly men and women [1]. It is a metabolic skeletal disorder caused by an imbalance between bone formation and resorption, leading to a loss of bone mass and quality, skeletal structure deterioration and an increased risk of fractures [2,3][2][3]. OP is classified into a primary and secondary form with distinct etiological backgrounds. Type 1 (primary) is typical of postmenopausal women in whom the decrease in estrogenic levels is associated with an inflammatory state linked to an increase in osteoclast activity and a consequent imbalance of bone metabolism, whereas type 2 (secondary) occurs in both males and females, but its pathologic mechanism has only partially been clarified [4].
Vascular calcification (VC) is defined as the ectopic deposition of mineral matrix in the vessel wall. It occurs prevalently in aging and primary chronic conditions (hypertension, diabetes mellitus and chronic kidney disease), representing an important risk factor for cardiovascular morbidity and mortality [5,6,7,8][5][6][7][8]. Previously, the calcification of the vessel wall was known as a passive, degenerative and uncontrolled process caused only by the abnormal precipitation of calcium crystal in the vasculature [9,10][9][10]. Nowadays, a growing body of evidence suggests that it is an active, regulated event that shares similar characteristics with bone formation and metabolism. In particular, its discovery in the calcified vessel of bone-related proteins, bone-like structures and osteoblastic like-cells derived from vascular smooth muscle cells (VSMCs) has highlighted the active and cell-mediated nature of this vascular process [11,12,13,14,15,16][11][12][13][14][15][16].
Although OP and VC produce differing pathophysiological effects, their onsets frequently coexist in aging, representing one of the main public health problems with significant morbidity and mortality [17].
For many years, their coexistence was considered independent and only related to age [18], but several studies have provided support for a close link between bone and vascular health (Table 1) [19,20][19][20].
Table 1. Clinical evidence linking bone loss to vascular calcification.
| Study | Name of the Study | Number of Patients Enrolled | Key Findings |
|---|
| [21] | Framingham Heart Study | 364 women and 190 men (28–62 years old) |
Bone loss was associated with progression of aortic calcification in women over 25 years |
| [22] | Women’s Health Across the Nation Study | 90 women (45–58 years old) |
Lower BMD was related to high aortic calcification |
| [23] | MESA Study | 946 women (mean age 65.5 years old) and 963 men (mean age 64.1 years old) |
Lower BMD was associated with greater coronary artery and abdominal aortic calcium score |
| [24] | Rotterdam Study | 582 men and 694 women all >55 years old |
BMD loss was significantly associated with higher follow-up coronary artery calcification |
In this regard, many findings suggest that bone loss in OP may promote and increase the risk of cardiovascular events and vascular atherosclerosis. In the Framingham, Women’s Health Across the Nation (SWAN), Multi-Ethnic Study of Atherosclerosis (MESA) and Rotterdam studies, loss of bone mineral density (BMD) was associated with the development and progression of aortic calcification as well as with a higher risk of cardiovascular disease (CVD) mortality [21,22,23,24,25,26,27][21][22][23][24][25][26][27]. On the other hand, a direct correlation between VC and risk of bone fracture was also found. The MINOS study, for example, emphasized that men with aortic calcification present a major risk of bone fracture [28]. This was also found in healthy post-menopausal women with aortic calcification associated with lower BMD and increased risk of femur fractures (2.3-fold increase) [29].
Different hypotheses have been proposed to better explain the link between bone and vascular system, which is commonly referred to as “bone-vascular crosstalk”.
First, bone loss and vascular calcification share common risk factors, including smoking, physical activity, alcohol intake, Type 2 diabetes, menopause and hypertension. In addition, both are characterized by chronic low-grade inflammation and oxidative stress and by the involvement of bone morphogenetic proteins (BMP), osteoprotegerin, and parathyroid hormone, thus also suggesting common pathophysiologic mechanisms [19].
2. Vitamin K, a Family of Essential Fat-Soluble Compounds
Vitamin K is a family of essential fat-soluble compounds first identified in the early 1930s by the Danish biochemist Hendrik Dam during his research on cholesterol metabolism [34][30]. He observed that chicks fed a low-fat and sterols-free diet showed increased bleeding, which did not disappear when cholesterol was replaced in the diet [35][31]. Successively, Dam identified the “anti-haemorrhagic factor” in a fat-soluble compound that he named “Koagulation vitamin” (abbreviated vitamin K) to indicate its ability to clot blood [36][32].
Vitamin K naturally exists in two main forms: Vitamin K1 and Vitamin K2 [37][33]. Structurally both shared the central 2-methyl-1,4 naphthoquinone ring, named “menadione”, with a side chain on the menadione 3-carbon position [32][34].
Vitamin K1, or phylloquinone, contains a phytyl chain of four isoprenoid residues. In contrast, Vitamin K2 presents a side chain based on the repeating, from 4 to 13, of unsaturated isoprenoid units [38][35] (Figure 1).
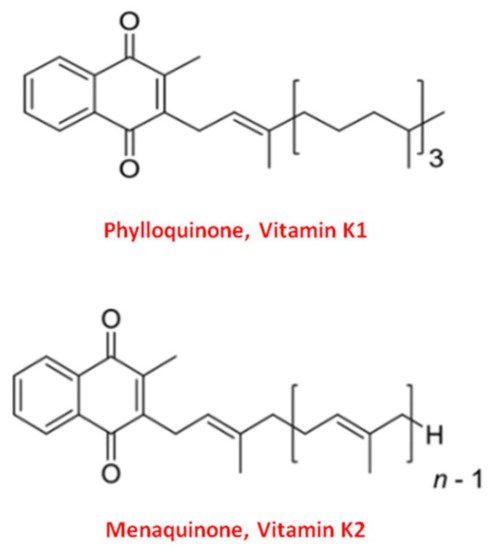
Figure 1. Molecular structure of the two main forms of Vitamin K. The upper structure represents Vitamin K1, also known as phylloquinone. The bottom structure is Vitamin K2, also known as menaquinone (MK).
All K-forms exert their biological function as cofactors for the Gamma-Glutamyl Carboxylase (GGCX), an enzyme which catalyzes the post-translational modification known as the “Vitamin K cycle” reaction [39][36]. More specifically, GGCX allows the conversion of the amino acid glutamate (Glu) into γ-carboxyglutamate (Gla) residues in at least another 14 specific proteins called “Vitamin K-dependent Proteins” (VKDPs), that, once activated, are able to bind calcium through their Gla residues [40][37].
Both Vitamin K1 and Vitamin K2 act as cofactors of GGCX in the “Vitamin K cycle”. However, Vitamin K1 triggers the activation of hepatic VKDPs implicated in the coagulation process (factor II, VII, IX and X). Whereas Vitamin K2 activates the VKDPs of extra-hepatic origin, such as Osteocalcin (OC) and Matrix Gla Protein (MGP) [37,41,42][33][38][39].
3. Vitamin K2 and Its Biomolecular Mechanisms of Action
The term VitK2 indicates a family of bioactive isoprenologs, also called “menaquinones” (MKs), which differ from each other with respect to the number of isoprenoid units in the side chain [32][34]. Thus, it is generally denoted as MK-n, where “n” (1–15) is the number of isoprenoid residues in the side chain [43,44,45][40][41][42]; for example, the isoforms menaquinone-4 (MK-4) and menaquinone-7 (MK-7) present four and seven isoprenoid units, respectively [43][40].
Most of the production of VitK2 in the human body take place at the intestinal level, where it is synthesized by intestinal bacteria of the genera Bacteroides, Lactococcus and Escherichia Coli [46,47][43][44]. However, the amount of VitK2-derived from intestinal bacteria is poorly absorbed and is not able to reach the optimal concentration required to exert the physiological functions [48,49][45][46]. Therefore, this vitamin should be supplemented daily with dietary sources such as animal-based foods (meat and egg yolk), bacterially fermented cheese, and the traditional Japanese dish called Natto, a fermented soybean in which the presence of Bacillus Subtilis reaches up to 1100 µg/100 g of VitK2 [50][47].
Regarding its metabolism, the various K2 isoforms show different bioavailability, and there is a direct correlation between their side chain length, lipophilicity, intestinal uptake and bioavailability in the human body [37,51][33][48].
As described in the previous paragraph, the compound members of VitK2 family are specific GGCX cofactors essential for the activation of extra-hepatic VKDPs. Specifically, through the activation of MPG and OC, VitK2 regulates the “calcium paradox” by reducing calcium deposition in the vessel wall and increasing it in the bone tissue, respectively. This results in the promotion of the bone mineralization process and a parallel inhibition of ectopic VC [38][35].
Osteocalcin, also known as bone γ-carboxyglutamate (Gla) protein or “Bone Gla Protein” (BGLP), was the first extra-hepatic VKDPs identified, and represents the most abundant, non-collagenous protein in the mineralized bone matrix [52,53][49][50]. It is a secretory small peptide of 49 amino acids and 5.6 kDa [54,55][51][52] synthesized by osteoblasts and resealed into bone microenvironment in two circulating forms: carboxylated (cOC) and undercarboxylated (ucOC) [56][53].
As shown in Figure 2, the carboxylated form plays an important role in the binding and precipitation of calcium-hydroxyapatite (Ca-HA), allowing bone matrix mineralization [57][54]. Once the mineralization process has been induced, cOC remains trapped in the bone matrix, and then it is released upon bone degradation into the circulation as ucOC [56,58][53][55]. Consequently, serum levels of cOC, ucOC and their ratio have to date been considered important biomarkers of bone turnover status, both in healthy and osteoporotic subjects [59,60][56][57].
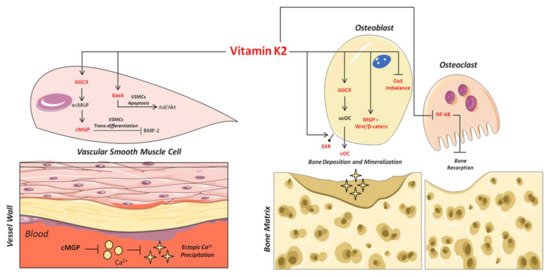
Figure 2. Mechanisms of action of VitK2 in “bone and vascular cross-talk”. At vascular level, VitK2, acting as cofactor for the enzyme GGCX, triggers the conversion of undercarboxylated MGP (ucMGP) in active carboxylated MGP (cMGP). The active cMGP could directly inhibit ectopic Ca2+ precipitation, but also VSMCs trans-differentiation through bone morphogenetic protein-2 (BMP-2). VitK2 can also inhibit VSMCs apoptosis through the Gas6/ AxL/Akt anti-apoptotic pathway. In bone tissue, VitK2 could promote osteoblasts proliferation and activity through MGP and Wnt/β-catenin pathway, control of oxidative stress (Ox-S) imbalance, via SXR receptor, and the well-established GGCX-dependent pathway. VitK2 may also exert a control of osteoclasts activities through the inhibition of NF-kB.
In addition, ucOC also plays an important function as a bone-derived hormone able to enhance insulin secretion, sensitivity, energy expenditure and glucose homeostasis [61,62,63][58][59][60]. Thus, it was recently designated as a predictor and potential therapeutic target of several metabolic diseases, including diabetes [64,65][61][62].
Similarly, Matrix Gla Protein belongs to the family of extra-hepatic VKDPs, but it plays a significant role in the prevention of ectopic calcification in vascular system. It is a secretory protein of 14 kDa, 88 amino acids and 5 Glu residues in positions 2, 37, 41, 47 and 52 [37][33].
Once synthesized by VSMCs in the vessel wall, MGP undergoes two types of post-translational modifications: γ-glutamate carboxylation and the serine phosphorylation [66,67][63][64]. Serine phosphorylation, in positions 3, 6 and 9, is catalyzed by the “Golgi-localized enzyme casein kinase” [68][65]; its precise function is not clear, although recent studies suggest that it may be implicated in MGP secretion into the extracellular micro-environment [69][66]. On the contrary, γ-carboxylation is necessary for the biological activation of MGP as an inhibitor of ectopic mineralization in the vessel wall [70,71][67][68].
The central role of MGP in vascular health was first demonstrated in 1997 through the development of MGP knock-out (−/−) mice. All mice lacking MGP died within 8 weeks of birth due to massive arterial calcification [72][69]. Subsequently, it was also found in humans that a loss-of-function mutation in the MGP gene results in Keutel syndrome [73][70], a rare autosomal recessive disease characterized by ectopic calcification of soft tissues [74][71].
Based on this, several mechanisms have been proposed to explain the inhibitory role of carboxylated MGP (cMGP) on ectopic vascular mineralization. First of all, the ability of cMGP to directly inhibit calcium-phosphate crystal precipitation was demonstrated [75][72] (Figure 2). Furthermore, its role in the inhibition of VSMC trans-differentiation into osteoblastic-like cells [76][73] was highlighted. Indeed, MGP is able to inhibit the osteoblast trans-differentiation of VSMCs through the bone morphogenetic protein-2 (BMP-2) (Figure 2). The latter is one of the main osteogenic transcription factors, and has also been found in calcified atherosclerotic plaque and medial calcified lesions, where it exerts its function as an activator of VSMC osteogenic trans-differentiation [13,76,77,78][13][73][74][75]. In this regard, the active carboxylated form of MGP inhibits BMP-2 expression and its osteoinductive properties [79,80,81][76][77][78].
Based on these findings, and given that only the active MGP exerts the inhibitory role on ectopic mineralization, the uncarboxylated form (ucMGP) is currently recognized as a specific diagnostic marker of VC and cardiovascular clinical outcomes [82][79].
References
- Eriksen, E.F.; Díez-Pérez, A.; Boonen, S. Update on long-term treatment with bisphosphonates for postmenopausal osteoporosis: A systematic review. Bone 2014, 58, 126–135.
- Hendrickx, G.; Boudin, E.; Van Hul, W. A look behind the scenes: The risk and pathogenesis of primary osteoporosis. Nat. Rev. Rheumatol. 2015, 11, 462–474.
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018, 182, 115–132.
- Nagy, E.E.; Nagy-Finna, C.; Popoviciu, H.-V.; Kovács, B. Soluble Biomarkers of Osteoporosis and Osteoarthritis, from Pathway Mapping to Clinical Trials: An Update. Clin. Interv. Aging 2020, 15, 501–518.
- Speer, M.Y.; Yang, H.-Y.; Brabb, T.; Leaf, E.; Look, A.; Lin, W.-L.; Frutkin, A.; Dichek, D.; Giachelli, C.M. Smooth Muscle Cells Give Rise to Osteochondrogenic Precursors and Chondrocytes in Calcifying Arteries. Circ. Res. 2009, 104, 733–741.
- Lampropoulos, C.E.; Papaioannou, I.; D’Cruz, D.P. Osteoporosis—A risk factor for cardiovascular disease? Nat. Rev. Rheumatol. 2012, 8, 587–598.
- Karwowski, W.; Naumnik, B.; Szczepański, M.; Myśliwiec, M. The mechanism of vascular calcification—A systematic review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, RA1–RA11.
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular Calcification: An Update on Mechanisms and Challenges in Treatment. Calcif. Tissue Int. 2013, 93, 365–373.
- Boström, K.I.; Jumabay, M.; Matveyenko, A.; Nicholas, S.B.; Yao, Y. Activation of Vascular Bone Morphogenetic Protein Signaling in Diabetes Mellitus. Circ. Res. 2011, 108, 446–457.
- Francesco, V.; Vasuri, F.; Fittipaldi, S.; Pasquinelli, G. Arterial calcification: Finger-pointing at resident and circulating stem cells. World J. Stem Cells 2014, 6, 540–551.
- Shanahan, C.M.; Cary, N.R. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation 1999, 100, 2168–2176.
- Moe, S.M.; Chen, N.X. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009, 75, 176–184.
- Tyson, K.L.; Reynolds, J.L. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 489–494.
- Bobryshev, Y.V. Transdifferentiation of smooth muscle cells into chondrocytes in atherosclerotic arteries in situ: Implications for diffuse intimal calcification. J. Pathol. 2005, 205, 641–650.
- Bostrom, K.I.; Rajamannan, N.M. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ. Res. 2011, 109, 564–577.
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2015, 25, 267–274.
- McFarlane, S.I.; Muniyappa, R. Osteoporosis and cardiovascular disease: Brittle bones and boned arteries, is there a link? Endocrine 2004, 23, 1–10.
- Sprini, D.; Rini, G.B. Correlation between osteoporosis and cardiovascular disease. Clin. Cases Miner. Bone Metab. 2014, 11, 117–119.
- Vassalle, C.; Mazzone, A. Bone loss and vascular calcification: A bi-directional interplay? Vascul. Pharmacol. 2016, 86, 77–86.
- Chen, Y.; Zhao, X. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1078–1093.
- Kiel, D.P.; Kauppila, L.I. Bone loss and the progression of abdominal aortic calcification over a 25 year period: The Framingham Heart Study. Calcif. Tissue Int. 2001, 68, 271–276.
- Farhat, G.N.; Strotmeyer, E.S. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: The health, aging, and body composition study. Calcif. Tissue Int. 2006, 79, 102–111.
- Hyder, J.A.; Allison, M.A. Association of coronary artery and aortic calcium with lumbar bone density: The MESA Abdominal Aortic Calcium Study. Am. J. Epidemiol. 2009, 169, 186–194.
- Campos-Obando, N.; Kavousi, M. Bone health and coronary artery calcification: The Rotterdam Study. Atherosclerosis 2015, 241, 278–283.
- Trivedi, D.P.; Khaw, K.T. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos. Int. 2001, 12, 259–265.
- Farhat, G.N.; Newman, A.B. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos. Int. 2007, 18, 999–1008.
- Choi, S.H.; An, J.H. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin. Endocrinol. 2009, 71, 644–651.
- Schulz, E.; Arfai, K. Aortic calcification and the risk of osteoporosis and fractures. J. Clin. Endocrinol. Metab. 2004, 89, 4246–4253.
- Bagger, Y.Z.; Tanko, L.B. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J. Intern. Med. 2006, 259, 598–605.
- Dam, H.; Schonheyder, F. The occurrence and chemical nature of vitamin K. Biochem. J. 1936, 30, 897–901.
- Dam, H. The formation of coprosterol in the intestine: The action of intestinal bacteria on cholesterol. Biochem. J. 1934, 28, 820–825.
- Dam, H. The antihaemorrhagic vitamin of the chick. Biochem. J. 1935, 29, 1273–1285.
- Willems, B.A.; Vermeer, C. The realm of vitamin K dependent proteins: Shifting from coagulation toward calcification. Mol. Nutr. Food Res. 2014, 58, 1620–1635.
- Shearer, M.J.; Newman, P. Metabolism and cell biology of vitamin K. Thromb. Haemost. 2008, 100, 530–547.
- Vermeer, C. Vitamin K: The effect on health beyond coagulation—An overview. Food Nutr. Res. 2012, 56.
- Tie, J.K.; Stafford, D.W. Structural and functional insights into enzymes of the vitamin K cycle. J. Thromb. Haemost. 2016, 14, 236–247.
- Hamidi, M.S.; Cheung, A.M. Vitamin K and musculoskeletal health in postmenopausal women. Mol. Nutr. Food Res. 2014, 58, 1647–1657.
- Schurgers, L.J. Vitamin K: Key vitamin in controlling vascular calcification in chronic kidney disease. Kidney Int. 2013, 83, 782–784.
- Simes, D.C.; Viegas, C.S.B. Vitamin K as a Powerful Micronutrient in Aging and Age-Related Diseases: Pros and Cons from Clinical Studies. Int. J. Mol. Sci. 2019, 20, 4150.
- Grober, U.; Reichrath, J. Vitamin K: An old vitamin in a new perspective. Dermatoendocrinology 2014, 6, e968490.
- Palermo, A.; Tuccinardi, D. Vitamin K and osteoporosis: Myth or reality? Metabolism 2017, 70, 57–71.
- Sato, T.; Inaba, N. MK-7 and Its Effects on Bone Quality and Strength. Nutrients 2020, 12, 965.
- Conly, J.M.; Stein, K. Quantitative and qualitative measurements of K vitamins in human intestinal contents. Am. J. Gastroenterol. 1992, 87, 311–316.
- Morishita, T.; Tamura, N. Production of menaquinones by lactic acid bacteria. J. Dairy Sci. 1999, 82, 1897–1903.
- Suttie, J.W. The importance of menaquinones in human nutrition. Annu. Rev. Nutr. 1995, 15, 399–417.
- Walther, B.; Karl, J.P. Menaquinones, bacteria, and the food supply: The relevance of dairy and fermented food products to vitamin K requirements. Adv. Nutr. 2013, 4, 463–473.
- Iwamoto, J. Vitamin K(2) therapy for postmenopausal osteoporosis. Nutrients 2014, 6, 1971–1980.
- Gijsbers, B.L.; Jie, K.S. Effect of food composition on vitamin K absorption in human volunteers. Br. J. Nutr. 1996, 76, 223–229.
- Young, M.F. Bone matrix proteins: Their function, regulation, and relationship to osteoporosis. Osteoporos. Int. 2003, 14 (Suppl. S3), S35–S42.
- Gorski, J.P. Biomineralization of bone: A fresh view of the roles of non-collagenous proteins. Front. Biosci. 2011, 16, 2598–2621.
- Gundberg, C.M.; Lian, J.B. Vitamin K-dependent carboxylation of osteocalcin: Friend or foe? Adv. Nutr. 2012, 3, 149–157.
- Booth, S.L.; Centi, A. The role of osteocalcin in human glucose metabolism: Marker or mediator? Nat. Rev. Endocrinol. 2013, 9, 43–55.
- Ferron, M.; Wei, J. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308.
- Zoch, M.L.; Clemens, T.L. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49.
- Lacombe, J.; Karsenty, G. In vivo analysis of the contribution of bone resorption to the control of glucose metabolism in mice. Mol. Metab. 2013, 2, 498–504.
- Kalaiselvi, V.S.; Prabhu, K. The association of serum osteocalcin with the bone mineral density in post menopausal women. J. Clin. Diagn. Res. 2013, 7, 814–816.
- Eastell, R.; Pigott, T. Diagnosis of endocrine disease: Bone turnover markers: Are they clinically useful? Eur. J. Endocrinol. 2018, 178, R19–R31.
- Lee, N.K.; Sowa, H. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469.
- Karsenty, G.; Oury, F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol. Cell. Endocrinol. 2014, 382, 521–526.
- Kanazawa, I. Osteocalcin as a hormone regulating glucose metabolism. World J. Diabetes 2015, 6, 1345–1354.
- Ducy, P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 2011, 54, 1291–1297.
- Yan, M.K.; Khalil, H. Vitamin supplements in type 2 diabetes mellitus management: A review. Diabetes Metab. Syndr. 2017, 11 (Suppl. S2), S589–S595.
- Schurgers, L.J.; Cranenburg, E.C. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603.
- Schurgers, L.J.; Uitto, J. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226.
- Price, P.A.; Rice, J.S. Conserved phosphorylation of serines in the Ser-X-Glu/Ser(P) sequences of the vitamin K-dependent matrix Gla protein from shark, lamb, rat, cow, and human. Protein Sci. 1994, 3, 822–830.
- Wajih, N.; Borras, T. Processing and transport of matrix γ-carboxyglutamic acid protein and bone morphogenetic protein-2 in cultured human vascular smooth muscle cells: Evidence for an uptake mechanism for serum fetuin. J. Biol. Chem. 2004, 279, 43052–43060.
- Chatrou, M.L.; Winckers, K. Vascular calcification: The price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. 2012, 26, 155–166.
- Scheiber, D.; Veulemans, V. High-Dose Menaquinone-7 Supplementation Reduces Cardiovascular Calcification in a Murine Model of Extraosseous Calcification. Nutrients 2015, 7, 6991–7011.
- Luo, G.; Ducy, P. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81.
- Munroe, P.B.; Olgunturk, R.O. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat. Genet. 1999, 21, 142–144.
- Hur, D.J.; Raymond, G.V. A novel MGP mutation in a consanguineous family: Review of the clinical and molecular characteristics of Keutel syndrome. Am. J. Med. Genet. A 2005, 135, 36–40.
- O’Young, J.; Liao, Y. Matrix Gla protein inhibits ectopic calcification by a direct interaction with hydroxyapatite crystals. J. Am. Chem. Soc. 2011, 133, 18406–18412.
- Steitz, S.A.; Speer, M.Y. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154.
- Engelse, M.A.; Neele, J.M. Vascular calcification: Expression patterns of the osteoblast-specific gene core binding factor alpha-1 and the protective factor matrix gla protein in human atherogenesis. Cardiovasc. Res. 2001, 52, 281–289.
- Shioi, A.; Taniwaki, H. Monckeberg’s medial sclerosis and inorganic phosphate in uremia. Am. J. Kidney Dis. 2001, 38, S47–S49.
- Wallin, R.; Cain, D. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2). Thromb. Haemost. 2000, 84, 1039–1044.
- Bostrom, K.; Tsao, D. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J. Biol. Chem. 2001, 276, 14044–14052.
- Zebboudj, A.F.; Shin, V. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J. Cell. Biochem. 2003, 90, 756–765.
- Puzantian, H.; Akers, S.R. Circulating Dephospho-Uncarboxylated Matrix Gla-Protein Is Associated with Kidney Dysfunction and Arterial Stiffness. Am. J. Hypertens. 2018, 31, 988–994.
