Lactiplantibacillus plantarum
(Basonym
Lactobacillus plantarum
) is a good candidate for developing oral vaccines because it survives gastrointestinal conditions transiently colonizing the intestinal tract, it beneficially modulates the mucosal immune responses not only locally (intestinal mucosa) but in distant mucosal sites as well (respiratory mucosa) and there are molecular techniques available for the manipulation of its genome.
- Lactiplantibacillus plantarum
- COVID-19
- SARS-CoV-2
- vaccine
- immunobiotics
- recombinant lactobacilli
- mucosal immunity
- antiviral immunity
1. Introduction
Coronaviruses are positive-sense single-stranded RNA (ssRNA) viruses with a wide range of hosts. To date, seven human coronaviruses (HCoV) were identified as human-pathogens; HCoV-229E, HCoV-OC43, HCoV-NL63 and HCoV-HKU1 (responsible for non-sever common cold), the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) isolated in 2003 in China and the Middle East Respiratory Syndrome coronavirus (MERS-CoV) that emerged in Middle Eastern countries in 2012 [1]. Both SARS-CoV and MERS-CoV are highly pathogenic viruses that caused nosocomial outbreaks with high case-fatality rates. The seventh and most recently identified human coronavirus is the SARS-CoV-2, responsible for the coronavirus disease 2019 (COVID-19).
COVID-19 emerged in December 2019 in Wuhan, China, and rapidly spread worldwide in a few months due to its high transmissibility and pathogenicity. Although SARS-CoV-2 induce a milder clinical commitment than SARS-CoV or and MERS-CoV, COVID-19 has affected more than 100 million people worldwide, causing the death of 2,217,005 persons according to the WHO’s situation report on 1 February 2021 (WHO, 2021) [2].
Several transmission-mitigation strategies have been implemented in most countries, including social distancing and lockdowns. In addition, a vaccine development race started as never seen before. Currently, several COVID-19 vaccines have finished the phase III clinical testing or been granted an emergency use authorization, including BBIBP-CorV (Sinopharm) and CoronaVac (Sinovac) in China, Pfizer-BioNTech COVID-19 vaccine (Pfizer) and mRNA-1273 vaccine (Moderna) in the United States, and Sputnik-V vaccine in Russia, offering hope for controlling the SARS-CoV-2 infection and stop the pandemic in the near future [3,4,5].
Several transmission-mitigation strategies have been implemented in most countries, including social distancing and lockdowns. In addition, a vaccine development race started as never seen before. Currently, several COVID-19 vaccines have finished the phase III clinical testing or been granted an emergency use authorization, including BBIBP-CorV (Sinopharm) and CoronaVac (Sinovac) in China, Pfizer-BioNTech COVID-19 vaccine (Pfizer) and mRNA-1273 vaccine (Moderna) in the United States, and Sputnik-V vaccine in Russia, offering hope for controlling the SARS-CoV-2 infection and stop the pandemic in the near future [3][4][5].
Since the protective immune responses against SARS-CoV-2 are poorly understood, it is unclear which vaccine strategies will be the most successful. The majority of COVID-19 vaccines have been designed to induce anti-SARS-CoV-2 neutralizing antibodies to prevent virus entry into the target cells. In some cases, vaccines are designed to induce both humoral and cellular immunity that could help limiting viral replication in the infected host [5]. Of note, most of the vaccines are designed for parenteral use, and therefore, are capable of mainly inducing systemic immunity despite of the fact that SARS-CoV-2 infects mucosal tissues and that human-to-human transmission is mediated by respiratory droplets and the fecal-oral transmission has not been ruled out [6,7]. The need to generate not only humoral but also cellular immunity against SARS-CoV-2 and to induce protective immunity in the mucosal surfaces where this virus initiates its replication allows us to speculate that this first generation of COVID-19 vaccines should be replaced later by a new generation of vaccines that allow overcoming the aforementioned limitations.
Since the protective immune responses against SARS-CoV-2 are poorly understood, it is unclear which vaccine strategies will be the most successful. The majority of COVID-19 vaccines have been designed to induce anti-SARS-CoV-2 neutralizing antibodies to prevent virus entry into the target cells. In some cases, vaccines are designed to induce both humoral and cellular immunity that could help limiting viral replication in the infected host [5]. Of note, most of the vaccines are designed for parenteral use, and therefore, are capable of mainly inducing systemic immunity despite of the fact that SARS-CoV-2 infects mucosal tissues and that human-to-human transmission is mediated by respiratory droplets and the fecal-oral transmission has not been ruled out [6][7]. The need to generate not only humoral but also cellular immunity against SARS-CoV-2 and to induce protective immunity in the mucosal surfaces where this virus initiates its replication allows us to speculate that this first generation of COVID-19 vaccines should be replaced later by a new generation of vaccines that allow overcoming the aforementioned limitations.
Protective mucosal immune responses are most effectively induced by mucosal immunization through oral or nasal routes, whereas injected vaccines are generally poor inducers of mucosal immunity. However, the induction of mucosal immune responses is challenging due to the physical-chemical barriers of the mucosal surfaces and the tendency to induce tolerance [8]. Therefore, mucosal vaccine delivery systems require high doses of antigens and efficient mucosal adjuvants. Lactic acid bacteria (LAB) have been proposed as both delivery vectors and mucosal adjuvants [9,10]. In the last decades, recombinant LAB have been tested as new-generation oral vaccine vectors due to their natural resistance to gastrointestinal conditions and their ability to modulate both intestinal innate and adaptive immune responses. In this sense,
Protective mucosal immune responses are most effectively induced by mucosal immunization through oral or nasal routes, whereas injected vaccines are generally poor inducers of mucosal immunity. However, the induction of mucosal immune responses is challenging due to the physical-chemical barriers of the mucosal surfaces and the tendency to induce tolerance [8]. Therefore, mucosal vaccine delivery systems require high doses of antigens and efficient mucosal adjuvants. Lactic acid bacteria (LAB) have been proposed as both delivery vectors and mucosal adjuvants [9][10]. In the last decades, recombinant LAB have been tested as new-generation oral vaccine vectors due to their natural resistance to gastrointestinal conditions and their ability to modulate both intestinal innate and adaptive immune responses. In this sense,
Lactiplantibacillus plantarum
(Basonym
Lactobacillus plantarum
) is a good candidate for developing oral vaccines because it survives gastrointestinal conditions transiently colonizing the intestinal tract, it beneficially modulates the mucosal immune responses not only locally (intestinal mucosa) but in distant mucosal sites as well (respiratory mucosa) and there are molecular techniques available for the manipulation of its genome.
2.
L. plantarum
as Modulators of Antiviral Immune Responses in Mucosal Tissues
The intestinal microbiota plays a key role in maintaining mucosal antiviral immunity in both local mucosal tissues (intestinal mucosa) and in distal mucosal sites (respiratory mucosa) [80,81,82,83]. Of note, most scientific works highlighted the ability of the intestinal microbiota to modulate the innate antiviral defense mechanisms of the gut and the respiratory tract immune by interacting with epithelial and antigen presenting cells. Furthermore, this modulation of the mucosal innate immune response also influences the mucosal antiviral cellular and humoral adaptive immune responses [82,84,85]. Remarkably, not all the members of the intestinal microbiota contribute equally to the beneficial modulation of the mucosal antiviral immunity. This opened the possibility of exploring particular strains of beneficial bacteria with immunomodulatory capacities, referred to as immunobiotics, in order to increase antiviral defenses in mucosal tissues [82,84,85]. Among these beneficial microorganisms with immunomodulatory capabilities, some
The intestinal microbiota plays a key role in maintaining mucosal antiviral immunity in both local mucosal tissues (intestinal mucosa) and in distal mucosal sites (respiratory mucosa) [11][12][13][14]. Of note, most scientific works highlighted the ability of the intestinal microbiota to modulate the innate antiviral defense mechanisms of the gut and the respiratory tract immune by interacting with epithelial and antigen presenting cells. Furthermore, this modulation of the mucosal innate immune response also influences the mucosal antiviral cellular and humoral adaptive immune responses [13][15][16]. Remarkably, not all the members of the intestinal microbiota contribute equally to the beneficial modulation of the mucosal antiviral immunity. This opened the possibility of exploring particular strains of beneficial bacteria with immunomodulatory capacities, referred to as immunobiotics, in order to increase antiviral defenses in mucosal tissues [13][15][16]. Among these beneficial microorganisms with immunomodulatory capabilities, some
L. plantarum strains are interesting alternatives to improve local and distal antiviral immune responses when orally administered [86,87,88].
strains are interesting alternatives to improve local and distal antiviral immune responses when orally administered [17][18][19].
2.1. Modulation of Intestinal Antiviral Immune Responses by L. plantarum
The interactions of epithelial cells with microorganisms reaching the intestinal mucosa play a central role in determining the type of immune responses that those microbes will trigger [89,90]. For viruses, the early intestinal innate response is the secretion of type I IFNs and the production of several ISGs that exert antiviral activities. Intestinal beneficial microorganisms have been shown to improve these innate antiviral mechanisms [82], and therefore, the microbe-intestinal epithelial cell interaction has been used as an effective tool for selecting immunobiotics to help fight intestinal viral infections. In this regard, we demonstrated that an originally established porcine intestinal epithelial cell line (PIE cells) is able to respond to ligands of the TLR3 and RIG-I receptors as well as to rotavirus infection [91,92]. Furthermore, the in vitro PIE cells system serves for screening of immunobiotic LAB strains with the capacity to differentially modulate IFN-β production and ISGs expression upon poly(I:C) stimulation or rotavirus challenge [82]. Our studies in PIE cells demonstrated that theThe interactions of epithelial cells with microorganisms reaching the intestinal mucosa play a central role in determining the type of immune responses that those microbes will trigger [20][21]. For viruses, the early intestinal innate response is the secretion of type I IFNs and the production of several ISGs that exert antiviral activities. Intestinal beneficial microorganisms have been shown to improve these innate antiviral mechanisms [13], and therefore, the microbe-intestinal epithelial cell interaction has been used as an effective tool for selecting immunobiotics to help fight intestinal viral infections. In this regard, we demonstrated that an originally established porcine intestinal epithelial cell line (PIE cells) is able to respond to ligands of the TLR3 and RIG-I receptors as well as to rotavirus infection [22][23]. Furthermore, the in vitro PIE cells system serves for screening of immunobiotic LAB strains with the capacity to differentially modulate IFN-β production and ISGs expression upon poly(I:C) stimulation or rotavirus challenge [13]. Our studies in PIE cells demonstrated that the
L. plantarumstrains CRL1506 and MPL16 increased IFN-γ and IFN-β levels with a concomitant up-regulation of the ISGs, while other
L. plantarum strains such as CRL681 could not [87,88,93]. Among the ISGs up-regulated bystrains such as CRL681 could not [18][19][24]. Among the ISGs up-regulated by
L. plantarum CRL1506 and MPL16, a notable effect was observed for Mx2 and RNAseL, which are important for the protection of the intestinal mucosa against viruses [94]. These and probably several other ISGs induced by CRL1506 and MPL16 strains through the IFN-α/β pathway would be related to the lower rotavirus replication found in lactobacilli-treated PIE cells. We confirmed in vivo the antiviral immunomodulatory properties ofCRL1506 and MPL16, a notable effect was observed for Mx2 and RNAseL, which are important for the protection of the intestinal mucosa against viruses [25]. These and probably several other ISGs induced by CRL1506 and MPL16 strains through the IFN-α/β pathway would be related to the lower rotavirus replication found in lactobacilli-treated PIE cells. We confirmed in vivo the antiviral immunomodulatory properties of
L. plantarum CRL1506 and MPL16 by using mice models [87,95,96]. Both strains significantly increased the production of intestinal type I IFNs and IFN-γ in mice after the challenge with the TLR3 agonist poly(I:C). In line with this, other studies reported the capacity ofCRL1506 and MPL16 by using mice models [18][26][27]. Both strains significantly increased the production of intestinal type I IFNs and IFN-γ in mice after the challenge with the TLR3 agonist poly(I:C). In line with this, other studies reported the capacity of
L. plantarumstrains to enhance the antiviral immunity in the intestinal epithelium (
Figure 3). The antiviral immunomodulatory properties of1). The antiviral immunomodulatory properties of
L. plantarum Lp-1 was studied in pig jejunal cells (IPEC-J2 cells) infected with the coronavirus transmissible gastroenteritis virus (TGEV), which is one of the most important gastrointestinal pathogens causing diarrhea, vomiting and high mortality in piglets [97]. The experimental data showed that the TGEV titers in IPEC-J2 cells treated withLp-1 was studied in pig jejunal cells (IPEC-J2 cells) infected with the coronavirus transmissible gastroenteritis virus (TGEV), which is one of the most important gastrointestinal pathogens causing diarrhea, vomiting and high mortality in piglets [28]. The experimental data showed that the TGEV titers in IPEC-J2 cells treated with
L. plantarum Lp-1 were significantly lower than controls. This effect was related to the ability of the Lp-1 strain to augment the expression of IFN-β, ZAP, MX2, MX1, PKR, OASL and ISG15 in IPEC-J2 cells after TGEV infection [97].Lp-1 were significantly lower than controls. This effect was related to the ability of the Lp-1 strain to augment the expression of IFN-β, ZAP, MX2, MX1, PKR, OASL and ISG15 in IPEC-J2 cells after TGEV infection [28].
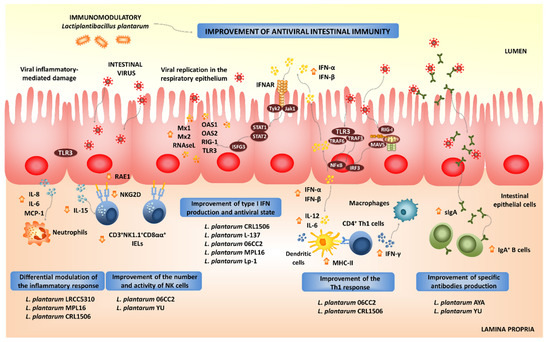
Beneficial effects of orally administered immunobiotic
strains on the resistance and immune responses against virus in the intestinal mucosa.
The activation of PRRs-mediated signaling pathways in intestinal epithelial cells not only stimulates the production of type I IFNs and ISGs, but also the expression of pro-inflammatory cytokines and chemokines. The activation of the NF-κB signaling pathway stimulates the production of inflammatory cytokines, including IL-1β, IL-8, GM-CSF, CCL-3 and CXCL-10 in the intestinal epithelium after viral infection [29][30]. This inflammatory response is the first line of host defense against viruses: it recruits and activates immune cells; however, if it is deregulated or it extends excessively in time, it may lead to tissue damage and epithelial barrier dysfunction [29][30]. Then, the efficient regulation of intestinal inflammatory responses induced by virus is essential to achieve full protection against the infections. Immunobiotics can also help in the modulation of intestinal inflammatory responses. We demonstrated that
L. plantarum strains CRL1506 and MPL16 differentially modulated the production of pro-inflammatory mediators in PIE cells upon the activation of TLR3 [87,88,93]. Moreover, both CRL1506 and MPL16 strains reduced TLR3-induced small intestinal injury in mice by regulating the production of pro-inflammatory cytokines and the interaction of intestinal epithelial cells and intraepithelial lymphocytes [87,95]. It was shown that the abnormal TLR3 signaling induce the expression of IL-15 in the intestinal epithelium, leading to the activation of CD3strains CRL1506 and MPL16 differentially modulated the production of pro-inflammatory mediators in PIE cells upon the activation of TLR3 [18][19][24]. Moreover, both CRL1506 and MPL16 strains reduced TLR3-induced small intestinal injury in mice by regulating the production of pro-inflammatory cytokines and the interaction of intestinal epithelial cells and intraepithelial lymphocytes [18][26]. It was shown that the abnormal TLR3 signaling induce the expression of IL-15 in the intestinal epithelium, leading to the activation of CD3
+NK1.1
+CD8αα
+ intraepithelial lymphocytes, which increase the apoptosis of intestinal epithelial cells induced by the perforin pathway [100]. In mice, the administration of poly(I:C) activates TLR3 in the intestinal mucosa, inducing villous atrophy and mucosal erosion [101]. In line with these findings, it was demonstrated that the blocking of the IL-15 receptor partially protected mice from poly(I:C)-induced small intestinal injury [101]. The activation of TLR3 stimulates intestinal epithelial cells to express retinoic acid early inducible 1 (RAE1) and induces CD3intraepithelial lymphocytes, which increase the apoptosis of intestinal epithelial cells induced by the perforin pathway [31]. In mice, the administration of poly(I:C) activates TLR3 in the intestinal mucosa, inducing villous atrophy and mucosal erosion [32]. In line with these findings, it was demonstrated that the blocking of the IL-15 receptor partially protected mice from poly(I:C)-induced small intestinal injury [32]. The activation of TLR3 stimulates intestinal epithelial cells to express retinoic acid early inducible 1 (RAE1) and induces CD3
+NK1.1
+CD8αα
+ cells to express NKG2D through IL-15 [102]. Then, the RAE1-NKG2D interaction has a prominent role in the intestinal injury. Interestingly, we demonstrated that mice pretreated withcells to express NKG2D through IL-15 [33]. Then, the RAE1-NKG2D interaction has a prominent role in the intestinal injury. Interestingly, we demonstrated that mice pretreated with
L. plantarumCRL1506 before TLR3 activation responded with reduced levels of IL-15 and RAE1 in intestinal epithelial cells and with diminished expression of NKG2D in CD3
+NK1.1
+CD8αα
+ intraepithelial lymphocytes [95]. Moreover, mice treated with CRL1506 or MPL16 strains before a poly(I:C) challenge significantly reduced the levels of intestinal TNF-α, IL-1β and IL-8 [87,95]. The beneficial effect of theintraepithelial lymphocytes [26]. Moreover, mice treated with CRL1506 or MPL16 strains before a poly(I:C) challenge significantly reduced the levels of intestinal TNF-α, IL-1β and IL-8 [18][26]. The beneficial effect of the
L. plantarumstrains in the intestinal inflammatory injury were translated into significant reductions of body weight loss and intestinal histological damage after poly(I:C) challenge.
L. plantarum LRCC5310, and particularly its exopolysaccharides (EPSs), induces an antagonistic effect against human rotavirus. MA104 cells treated with the LRCC5310 strain or its EPSs had significantly lower cytopathic alterations and reduced viral replication when compared to untreated controls [103]. In addition, orally administeredLRCC5310, and particularly its exopolysaccharides (EPSs), induces an antagonistic effect against human rotavirus. MA104 cells treated with the LRCC5310 strain or its EPSs had significantly lower cytopathic alterations and reduced viral replication when compared to untreated controls [34]. In addition, orally administered
L. plantarum LRCC5310 increased the protection of young mice against rotavirus infection reducing the duration of diarrhea and viral shedding and preventing the destruction of the intestinal epithelium integrity [103]. Complementary in vitro studies demonstrated thatLRCC5310 increased the protection of young mice against rotavirus infection reducing the duration of diarrhea and viral shedding and preventing the destruction of the intestinal epithelium integrity [34]. Complementary in vitro studies demonstrated that
L. plantarum LRCC5310 EPS increases IL-10 and reduces IL-1β and TNF-α in both intestinal epithelial cells and macrophages [103]. A clinical trial whereLRCC5310 EPS increases IL-10 and reduces IL-1β and TNF-α in both intestinal epithelial cells and macrophages [34]. A clinical trial where
L. plantarum LRCC5310 was administered in infants with rotavirus enteritis did not show significant differences between control and LRCC5310-treated groups at the beginning of the study in terms of clinical symptoms and laboratory inflammation markers. However, at the end of the trial, there was a significant reduction in the severity and duration of diarrhea as well as rotavirus titers in LRCC5310-treated children when compared to controls [104].LRCC5310 was administered in infants with rotavirus enteritis did not show significant differences between control and LRCC5310-treated groups at the beginning of the study in terms of clinical symptoms and laboratory inflammation markers. However, at the end of the trial, there was a significant reduction in the severity and duration of diarrhea as well as rotavirus titers in LRCC5310-treated children when compared to controls [35].
We also demonstrated that
L. plantarum CRL1506 modulated the expression of pro-inflammatory cytokines and influenced the activation and maturation of intestinal antigen presenting cells [93]. It has been reported that DCs produce IFN-α/β and undergo maturation in response to type I IFNs. Moreover, IFN-α/β have been shown to potently enhance the activation of DCs in vivo, serving as an important link between innate and adaptive immunity in the context of viral infections [105]. In fact, the in vitro treatment of DCs with type I IFNs activates these cells and increases their capacity to initiate T cell responses. DCs stimulated with type I IFNs have improved expression of MHC-II as well as the co-stimulatory molecules CD40 and CD86. Furthermore, IFN-treated DCs had a higher ability to stimulate CD4CRL1506 modulated the expression of pro-inflammatory cytokines and influenced the activation and maturation of intestinal antigen presenting cells [24]. It has been reported that DCs produce IFN-α/β and undergo maturation in response to type I IFNs. Moreover, IFN-α/β have been shown to potently enhance the activation of DCs in vivo, serving as an important link between innate and adaptive immunity in the context of viral infections [36]. In fact, the in vitro treatment of DCs with type I IFNs activates these cells and increases their capacity to initiate T cell responses. DCs stimulated with type I IFNs have improved expression of MHC-II as well as the co-stimulatory molecules CD40 and CD86. Furthermore, IFN-treated DCs had a higher ability to stimulate CD4
+and CD8
+ T cells that produce IFN-γ [106,107]. In line with these reports, our in vitro and in vivo results demonstrated the immunobioticT cells that produce IFN-γ [37][38]. In line with these reports, our in vitro and in vivo results demonstrated the immunobiotic
L. plantarum strains CRL1506 and MPL16 were able to improve intestinal Th1 response, as evidenced by the augmented expression of MHC-II, IL-1β, IL-6 and IFN-γ in the intestinal DCs [87,88,93]. Thus,strains CRL1506 and MPL16 were able to improve intestinal Th1 response, as evidenced by the augmented expression of MHC-II, IL-1β, IL-6 and IFN-γ in the intestinal DCs [18][19][24]. Thus,
L. plantarumwould be capable of stimulating the intestinal adaptive immunity through its ability to modulate antigen presentation in DCs in a type I IFN-dependent manner. Moreover, this hypothesis prompted us to evaluate whether orally administered
L. plantarum CRL1506 or its non-viable bacterium-like particles (BLPs) were able to modify the immune response to an oral vaccine [108]. In our hands,CRL1506 or its non-viable bacterium-like particles (BLPs) were able to modify the immune response to an oral vaccine [39]. In our hands,
L. plantarumCRL1506 or its BLPs showed adjuvant capacities when used together with a rotavirus vaccine. Immunization of mice with
L. plantarum CRL1506 or its BLPs significantly improved the specific Th1 mucosal and systemic immune responses generated against the rotavirus antigens. Furthermore, our immunization protocol not only stimulated the cellular immunity but also increased levels of intestinal IgA- and serum IgG-specific antibodies were found in animals immunized with rotavirus vaccine and the CRL1506 strain [108]. These results provide additional evidence for the hypothesis that gives a key role to type I IFNs in the efficient generation of adaptive responses induced by immunobioticCRL1506 or its BLPs significantly improved the specific Th1 mucosal and systemic immune responses generated against the rotavirus antigens. Furthermore, our immunization protocol not only stimulated the cellular immunity but also increased levels of intestinal IgA- and serum IgG-specific antibodies were found in animals immunized with rotavirus vaccine and the CRL1506 strain [39]. These results provide additional evidence for the hypothesis that gives a key role to type I IFNs in the efficient generation of adaptive responses induced by immunobiotic
L. plantarum treatments since it was demonstrated that type I IFNs promote antibody responses in vivo [109]. In fact, type I IFNs were shown to increase the synthesis of antigen-specific antibodies of all subclasses of IgG and induced IgG2a and IgG3 antibodies far more effectively than widely used adjuvants [109]. In line with our results, the oral administration oftreatments since it was demonstrated that type I IFNs promote antibody responses in vivo [40]. In fact, type I IFNs were shown to increase the synthesis of antigen-specific antibodies of all subclasses of IgG and induced IgG2a and IgG3 antibodies far more effectively than widely used adjuvants [40]. In line with our results, the oral administration of
L. plantarum 06CC2 significantly increased IFN-γ and IL-12p40 expression in Peyer’s patches of unchallenged [110] as well as in Herpes Simplex Virus type 1 (HSV-1)-infected mice [111]. Furthermore, the lactobacilli treatment improved splenic NK cell activity and IFN-γ-producing cells. This immunomodulatory effect induced by the 06CC2 strain correlated with reduced HSV-1 titers in skin lesions.06CC2 significantly increased IFN-γ and IL-12p40 expression in Peyer’s patches of unchallenged [41] as well as in Herpes Simplex Virus type 1 (HSV-1)-infected mice [42]. Furthermore, the lactobacilli treatment improved splenic NK cell activity and IFN-γ-producing cells. This immunomodulatory effect induced by the 06CC2 strain correlated with reduced HSV-1 titers in skin lesions.
Of note, our and other groups proved that
L. plantarumstrains had different adjuvant capacities in terms of their ability to improve the intestinal cellular and humoral immune responses triggered by virus infection or vaccination. Both humoral and cellular intestinal specific immune responses were significantly upgraded in mice immunized with rotavirus vaccine and
L. plantarumCRL1506 but not in animals treated with
L. plantarum CRL1905 or its BLPs, indicating that distinct strains function differently as mucosal adjuvants [108]. The final outcome of cellular response against beneficial immunomodulatory lactobacilli depends on the combination of different microbial-associated molecular patterns (MAMPs) that can interact with various PRRs to trigger different signaling pathways [112]. The unique combination of cellular and molecular interactions established between certain lactobacilli strains with non-immune and immune cells explains why the immunomodulatory properties of LAB in general, andCRL1905 or its BLPs, indicating that distinct strains function differently as mucosal adjuvants [39]. The final outcome of cellular response against beneficial immunomodulatory lactobacilli depends on the combination of different microbial-associated molecular patterns (MAMPs) that can interact with various PRRs to trigger different signaling pathways [43]. The unique combination of cellular and molecular interactions established between certain lactobacilli strains with non-immune and immune cells explains why the immunomodulatory properties of LAB in general, and
L. plantarumin particular, are a strain dependent characteristic. Then, an efficient selection of the most effective
L. plantarumstrains with the ability to beneficially modulate intestinal immunity is necessary, in order to use them in the development of functional foods or as adjuvants for mucosal vaccines. This becomes more relevant if the desired functional food or vaccine formulation aims to confer immunity not only in the intestinal mucosa but also in distant mucosal sites such as the respiratory tract, since this effect is only achieved by a small group of strains, as outlined below.
2.2. Modulation of Respiratory Antiviral Immune Responses by L. plantarum
Experiments in animal models and human clinical trials have demonstrated that certain LAB strains, when orally administered, are effective in modulating the respiratory immunity and enhancing the resistance against bacterial and viral infections. This group of immunobiotic LAB improve the resistance of children, adults and the elderly to respiratory infections such as the pneumococcal pneumonia-, the common cold- and influenza-like symptoms [85]. Among the LAB strains that possess this unique immunomodulatory property, there is a group of strains belonging to the species
Experiments in animal models and human clinical trials have demonstrated that certain LAB strains, when orally administered, are effective in modulating the respiratory immunity and enhancing the resistance against bacterial and viral infections. This group of immunobiotic LAB improve the resistance of children, adults and the elderly to respiratory infections such as the pneumococcal pneumonia-, the common cold- and influenza-like symptoms [16]. Among the LAB strains that possess this unique immunomodulatory property, there is a group of strains belonging to the species
L. plantarum
(
Figure 4).
2).
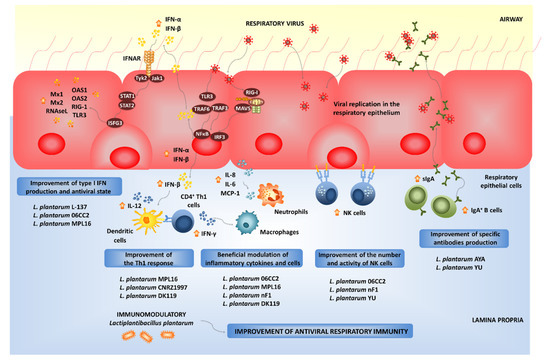
Beneficial effects of orally administered immunobiotic
strains on the resistance and immune responses against viruses in the respiratory mucosa.
Not all lactobacilli strains possessing immunomodulatory abilities in the intestinal mucosa are capable of stimulating the respiratory immunity when orally administered [110,111,112,113,114,115,116]. Orally administered
Not all lactobacilli strains possessing immunomodulatory abilities in the intestinal mucosa are capable of stimulating the respiratory immunity when orally administered [41][42][43][44][45][46][47]. Orally administered
L. plantarum
MPL16 but not
L. plantarum
CRL1506 was capable of modulating respiratory immunity. The MPL16 strain is capable of improving the levels of IFN-γ and type I IFNs in the respiratory tract, indicating its ability to modulate the function of CD4
+
IFN-γ
+
T cells and CD11c
+
SiglecF
+
alveolar macrophages. Moreover, the increased levels of respiratory IFN-γ and IFN-β found in
L. plantarum MPL16-treated mice correlated with the improved resistance of mice to RSV infection [87].
MPL16-treated mice correlated with the improved resistance of mice to RSV infection [18].
The strain-dependent ability of orally administered LAB to modulate the respiratory immunity was also confirmed by other research groups. The capacity of several
L. plantarum strains to modulate the respiratory immunity when orally administered was evaluated in a lethal model of IFV pneumonia [110]. Among the strains evaluated,
strains to modulate the respiratory immunity when orally administered was evaluated in a lethal model of IFV pneumonia [41]. Among the strains evaluated,
L. plantarum
06CC2 stood out for its ability to improve the survival of mice infected with IFV, while the
L. plantarum
strains 05AM23, 06TCa8, 06TCa40 and 06CC9 induced no protective effect. Orally administered 06CC2 strain reduced IFV titers in lungs and improved Th1 response in the respiratory tract and NK cell activity in both lungs and spleens. In addition, in a large-scale screening of immunomodulatory LAB, several
Lactiplantibacillus strains were evaluated in their ability to modulate immunity in both TNF-α-activated HT-29 cells and peripheral blood mononuclear cells [116]. Among the evaluated strains,
strains were evaluated in their ability to modulate immunity in both TNF-α-activated HT-29 cells and peripheral blood mononuclear cells [47]. Among the evaluated strains,
L. plantarum
CNRZ1997 was able to significantly increase the production of inflammatory cytokines in both epithelial and immune cells, while other
L. plantarum
strains showed no effect or demonstrated to have an anti-inflammatory capacity. Interestingly, the work demonstrated that the orally administered CNRZ1997 strain was capable of reducing the replication of IFV in the respiratory tract of mice. The oral treatment with
L. plantarum
CNRZ1997 was as effective as the oral treatments with
L. rhamnosus
GG or
L. casei DN114-001 (two commercial probiotic strains with anti-IFV properties in preclinical and human trials) to protect mice against IFV infection [84].
DN114-001 (two commercial probiotic strains with anti-IFV properties in preclinical and human trials) to protect mice against IFV infection [15].
It seems that viability is not a requisite for the beneficial modulation of the antiviral respiratory immunity, as was seen with heat-killed
L. plantarum L-137, which improved Th1 immunity in healthy subjects [117]. The strain was capable of increasing concanavalin A-induced proliferation of blood lymphocytes and enhancing the percentages of CD4
L-137, which improved Th1 immunity in healthy subjects [48]. The strain was capable of increasing concanavalin A-induced proliferation of blood lymphocytes and enhancing the percentages of CD4
+
IFN-γ
+
T cells. Orally administered heat-killed
L. plantarum L-137 beneficially modulated antiviral immunity in mice [118] and pigs [119], and stimulated the production of IFN-β in mice following IFV infection [118]. Further studies in pigs demonstrated that the L-137 strain significantly improved the expression of IFN-β in blood cells [119]. The treatment with
L-137 beneficially modulated antiviral immunity in mice [49] and pigs [50], and stimulated the production of IFN-β in mice following IFV infection [49]. Further studies in pigs demonstrated that the L-137 strain significantly improved the expression of IFN-β in blood cells [50]. The treatment with
L. plantarum L-137 was translated into a higher body weight gain of pigs when compared to controls, indicating an improved health status. The impact of the L-137 strain on upper respiratory tract infections was evaluated in human subjects with high psychological stress levels [120].
L-137 was translated into a higher body weight gain of pigs when compared to controls, indicating an improved health status. The impact of the L-137 strain on upper respiratory tract infections was evaluated in human subjects with high psychological stress levels [51].
L. plantarum
L-137 reduced the incidence, duration and severity of respiratory infections as well as the duration of medication. Similarly, the consumption of
L. plantarum nF1-fortified yogurt improved IL-12 and IFN-γ production and NK cell activity in elderly subjects, indicating its potential to stimulate antiviral immunity [121]. In fact, the oral treatment of mice with heat-treated
nF1-fortified yogurt improved IL-12 and IFN-γ production and NK cell activity in elderly subjects, indicating its potential to stimulate antiviral immunity [52]. In fact, the oral treatment of mice with heat-treated
L. plantarum nF1 significantly increased the expression of IFN-γ, IL-2 and IL-12 in the spleen, the NK cell membrane marker Klrb1 and the NK and T cells activation marker CD69 after the challenge with IFV [122]. In line with these findings, a higher splenic NK activity was observed in nF1-treated mice. Those immunological changes induced by
nF1 significantly increased the expression of IFN-γ, IL-2 and IL-12 in the spleen, the NK cell membrane marker Klrb1 and the NK and T cells activation marker CD69 after the challenge with IFV [53]. In line with these findings, a higher splenic NK activity was observed in nF1-treated mice. Those immunological changes induced by
L. plantarum nF1 correlated with a reduction of IFV titers and an improved survival of mice [122,123].
nF1 correlated with a reduction of IFV titers and an improved survival of mice [53][54].
Alveolar macrophages are key cells in the beneficial modulation of respiratory immunity induced by gut microorganisms. It was shown that the intestinal microbiota help to maintain the optimal antiviral functions of alveolar macrophages. Beneficial gut microbes are involved in the efficient capacity of alveolar macrophages to produce type I IFNs and antiviral factors including IRF7, IFNGR1, STAT1, STAT2, IFIT3, MX1 and OAS1 that improve the resistance to respiratory viral infections [81]. We have recently demonstrated that immunobiotics with the ability of modulating the respiratory immunity are able to functionally modulate the alveolar macrophages response to viral challenges. In fact, our results indicate that alveolar macrophages greatly contribute to the augment of IFN-γ and IFN-β in the respiratory tract of mice orally treated with
Alveolar macrophages are key cells in the beneficial modulation of respiratory immunity induced by gut microorganisms. It was shown that the intestinal microbiota help to maintain the optimal antiviral functions of alveolar macrophages. Beneficial gut microbes are involved in the efficient capacity of alveolar macrophages to produce type I IFNs and antiviral factors including IRF7, IFNGR1, STAT1, STAT2, IFIT3, MX1 and OAS1 that improve the resistance to respiratory viral infections [12]. We have recently demonstrated that immunobiotics with the ability of modulating the respiratory immunity are able to functionally modulate the alveolar macrophages response to viral challenges. In fact, our results indicate that alveolar macrophages greatly contribute to the augment of IFN-γ and IFN-β in the respiratory tract of mice orally treated with
L. rhamnosus CRL1505 [115] or
CRL1505 [46] or
L. plantarum
MPL16 (submitted for publication). Moreover, an improved expression of IFNAR1, Mx2, OAS1, OAS2, RNAseL and IFITM3 in alveolar macrophages after the oral treatment with
L. rhamnosus
CRL1505 or
L. plantarum
MPL16 was detected in our experiments. In line with our results, it was reported that mice orally treated with
L. plantarum DK119 had higher BAL IL-2 and IFN-γ levels, and a low degree of inflammation upon IFV infection [124]. The lactobacilli treatment reduced viral loads in the lungs and improved survival of infected mice. In contrast, the levels of IL-6 and TNF-α in the respiratory tract of DK119-treated mice were lower compared to those from control mice after IFV infection. Consistent with this pattern of cytokines, a significantly reduced degree of inflammation was observed in mice receiving
DK119 had higher BAL IL-2 and IFN-γ levels, and a low degree of inflammation upon IFV infection [55]. The lactobacilli treatment reduced viral loads in the lungs and improved survival of infected mice. In contrast, the levels of IL-6 and TNF-α in the respiratory tract of DK119-treated mice were lower compared to those from control mice after IFV infection. Consistent with this pattern of cytokines, a significantly reduced degree of inflammation was observed in mice receiving
L. plantarum
DK119. In mice treated with clodronate liposomes to induce the depletion of CD3
-
CD11b
-
CD11c
+
F4/80
+
alveolar macrophages, the administration of the DK119 strain could not induce modifications in the severe weight loss and mortality induced by IFV infection, indicating that alveolar macrophages have a key role in
L. plantarum DK119-mediated protection [124].
DK119-mediated protection [55].
The exact nature of the cellular and molecular signals used by orally administered immunobiotics to modulate the respiratory antiviral immunity remain to be determined. Our recent experiments blocking CD4
+ T cells and IFN-γ allow the speculation of a potential mechanism that could explain the remote effect induced by orally administered immunobiotics [115]. The existence of the so-called common mucosal immune system implies that the immune cells activated in one mucosal tissue can mobilize and reach distant mucosal sites, where they can influence immune responses. Then, the mobilization of B and T cells from the intestinal mucosa to the respiratory tract could be involved in the beneficial effects exerted by orally administered immunobiotics [85,115]. Strains such as
T cells and IFN-γ allow the speculation of a potential mechanism that could explain the remote effect induced by orally administered immunobiotics [46]. The existence of the so-called common mucosal immune system implies that the immune cells activated in one mucosal tissue can mobilize and reach distant mucosal sites, where they can influence immune responses. Then, the mobilization of B and T cells from the intestinal mucosa to the respiratory tract could be involved in the beneficial effects exerted by orally administered immunobiotics [16][46]. Strains such as
L. plantarum
MPL16 would induce the mobilization CD4
+
IFN-γ
+
T cells from the intestine to the lungs, and the local production of IFN-γ would modulate the respiratory tract innate immune microenvironment, leading to the activation of local immune cells such as alveolar macrophages. As mentioned in the previous section,
L. plantarum 06CC2 enhances the expression of IFN-γ and IL-12 in Peyer’s patches, as well as in the respiratory tract supporting the hypothesis that the 06CC2 strain elicited its protective effect in the respiratory tract through intestinal immunity [110]. On the other hand,
06CC2 enhances the expression of IFN-γ and IL-12 in Peyer’s patches, as well as in the respiratory tract supporting the hypothesis that the 06CC2 strain elicited its protective effect in the respiratory tract through intestinal immunity [41]. On the other hand,
L. plantarum YU was shown to strongly induce the in vitro production of IL-12 by antigen presenting cells from murine Peyer’s patches, mesenteric lymph nodes and spleen [125], which was associated to TLR2 stimulation. Orally administered
YU was shown to strongly induce the in vitro production of IL-12 by antigen presenting cells from murine Peyer’s patches, mesenteric lymph nodes and spleen [56], which was associated to TLR2 stimulation. Orally administered
L. plantarum
YU was able to reduce body weight loss and lung viral replication after a challenge with IFV, to enhance intestinal IgA concentration and splenic NK cell activity. Of note, the levels of IFV-specific secretory IgA in BAL samples were also increased by the oral treatment with
L. plantarum YU [125]. Several LAB strains were evaluated according to their ability to stimulate the production of IgA in primary cultures of immune cells isolated from mice Peyer’s patches [126]. Among the strains evaluated,
YU [56]. Several LAB strains were evaluated according to their ability to stimulate the production of IgA in primary cultures of immune cells isolated from mice Peyer’s patches [57]. Among the strains evaluated,
L. plantarum
AYA and N63 had the ability to significantly increase IgA levels in vitro. However, when the two strains were evaluated in vivo, only
L. plantarum
AYA increased the levels of intestinal IgA in orally treated mice. Of note, the immunomodulatory effect was obtained with both the viable bacterium as well as the heat-killed
L. plantarum
AYA. The oral administration of the AYA strain stimulated DCs by modulating their expression of IL-6 and significantly increased the numbers of IgA
+
B220
+ cells in mice Peyer´s patches. Mice treated with AYA strain and then challenged with IFV had an improved resistance to the viral infection. This protective effect was associated to the induction of higher levels of IgA in the respiratory tract [126]. The work suggested that
cells in mice Peyer´s patches. Mice treated with AYA strain and then challenged with IFV had an improved resistance to the viral infection. This protective effect was associated to the induction of higher levels of IgA in the respiratory tract [57]. The work suggested that
L. plantarum
AYA stimulate IL-6 production in intestinal DCs, which in turn promotes the differentiation of IgA
+
B cells into plasma cells improving intestinal IgA production (
Figure 3). Moreover,
1). Moreover,
L. plantarum
AYA would also induce the mobilization of IgA
+
B cells from the intestinal mucosa to the respiratory tract, enhancing the IgA production in the context of a viral respiratory infection (
).
All in all, these data indicate that appropriate immunobiotic
L. plantarum
strains could be used to improve antiviral immunity on both the intestinal and the respiratory mucosa at the same time, and therefore, they are an interesting biotechnological resource for the development of mucosal vaccines.
References
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534.
- WHO. Weekly Operational Update on COVID-19-1. February 2021. Available online: (accessed on 1 February 2021).
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632.
- Frederiksen, L.S.F.; Zhang, Y.; Foged, C.; Thakur, A. The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front. Immunol. 2020, 11, 1817.
- Moore, J.P.; Klasse, P.J. COVID-19 Vaccines:’’Warp Speed’’ Needs Mind Melds, Not Warped Minds. J. Virol. 2020, 94.
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435.
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505.
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.J. Mucosal vaccines: Strategies and challenges. Immunol. Lett. 2020, 217, 116–125.
- Medina, M.; Vintini, E.; Villena, J.; Raya, R.; Alvarez, S. Lactococcus lactis as an adjuvant and delivery vehicle of antigens against pneumococcal respiratory infections. Bioeng. Bugs 2010, 1, 313–325.
- Villena, J.; Oliveira, M.L.; Ferreira, P.C.; Salva, S.; Alvarez, S. Lactic acid bacteria in the prevention of pneumococcal respiratory infection: Future opportunities and challenges. Int. Immunopharmacol. 2011, 11, 1633–1645.
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359.
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170.
- Villena, J.; Vizoso-Pinto, M.G.; Kitazawa, H. Intestinal Innate Antiviral Immunity and Immunobiotics: Beneficial Effects against Rotavirus Infection. Front. Immunol. 2016, 7, 563.
- Bradley, K.C.; Finsterbusch, K.; Schnepf, D.; Crotta, S.; Llorian, M.; Davidson, S.; Fuchs, S.Y.; Staeheli, P.; Wack, A. Microbiota-Driven Tonic Interferon Signals in Lung Stromal Cells Protect from Influenza Virus Infection. Cell Rep. 2019, 28, 245–256.e244.
- Zelaya, H.; Alvarez, S.; Kitazawa, H.; Villena, J. Respiratory Antiviral Immunity and Immunobiotics: Beneficial Effects on Inflammation-Coagulation Interaction during Influenza Virus Infection. Front. Immunol. 2016, 7, 633.
- Villena, J.; Kitazawa, H. The Modulation of Mucosal Antiviral Immunity by Immunobiotics: Could They Offer Any Benefit in the SARS-CoV-2 Pandemic? Front. Physiol. 2020, 11, 699.
- Mizuno, H.; Arce, L.; Tomotsune, K.; Albarracin, L.; Funabashi, R.; Vera, D.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Sasaki, Y.; et al. Lipoteichoic Acid Is Involved in the Ability of the Immunobiotic Strain Lactobacillus plantarum CRL1506 to Modulate the Intestinal Antiviral Innate Immunity Triggered by TLR3 Activation. Front. Immunol. 2020, 11, 571.
- Albarracin, L.; Garcia-Castillo, V.; Masumizu, Y.; Indo, Y.; Islam, M.A.; Suda, Y.; Garcia-Cancino, A.; Aso, H.; Takahashi, H.; Kitazawa, H.; et al. Efficient Selection of New Immunobiotic Strains With Antiviral Effects in Local and Distal Mucosal Sites by Using Porcine Intestinal Epitheliocytes. Front. Immunol. 2020, 11, 543.
- Albarracin, L.; Kobayashi, H.; Iida, H.; Sato, N.; Nochi, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Transcriptomic Analysis of the Innate Antiviral Immune Response in Porcine Intestinal Epithelial Cells: Influence of Immunobiotic Lactobacilli. Front. Immunol. 2017, 8, 57.
- Parashar, U.D.; Gibson, C.J.; Bresee, J.S.; Glass, R.I. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 2006, 12, 304–306.
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From pathogenesis to vaccination. Gastroenterology 2009, 136, 1939–1951.
- Hosoya, S.; Villena, J.; Shimazu, T.; Tohno, M.; Fujie, H.; Chiba, E.; Shimosato, T.; Aso, H.; Suda, Y.; Kawai, Y.; et al. Immunobiotic lactic acid bacteria beneficially regulate immune response triggered by poly(I:C) in porcine intestinal epithelial cells. Vet. Res. 2011, 42, 111.
- Ishizuka, T.; Kanmani, P.; Kobayashi, H.; Miyazaki, A.; Soma, J.; Suda, Y.; Aso, H.; Nochi, T.; Iwabuchi, N.; Xiao, J.Z.; et al. Immunobiotic Bifidobacteria Strains Modulate Rotavirus Immune Response in Porcine Intestinal Epitheliocytes via Pattern Recognition Receptor Signaling. PLoS ONE 2016, 11, e0152416.
- Villena, J.; Chiba, E.; Vizoso-Pinto, M.G.; Tomosada, Y.; Takahashi, T.; Ishizuka, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H. Immunobiotic Lactobacillus rhamnosus strains differentially modulate antiviral immune response in porcine intestinal epithelial and antigen presenting cells. BMC Microbiol. 2014, 14, 126.
- Morelli, M.; Ogden, K.M.; Patton, J.T. Silencing the alarms: Innate immune antagonism by rotavirus NSP1 and VP3. Virology 2015, 479–480, 75–84.
- Tada, A.; Zelaya, H.; Clua, P.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Immunobiotic Lactobacillus strains reduce small intestinal injury induced by intraepithelial lymphocytes after Toll-like receptor 3 activation. Inflamm. Res. 2016, 65, 771–783.
- Villena, J.; Chiba, E.; Tomosada, Y.; Salva, S.; Marranzino, G.; Kitazawa, H.; Alvarez, S. Orally administered Lactobacillus rhamnosus modulates the respiratory immune response triggered by the viral pathogen-associated molecular pattern poly(I:C). BMC Immunol. 2012, 13, 53.
- Wang, K.; Ran, L.; Yan, T.; Niu, Z.; Kan, Z.; Zhang, Y.; Yang, Y.; Xie, L.; Huang, S.; Yu, Q.; et al. Anti-TGEV Miller Strain Infection Effect of Lactobacillus plantarum Supernatant Based on the JAK-STAT1 Signaling Pathway. Front. Microbiol. 2019, 10, 2540.
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696.
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280.
- Kinoshita, N.; Hiroi, T.; Ohta, N.; Fukuyama, S.; Park, E.J.; Kiyono, H. Autocrine IL-15 mediates intestinal epithelial cell death via the activation of neighboring intraepithelial NK cells. J. Immunol. 2002, 169, 6187–6192.
- Zhou, R.; Wei, H.; Sun, R.; Tian, Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J. Immunol. 2007, 178, 4548–4556.
- Zhou, R.; Wei, H.; Sun, R.; Zhang, J.; Tian, Z. NKG2D recognition mediates Toll-like receptor 3 signaling-induced breakdown of epithelial homeostasis in the small intestines of mice. Proc. Natl. Acad. Sci. USA 2007, 104, 7512–7515.
- Kim, K.; Lee, G.; Thanh, H.D.; Kim, J.H.; Konkit, M.; Yoon, S.; Park, M.; Yang, S.; Park, E.; Kim, W. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. J. Dairy Sci. 2018, 101, 5702–5712.
- Shin, D.Y.; Yi, D.Y.; Jo, S.; Lee, Y.M.; Kim, J.H.; Kim, W.; Park, M.R.; Yoon, S.M.; Kim, Y.; Yang, S.; et al. Effect of a new Lactobacillus plantarum product, LRCC5310, on clinical symptoms and virus reduction in children with rotaviral enteritis. Medicine 2020, 99, e22192.
- Le Bon, A.; Tough, D.F. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 2002, 14, 432–436.
- Montoya, M.; Schiavoni, G.; Mattei, F.; Gresser, I.; Belardelli, F.; Borrow, P.; Tough, D.F. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 2002, 99, 3263–3271.
- Yoo, J.K.; Baker, D.P.; Fish, E.N. Interferon-beta modulates type 1 immunity during influenza virus infection. Antivir. Res. 2010, 88, 64–71.
- Raya Tonetti, F.; Arce, L.; Salva, S.; Alvarez, S.; Takahashi, H.; Kitazawa, H.; Vizoso-Pinto, M.G.; Villena, J. Immunomodulatory Properties of Bacterium-Like Particles Obtained From Immunobiotic Lactobacilli: Prospects for Their Use as Mucosal Adjuvants. Front. Immunol. 2020, 11, 15.
- Le Bon, A.; Schiavoni, G.; D’Agostino, G.; Gresser, I.; Belardelli, F.; Tough, D.F. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 2001, 14, 461–470.
- Takeda, S.; Takeshita, M.; Kikuchi, Y.; Dashnyam, B.; Kawahara, S.; Yoshida, H.; Watanabe, W.; Muguruma, M.; Kurokawa, M. Efficacy of oral administration of heat-killed probiotics from Mongolian dairy products against influenza infection in mice: Alleviation of influenza infection by its immunomodulatory activity through intestinal immunity. Int. Immunopharmacol. 2011, 11, 1976–1983.
- Matsusaki, T.; Takeda, S.; Takeshita, M.; Arima, Y.; Tsend-Ayush, C.; Oyunsuren, T.; Sugita, C.; Yoshida, H.; Watanabe, W.; Kurokawa, M. Augmentation of T helper type 1 immune response through intestinal immunity in murine cutaneous herpes simplex virus type 1 infection by probiotic Lactobacillus plantarum strain 06CC2. Int. Immunopharmacol. 2016, 39, 320–327.
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184.
- Chiba, E.; Tomosada, Y.; Vizoso-Pinto, M.G.; Salva, S.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Immunobiotic Lactobacillus rhamnosus improves resistance of infant mice against respiratory syncytial virus infection. Int. Immunopharmacol. 2013, 17, 373–382.
- Zelaya, H.; Tsukida, K.; Chiba, E.; Marranzino, G.; Alvarez, S.; Kitazawa, H.; Aguero, G.; Villena, J. Immunobiotic lactobacilli reduce viral-associated pulmonary damage through the modulation of inflammation-coagulation interactions. Int. Immunopharmacol. 2014, 19, 161–173.
- Garcia-Castillo, V.; Tomokiyo, M.; Raya Tonetti, F.; Islam, M.A.; Takahashi, H.; Kitazawa, H.; Villena, J. Alveolar Macrophages Are Key Players in the Modulation of the Respiratory Antiviral Immunity Induced by Orally Administered Lacticaseibacillus rhamnosus CRL1505. Front. Immunol. 2020, 11, 568636.
- Kechaou, N.; Chain, F.; Gratadoux, J.J.; Blugeon, S.; Bertho, N.; Chevalier, C.; Le Goffic, R.; Courau, S.; Molimard, P.; Chatel, J.M.; et al. Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl. Environ. Microbiol. 2013, 79, 1491–1499.
- Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Yoshikai, Y.; Tsuru, T. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J. Nutr. 2006, 136, 3069–3073.
- Maeda, N.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Kase, T.; Yoshikai, Y. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 2009, 9, 1122–1125.
- Arimori, Y.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Shidara, O.; Ichikawa, H.; Yoshikai, Y. Daily intake of heat-killed Lactobacillus plantarum L-137 enhances type I interferon production in healthy humans and pigs. Immunopharmacol. Immunotoxicol. 2012, 34, 937–943.
- Hirose, Y.; Yamamoto, Y.; Yoshikai, Y.; Murosaki, S. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. J. Nutr. Sci. 2013, 2, e39.
- Lee, A.; Lee, Y.J.; Yoo, H.J.; Kim, M.; Chang, Y.; Lee, D.S.; Lee, J.H. Consumption of Dairy Yogurt Containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and Heat-Treated Lactobacillus plantarum Improves Immune Function Including Natural Killer Cell Activity. Nutrients 2017, 9, 558.
- Kim, D.H.; Chung, W.C.; Chun, S.H.; Han, J.H.; Song, M.J.; Lee, K.W. Enhancing the natural killer cell activity and anti-influenza effect of heat-treated Lactobacillus plantarum nF1-fortified yogurt in mice. J. Dairy Sci. 2018, 101, 10675–10684.
- Park, S.; Kim, J.I.; Bae, J.Y.; Yoo, K.; Kim, H.; Kim, I.H.; Park, M.S.; Lee, I. Effects of heat-killed Lactobacillus plantarum against influenza viruses in mice. J. Microbiol. 2018, 56, 145–149.
- Park, M.K.; Ngo, V.; Kwon, Y.M.; Lee, Y.T.; Yoo, S.; Cho, Y.H.; Hong, S.M.; Hwang, H.S.; Ko, E.J.; Jung, Y.J.; et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS ONE 2013, 8, e75368.
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum strain YU from fermented foods activates Th1 and protective immune responses. Int. Immunopharmacol. 2011, 11, 2017–2024.
- Kikuchi, Y.; Kunitoh-Asari, A.; Hayakawa, K.; Imai, S.; Kasuya, K.; Abe, K.; Adachi, Y.; Fukudome, S.; Takahashi, Y.; Hachimura, S. Oral administration of Lactobacillus plantarum strain AYA enhances IgA secretion and provides survival protection against influenza virus infection in mice. PLoS ONE 2014, 9, e86416.
